Complications Following Inappropriate Intravitreal Triamcinolone Acetonide Injection 115
Figure 1. Fundus image at initial examination. On the first
examination, central retinal vein occlusion was observed (a).
Optical coherence tomography revealed macular edema (b).
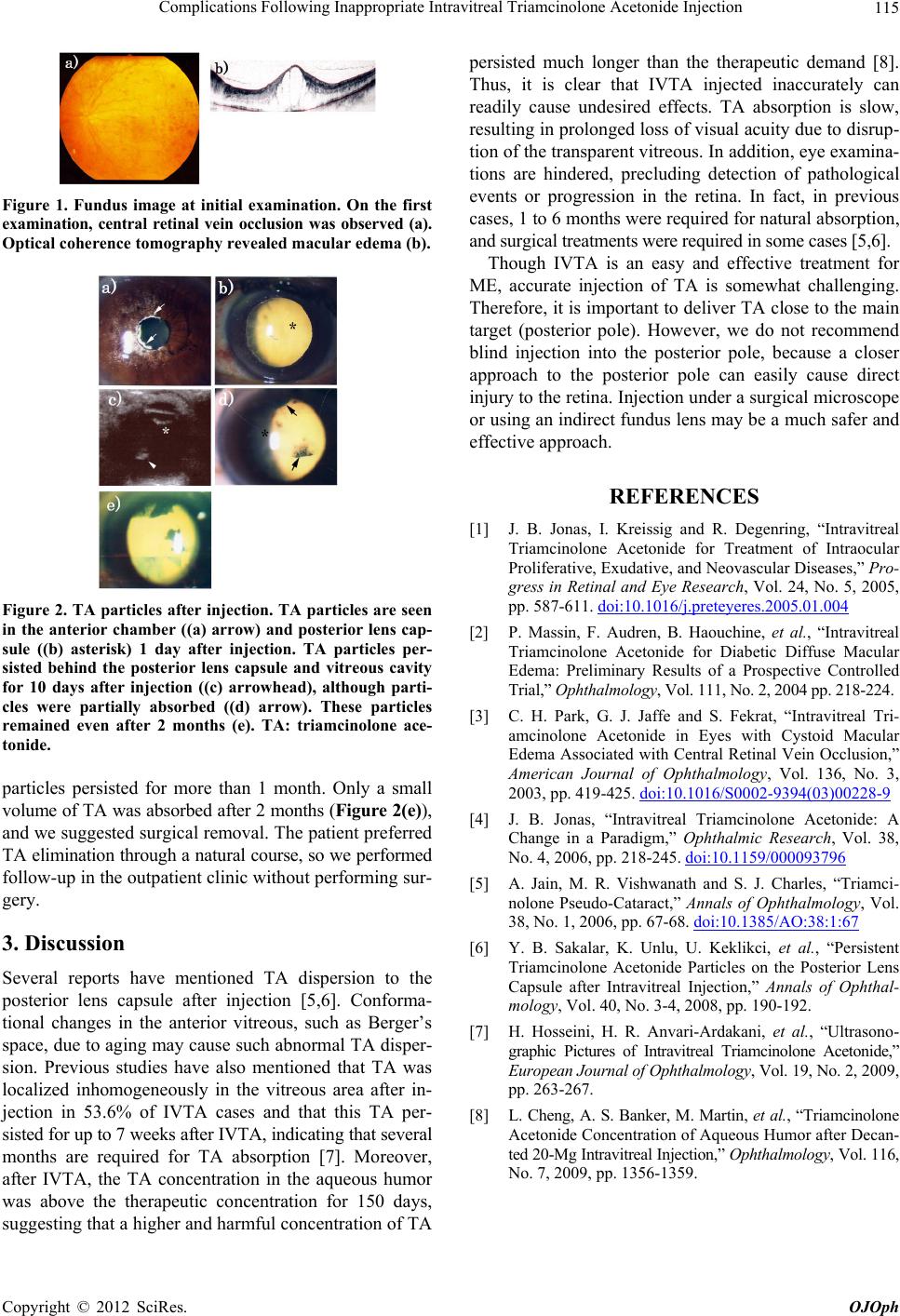
Figure 2. TA particles after injection. TA particles are seen
in the anterior chamber ((a) arrow) and posterior lens cap-
sule ((b) asterisk) 1 day after injection. TA particles per-
sisted behind the posterior lens capsule and vitreous cavity
for 10 days after injection ((c) arrowhead), although parti-
cles were partially absorbed ((d) arrow). These particles
remained even after 2 months (e). TA: triamcinolone ace-
tonide.
particles persisted for more than 1 month. Only a small
volume of TA was absorbed after 2 months (Figure 2(e)),
and we suggested surgical removal. The patient preferred
TA elimination through a natural course, so we performed
follow-up in the outpatient clinic without performing su r-
gery.
3. Discussion
Several reports have mentioned TA dispersion to the
posterior lens capsule after injection [5,6]. Conforma-
tional changes in the anterior vitreous, such as Berger’s
space, due to aging may cause such abnormal TA disper-
sion. Previous studies have also mentioned that TA was
localized inhomogeneously in the vitreous area after in-
jection in 53.6% of IVTA cases and that this TA per-
sisted for up to 7 weeks after IVTA, indicating that several
months are required for TA absorption [7]. Moreover,
after IVTA, the TA concentration in the aqueous humor
was above the therapeutic concentration for 150 days,
suggesting that a higher and harmful concentration of TA
persisted much longer than the therapeutic demand [8].
Thus, it is clear that IVTA injected inaccurately can
readily cause undesired effects. TA absorption is slow,
resulting in prolong ed loss of visual acuity due to disrup-
tion of the transparent vitreous. In addition , eye examina-
tions are hindered, precluding detection of pathological
events or progression in the retina. In fact, in previous
cases, 1 to 6 months were required for natural absorption,
and surgical treatments were required in some cases [5,6].
Though IVTA is an easy and effective treatment for
ME, accurate injection of TA is somewhat challenging.
Therefore, it is important to deliv er TA close to the main
target (posterior pole). However, we do not recommend
blind injection into the posterior pole, because a closer
approach to the posterior pole can easily cause direct
injury to the retina. Injection under a surg ical microscope
or using an indirect fundus lens may be a much safer and
effective approach.
REFERENCES
[1] J. B. Jonas, I. Kreissig and R. Degenring, “Intravitreal
Triamcinolone Acetonide for Treatment of Intraocular
Proliferative, Exudative, and Neovascular Diseases,” Pro-
gress in Retinal and Eye Research, Vol. 24, No. 5, 2005,
pp. 587-611. doi:10.1016/j.preteyeres.2005.01.004
[2] P. Massin, F. Audren, B. Haouchine, et al., “Intravitreal
Triamcinolone Acetonide for Diabetic Diffuse Macular
Edema: Preliminary Results of a Prospective Controlled
Trial,” Ophthalmology, Vol. 111, No. 2, 2004 pp. 218-224.
[3] C. H. Park, G. J. Jaffe and S. Fekrat, “Intravitreal Tri-
amcinolone Acetonide in Eyes with Cystoid Macular
Edema Associated with Central Retinal Vein Occlusion,”
American Journal of Ophthalmology, Vol. 136, No. 3,
2003, pp. 419-425. doi:10.1016/S0002-9394(03)00228-9
[4] J. B. Jonas, “Intravitreal Triamcinolone Acetonide: A
Change in a Paradigm,” Ophthalmic Research, Vol. 38,
No. 4, 2006, pp. 218-245. doi:10.1159/000093796
[5] A. Jain, M. R. Vishwanath and S. J. Charles, “Triamci-
nolone Pseudo-Cataract,” Annals of Ophthalmology, Vol.
38, No. 1, 2006, pp. 67-68. doi:10.1385/AO:38:1:67
[6] Y. B. Sakalar, K. Unlu, U. Keklikci, et al., “Persistent
Triamcinolone Acetonide Particles on the Posterior Lens
Capsule after Intravitreal Injection,” Annals of Ophthal-
mology, Vol. 40, No. 3-4, 2008, pp. 190-192.
[7] H. Hosseini, H. R. Anvari-Ardakani, et al., “Ultrasono-
graphic Pictures of Intravitreal Triamcinolone Acetonide,”
European Journal of Ophthalmology, Vol. 19, No. 2, 2009,
pp. 263-267.
[8] L. Cheng, A. S. Banker, M. Martin, et al., “Triamcinolone
Acetonide Concentration of Aqueous Humor after Decan-
ted 20-Mg Intravitreal Injection,” Ophthalmology, Vol. 116,
No. 7, 2009, pp. 1356-1359.
Copyright © 2012 SciRes. OJOph