 Atmospheric and Climate Sciences, 2012, 2, 546-561 http://dx.doi.org/10.4236/acs.2012.24050 Published Online October 2012 (http://www.SciRP.org/journal/acs) Effects of Changed Climate Conditions on Tropospheric Ozone over Three Centuries Gitte Brandt Hedegaard1,2,3, Jesper Heile Christensen1, Camilla Geels1, Allan Gross1, Kaj Mantzius Hansen1, Wilhelm May2, Azimeh Zare1,4, Jørgen Brandt1 1Department of Environmental Science, Aarhus University, Roskilde, Denmark 2Danish Climate Centre, Danish Meteorological Institute, Copenhagen, Denmark 3Centre for Environmental and Climate Research, Lund University, Lund, Sweden 4Institute of Geophysics, University of Tehran, Tehran, Iran Email: jbr@dmu.dk, gitte.brandt_hedegaard@cec.lu.se Received June 8, 2012; revised July 10, 2012; accepted July 21, 2012 ABSTRACT The ozone chemistry in four decades (1890s, 1990s, 2090s and 2190s) representing the changes over three centuries has been simulated using the chemistry version of the atmospheric long-range transport model: the Danish Eulerian Hemi- spheric Model (DEHM) forced with meteorology projected by the ECHAM5/MPI-OM coupled Atmosphere-Ocean General Circulation Model. The largest changes in meteorology, ozone and its precursors are found in the 21st century, however, also significant changes are found in the 22nd century. At surface level the ozone concentration is projected to increase due to climate change in the areas where substantial amounts of ozone precursors are emitted. Elsewhere a sig- nificant decrease is projected at the surface. In the free troposphere a general increase is found in the entire Northern Hemisphere except in the tropics, where the ozone concentration is decreasing. In the Arctic the ozone concentration will increase in the entire air column, which most likely is due to changes in atmospheric transport. Changes in tem- perature, humidity and the naturally emitted Volatile Organic Compounds (VOCs) are governing the changes in ozone both in the past, present and future century. Keywords: Ozone; Climate Change; Air Quality; Modelling 1. Introduction Since the industrialization the concentration of green- house gases in the atmosphere has increased. The green- house gases affect our climate, which has been undergo- ing a continuous change into what we experience today. Atmospheric chemistry is highly dependent on tempera- ture, humidity and solar radiation and the observed war- ming will inherently affect the chemical composition of the atmosphere. The effect of changes in meteorology on air pollution levels implies that even though we today decide to keep the anthropogenic emissions of air pollutants constant, the air pollution levels will change anyway in response to climate change. This paper focus on ozone, which in the lower tropo- sphere acts as a toxic gas that through respiratory and cardiovascular diseases can lead to premature death of humans. Furthermore ozone is harmful to plants in high concentrations and can diminish the crop yield substan- tially. Since air pollutants like ozone can have tremen- dous effect on human health, agriculture, the terrestrial and marine eco-systems etc., it is important to project the future air pollution levels. These findings can be used to develop and implement new air pollution legislation, which hopefully will minimize the negative conse- quences of human interference with the environment. So far the research community has concentrated on air quality in the 21st century [1-6]. Here we use a 340-year long climate simulation to investigate ozone and its re- lated precursors in the past, present and future. We focus on the impacts of climate change alone by keeping the anthropogenic emissions constant at a year 2000 level. In Section 2 the climate model and the applied climate simulation are described. Section 3 describes the applied chemistry model and the experimental setup and finally the results are displayed and discussed in relation to the chemical reactions included in the model in Sections 4 and 5. In Section 6 the findings are summarized together with some future perspectives. 2. Climate Projections Using ECHAM5/MPI-OM The coupled atmosphere-ocean model ECHAM5/ MPI- OM used to drive the chemical transport model DEHM C opyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 547 consists of the atmospheric general circulation model ECHAM5 [7,8] and the ocean-sea-ice model MPI-OM [9]. The atmospheric model ECHAM5 is horizontally defined in a spectral grid with truncation T63. Vertically the model is defined in a hybrid sigma-pressure system and divided into 31 layers with the top layer at 10 hPa. State-of-the-art parameterizations are used for shortwave and long-wave radiation, stratiform clouds, boundary layer and land-surface processes and for describing grav- ity wave drag in the model. A description of the aerosol effect included in this model version can be found in [10]. The ocean-sea-ice model has a horizontal resolution of 1.5˚ × 1.5˚ and is vertically discretized into 40 z-levels. Concentration and thickness of sea ice is treated interac- tively in the model by a dynamic and thermodynamic sea-ice model. For further details of the ocean-sea-ice model, see [9]. The atmosphere model ECHAM5 and the ocean-sea- ice model MPI-OM is interactively coupled and ex- change information regarding sea-surface temperature, sea-ice concentration and thickness, wind stress, heat and freshwater once a day. Further details of the coupling can be found in [10] and [11]. The model does not employ flux adjustments. The coupling of the atmosphere and ocean model has been tested by [11] and is found to per- form well with respect to sea surface temperatures, sea- ice conditions, meridional heat transport and transport of freshwater [10]. The specific climate simulation used in this experi- ment is forced with emissions, based on realistic estima- tions until year 2000 and emissions according to the SRES A1B scenario in the period 2000-2100. In the final period 2101-2200 all the emissions have been fixed at a 2100-level. The SRES A1B scenario assumes a future world with very rapid economic growth and a rapid in- troduction of new and more efficient technologies bal- anced between both fossil—and non-fossil intensive en- ergy sources. The population growth peaks in about 2050 and declines hereafter [12]. It should be noted that this forcing from emissions only applies to the projected me- teorology. The anthropogenic emissions used in the DEHM model are kept constant in order to isolate the signal from climate change on the air pollution levels. Projected Meteorology The climate simulation applied in this study was part of the 4th IPCC Assessment Report (AR4) multi-model ensemble study. In the current simulation the global tem- perature is projected to increase by 3.0˚C by the end of the 21st century and by 4.3˚C by the end of the 22nd century, both relative to the period 1971-2000 [10]. This increase is a little higher than the average value (2.7˚C and 3.4˚C, respectively) projected by the multi-model ensemble following the SRES A1B scenario in the AR4 [13]. However, it is well within the standard deviation of the IPCC AR4 multi-model ensemble by the end of the 21st century. In Figure 1 the mean temperature at the lowest model level of the four decades (1890s, 1990s, 2090s and 2190s) considered in this study is plotted (up- per panel) together with the absolute change between these decadal mean values (center panel) and the signify- cance of these changes (lower panel) using a student’s t-test [14]. Temporally the temperature is increasing sig- nificantly in the two future decades (2090s and 2190s) relative to the 1990s. The ECHAM5/MPI-OM model si- mulation generally also projects a temperature increase in the 20th century (represented by the difference between the 1990s and the 1890s), however, this increase is only significant in the tropics. Further, a temperature decrease is projected over the North Atlantic storm tracks in the former period (1990s minus 1890s). The absolute largest temperature increase is found in the 21st century (see centre plot of Figure 1). This is in line with results from [10] who found that the changes in the global annual mean near-surface temperature is larg- est around year 2060 with a warming rate of more than. 4.5˚C. This high warming rate is due to a strong increase in all greenhouse gases except methane and a marked reduction in the anthropogenic sulphur emissions ac- cording to the SRES A1B emission scenario. Focus- sing on the 21st century (2090s-1990s) the temperature in- crease is largest in the Arctic region, where it locally exceeds 9˚C. Over land areas in general the temperature increase ranges from 3˚C to 6˚C and over the ocean the increase is more modest in the range 1˚C to 4˚C. The projected temperature change found in the 21st century is continuing into the 21st century with reduced strength. In Figure 2 results for the specific humidity is shown. The specific humidity is closely related to the atmos- pheric temperature. At saturation the specific humidity is a quasi-exponential function of temperature according to the Clausius-Clapeyron equation [15]. This exponential dependency implies that the change in humidity is sig- nificantly largest at low latitudes (where the highest temperature is projected). The specific humidity distribu- tion follows the latitudes very closely (Figure 2). As the projected temperature field (Figure 1), the specific hu- midity also shows the absolute largest changes within the 21st century (middle panel of Figure 2). In the 21st cen- tury the projected change in specific humidity is only significant in the tropical region. Vertically the largest change in specific humidity is confined to the lowest part of the troposphere where most water vapour in the at- mosphere is found, in contrast to the vertical profile of temperature change, which is rather uniform. The shortwave radiation depends on both space and time and is derived from the cloud cover calculated by Copyright © 2012 SciRes. ACS 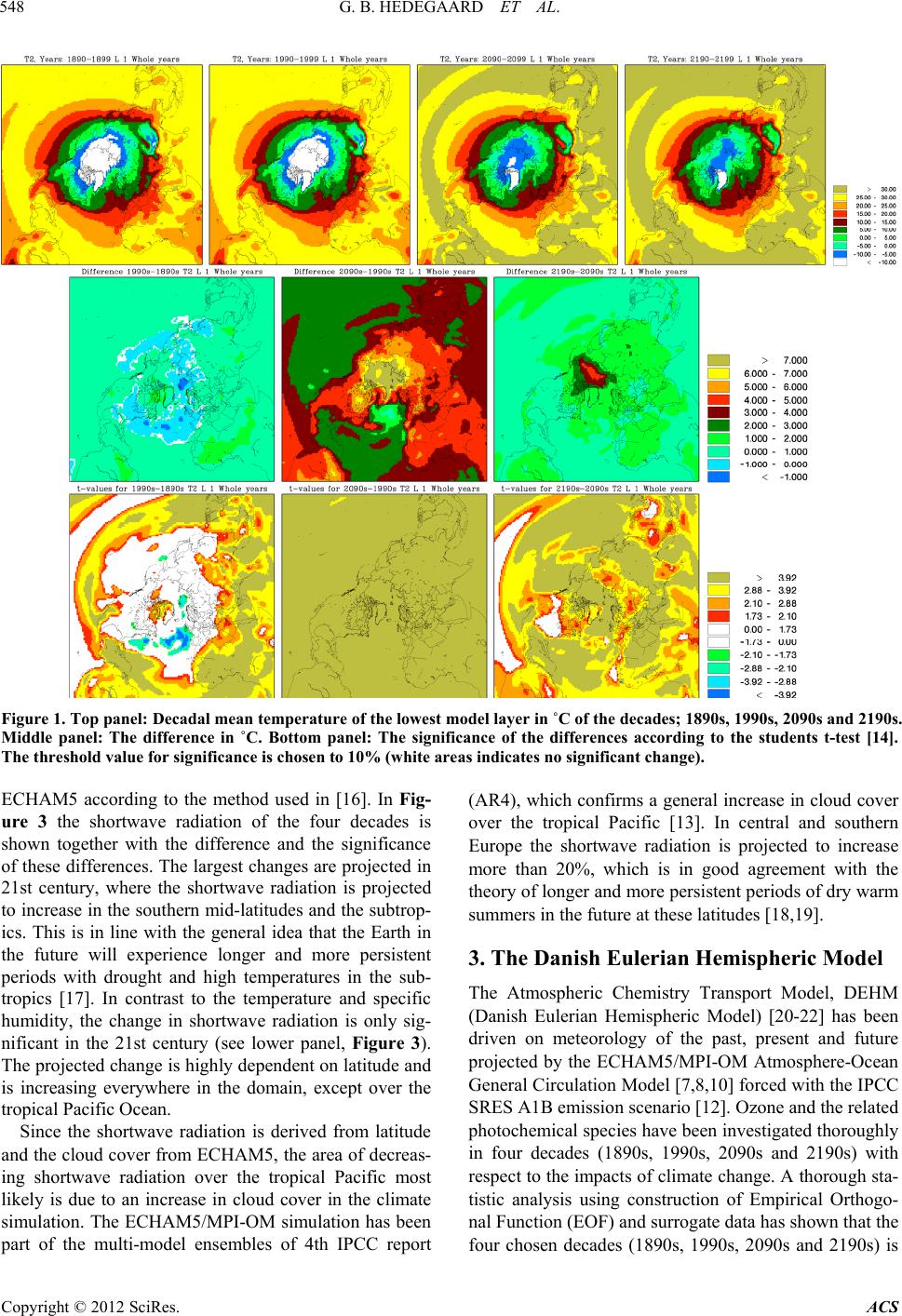 G. B. HEDEGAARD ET AL. Copyright © 2012 SciRes. ACS 548 Figure 1. Top panel: Decadal mean temperature of the lowest model layer in ˚C of the decades; 1890s, 1990s, 2090s and 2190s. Middle panel: The difference in ˚C. Bottom panel: The significance of the differences according to the students t-test [14]. The threshold value for significance is chosen to 10% (white areas indicates no significant change). ECHAM5 according to the method used in [16]. In Fig- ure 3 the shortwave radiation of the four decades is shown together with the difference and the significance of these differences. The largest changes are projected in 21st century, where the shortwave radiation is projected to increase in the southern mid-latitudes and the subtrop- ics. This is in line with the general idea that the Earth in the future will experience longer and more persistent periods with drought and high temperatures in the sub- tropics [17]. In contrast to the temperature and specific humidity, the change in shortwave radiation is only sig- nificant in the 21st century (see lower panel, Figure 3). The projected change is highly dependent on latitude and is increasing everywhere in the domain, except over the tropical Pacific Ocean. (AR4), which confirms a general increase in cloud cover over the tropical Pacific [13]. In central and southern Europe the shortwave radiation is projected to increase more than 20%, which is in good agreement with the theory of longer and more persistent periods of dry warm summers in the future at these latitudes [18,19]. 3. The Danish Eulerian Hemispheric Model The Atmospheric Chemistry Transport Model, DEHM (Danish Eulerian Hemispheric Model) [20-22] has been driven on meteorology of the past, present and future projected by the ECHAM5/MPI-OM Atmosphere-Ocean General Circulation Model [7,8,10] forced with the IPCC SRES A1B emission scenario [12]. Ozone and the related photochemical species have been investigated thoroughly in four decades (1890s, 1990s, 2090s and 2190s) with respect to the impacts of climate change. A thorough sta- tistic analysis using construction of Empirical Orthogo- nal Function (EOF) and surrogate data has shown that the four chosen decades (1890s, 1990s, 2090s and 2190s) is Since the shortwave radiation is derived from latitude and the cloud cover from ECHAM5, the area of decreas- ing shortwave radiation over the tropical Pacific most likely is due to an increase in cloud cover in the climate simulation. The ECHAM5/MPI-OM simulation has been part of the multi-model ensembles of 4th IPCC report 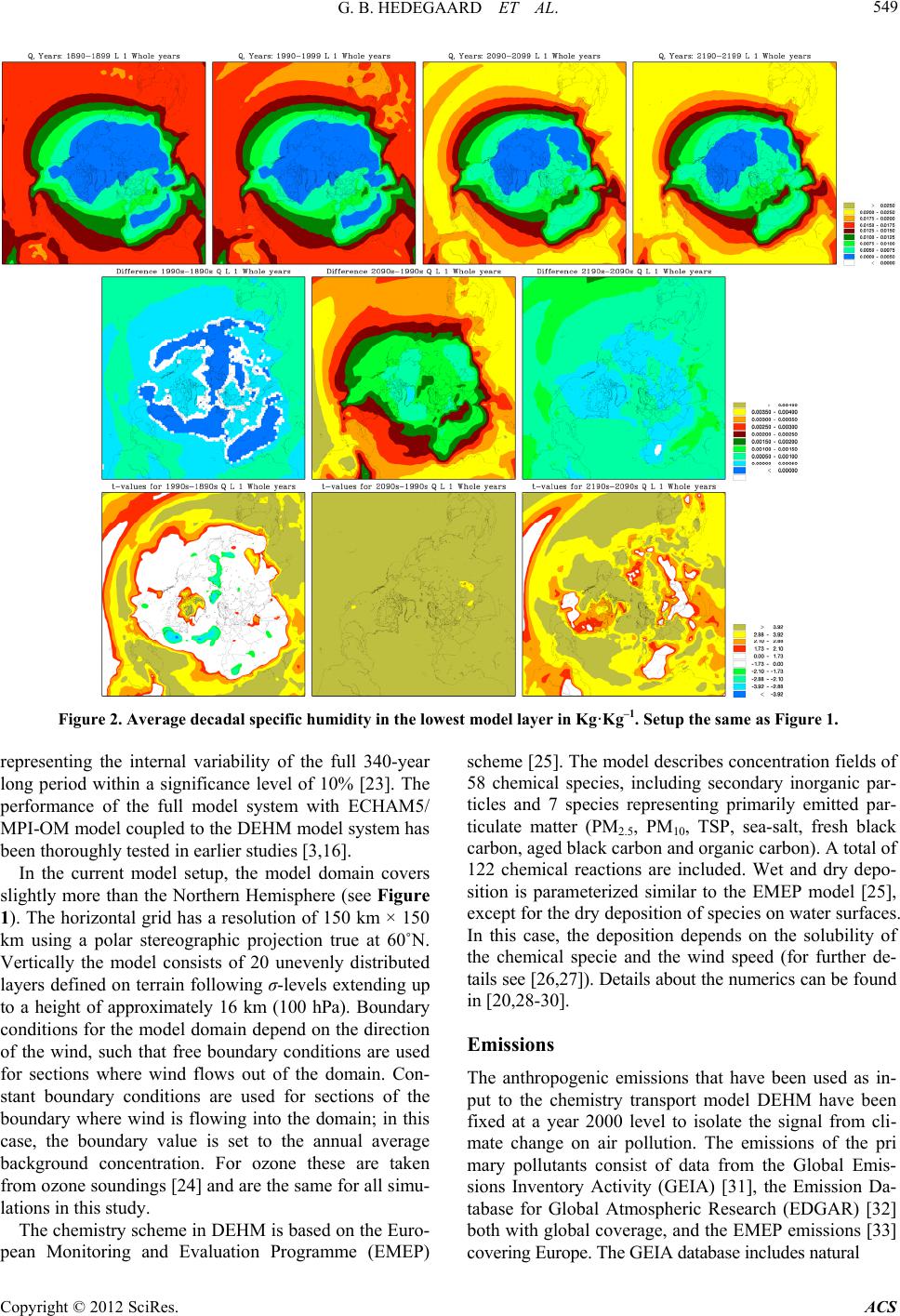 G. B. HEDEGAARD ET AL. 549 Figure 2. Average decadal specific humidity in the lowest model layer in Kg·Kg–1. Setup the same as Figure 1. representing the internal variability of the full 340-year long period within a significance level of 10% [23]. The performance of the full model system with ECHAM5/ MPI-OM model coupled to the DEHM model system has been thoroughly tested in earlier studies [3,16]. In the current model setup, the model domain covers slightly more than the Northern Hemisphere (see Figure 1). The horizontal grid has a resolution of 150 km × 150 km using a polar stereographic projection true at 60˚N. Vertically the model consists of 20 unevenly distributed layers defined on terrain following σ-levels extending up to a height of approximately 16 km (100 hPa). Boundary conditions for the model domain depend on the direction of the wind, such that free boundary conditions are used for sections where wind flows out of the domain. Con- stant boundary conditions are used for sections of the boundary where wind is flowing into the domain; in this case, the boundary value is set to the annual average background concentration. For ozone these are taken from ozone soundings [24] and are the same for all simu- lations in this study. The chemistry scheme in DEHM is based on the Euro- pean Monitoring and Evaluation Programme (EMEP) scheme [25]. The model describes concentration fields of 58 chemical species, including secondary inorganic par- ticles and 7 species representing primarily emitted par- ticulate matter (PM2.5, PM10, TSP, sea-salt, fresh black carbon, aged black carbon and organic carbon). A total of 122 chemical reactions are included. Wet and dry depo- sition is parameterized similar to the EMEP model [25], except for the dry deposition of species on water surfaces. In this case, the deposition depends on the solubility of the chemical specie and the wind speed (for further de- tails see [26,27]). Details about the numerics can be found in [20,28-30]. Emissions The anthropogenic emissions that have been used as in- put to the chemistry transport model DEHM have been fixed at a year 2000 level to isolate the signal from cli- mate change on air pollution. The emissions of the pri mary pollutants consist of data from the Global Emis- sions Inventory Activity (GEIA) [31], the Emission Da- tabase for Global Atmospheric Research (EDGAR) [32] both with global coverage, and the EMEP emissions [33] covering Europe. The GEIA database includes natural Copyright © 2012 SciRes. ACS 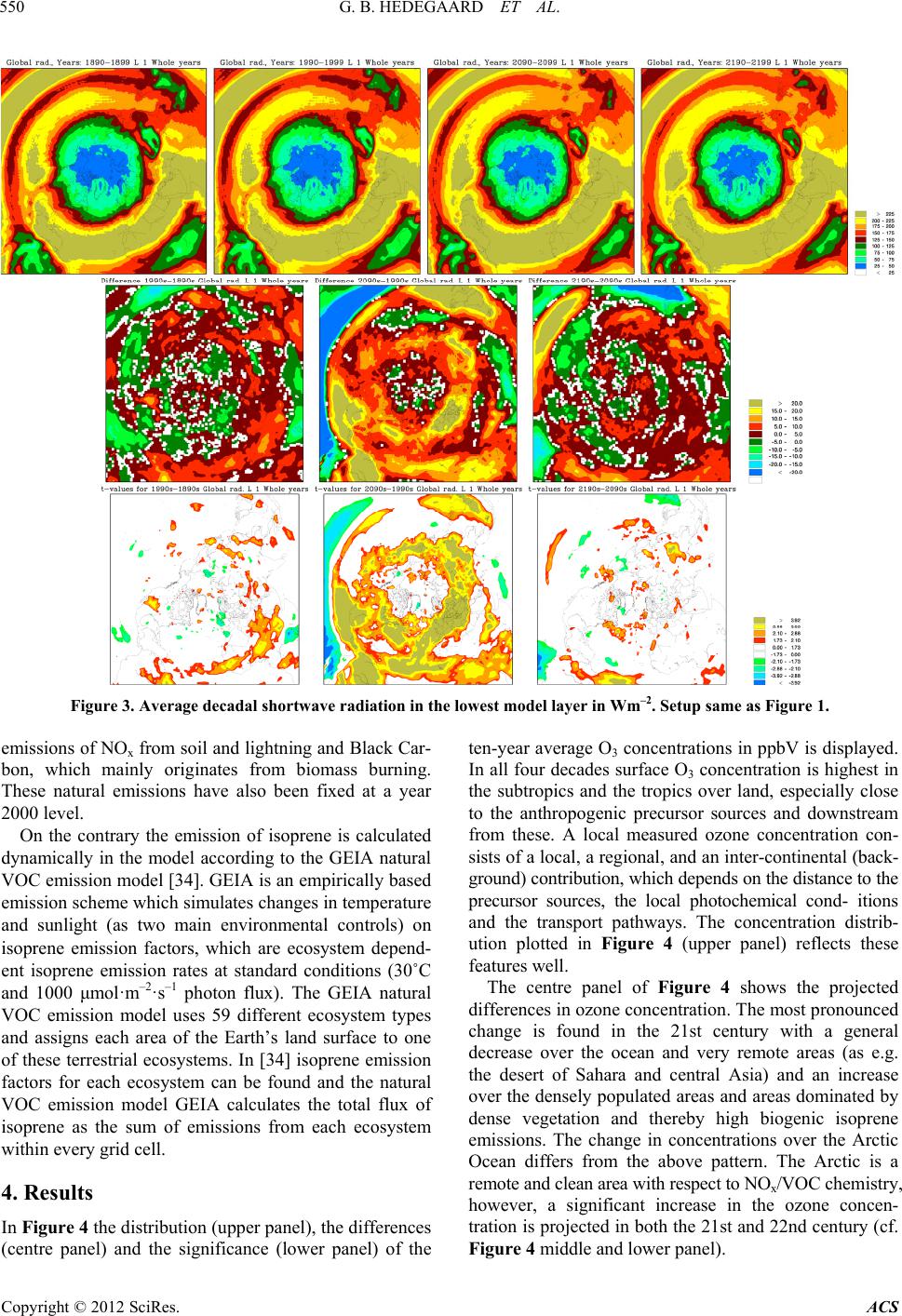 G. B. HEDEGAARD ET AL. 550 Figure 3. Average decadal shortwave radiation in the lowest model layer in Wm–2. Setup same as Figure 1. emissions of NOx from soil and lightning and Black Car- bon, which mainly originates from biomass burning. These natural emissions have also been fixed at a year 2000 level. On the contrary the emission of isoprene is calculated dynamically in the model according to the GEIA natural VOC emission model [34]. GEIA is an empirically based emission scheme which simulates changes in temperature and sunlight (as two main environmental controls) on isoprene emission factors, which are ecosystem depend- ent isoprene emission rates at standard conditions (30˚C and 1000 μmol· m –2·s–1 photon flux). The GEIA natural VOC emission model uses 59 different ecosystem types and assigns each area of the Earth’s land surface to one of these terrestrial ecosystems. In [34] isoprene emission factors for each ecosystem can be found and the natural VOC emission model GEIA calculates the total flux of isoprene as the sum of emissions from each ecosystem within every grid cell. 4. Results In Figure 4 the distribution (upper panel), the differences (centre panel) and the significance (lower panel) of the ten-year average O3 concentrations in ppbV is displayed. In all four decades surface O3 concentration is highest in the subtropics and the tropics over land, especially close to the anthropogenic precursor sources and downstream from these. A local measured ozone concentration con- sists of a local, a regional, and an inter-continental (back- ground) contribution, which depends on the distance to the precursor sources, the local photochemical cond- itions and the transport pathways. The concentration distrib- ution plotted in Figure 4 (upper panel) reflects these features well. The centre panel of Figure 4 shows the projected differences in ozone concentration. The most pronounced change is found in the 21st century with a general decrease over the ocean and very remote areas (as e.g. the desert of Sahara and central Asia) and an increase over the densely populated areas and areas dominated by dense vegetation and thereby high biogenic isoprene emissions. The change in concentrations over the Arctic Ocean differs from the above pattern. The Arctic is a remote and clean area with respect to NOx/VOC chemistry, however, a significant increase in the ozone concen- tration is projected in both the 21st and 22nd century (cf. Figure 4 middle and lower panel). Copyright © 2012 SciRes. ACS 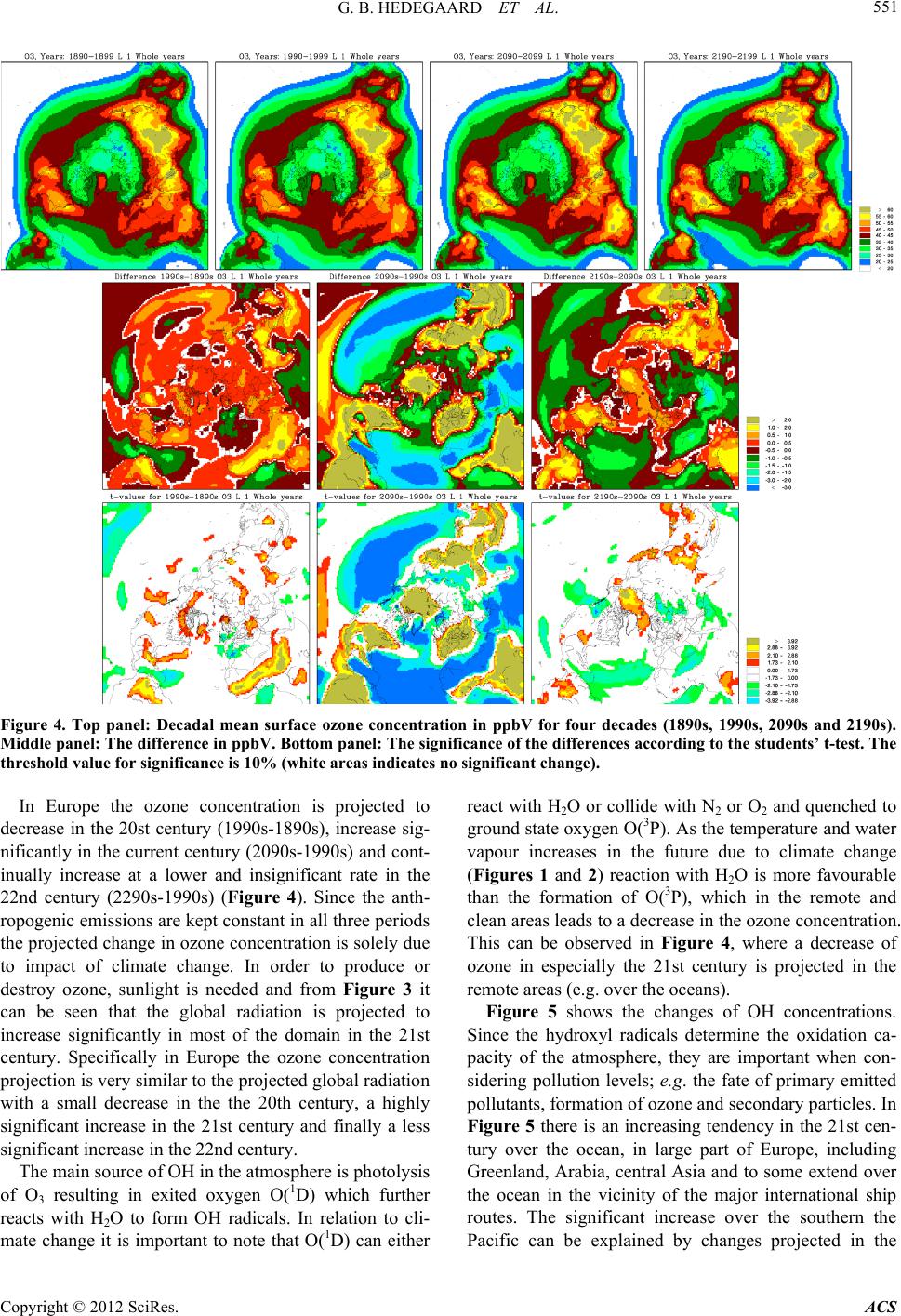 G. B. HEDEGAARD ET AL. 551 Figure 4. Top panel: Decadal mean surface ozone concentration in ppbV for four decades (1890s, 1990s, 2090s and 2190s). Middle panel: The difference in ppbV. Bottom panel: The significance of the differences according to the students’ t-test. The threshold value for significance is 10% (white areas indicates no significant change). In Europe the ozone concentration is projected to decrease in the 20st century (1990s-1890s), increase sig- nificantly in the current century (2090s-1990s) and cont- inually increase at a lower and insignificant rate in the 22nd century (2290s-1990s) (Figure 4). Since the anth- ropogenic emissions are kept constant in all three periods the projected change in ozone concentration is solely due to impact of climate change. In order to produce or destroy ozone, sunlight is needed and from Figure 3 it can be seen that the global radiation is projected to increase significantly in most of the domain in the 21st century. Specifically in Europe the ozone concentration projection is very similar to the projected global radiation with a small decrease in the the 20th century, a highly significant increase in the 21st century and finally a less significant increase in the 22nd century. The main source of OH in the atmosphere is photolysis of O3 resulting in exited oxygen O(1D) which further reacts with H2O to form OH radicals. In relation to cli- mate change it is important to note that O(1D) can either react with H2O or collide with N2 or O2 and quenched to ground state oxygen O(3P). As the temperature and water vapour increases in the future due to climate change (Figures 1 and 2) reaction with H2O is more favourable than the formation of O(3P), which in the remote and clean areas leads to a decrease in the ozone concentration. This can be observed in Figure 4, where a decrease of ozone in especially the 21st century is projected in the remote areas (e.g. over the oceans). Figure 5 shows the changes of OH concentrations. Since the hydroxyl radicals determine the oxidation ca- pacity of the atmosphere, they are important when con- sidering pollution levels; e.g. the fate of primary emitted pollutants, formation of ozone and secondary particles. In Figure 5 there is an increasing tendency in the 21st cen- tury over the ocean, in large part of Europe, including Greenland, Arabia, central Asia and to some extend over the ocean in the vicinity of the major international ship routes. The significant increase over the southern the Pacific can be explained by changes projected in the Copyright © 2012 SciRes. ACS 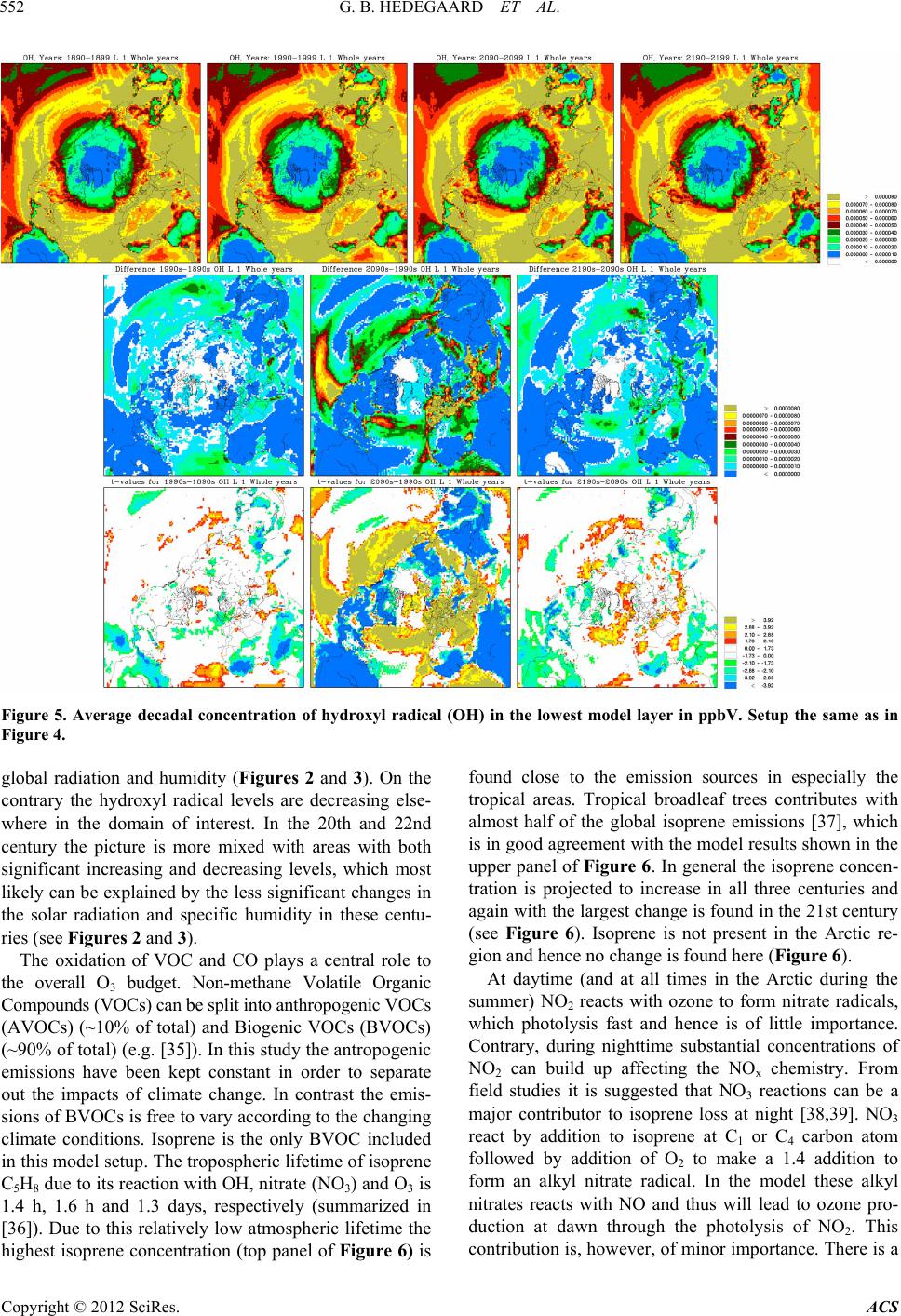 G. B. HEDEGAARD ET AL. 552 Figure 5. Average decadal concentration of hydroxyl radical (OH) in the lowest model layer in ppbV. Setup the same as in Figure 4. global radiation and humidity (Figures 2 and 3). On the contrary the hydroxyl radical levels are decreasing else- where in the domain of interest. In the 20th and 22nd century the picture is more mixed with areas with both significant increasing and decreasing levels, which most likely can be explained by the less significant changes in the solar radiation and specific humidity in these centu- ries (see Figures 2 and 3). The oxidation of VOC and CO plays a central role to the overall O3 budget. Non-methane Volatile Organic Compounds (VOCs) can be split into anthropogenic VOCs (AVOCs) (~10% of total) and Biogenic VOCs (BVOCs) (~90% of total) (e.g. [35]). In this study the antropogenic emissions have been kept constant in order to separate out the impacts of climate change. In contrast the emis- sions of BVOCs is free to vary according to the changing climate conditions. Isoprene is the only BVOC included in this model setup. The tropospheric lifetime of isoprene C5H8 due to its reaction with OH, nitrate (NO3) and O3 is 1.4 h, 1.6 h and 1.3 days, respectively (summarized in [36]). Due to this relatively low atmospheric lifetime the highest isoprene concentration (top panel of Figure 6) is found close to the emission sources in especially the tropical areas. Tropical broadleaf trees contributes with almost half of the global isoprene emissions [37], which is in good agreement with the model results shown in the upper panel of Figure 6. In general the isoprene concen- tration is projected to increase in all three centuries and again with the largest change is found in the 21st century (see Figure 6). Isoprene is not present in the Arctic re- gion and hence no change is found here (Figure 6). At daytime (and at all times in the Arctic during the summer) NO2 reacts with ozone to form nitrate radicals, which photolysis fast and hence is of little importance. Contrary, during nighttime substantial concentrations of NO2 can build up affecting the NOx chemistry. From field studies it is suggested that NO3 reactions can be a major contributor to isoprene loss at night [38,39]. NO3 react by addition to isoprene at C1 or C4 carbon atom followed by addition of O2 to make a 1.4 addition to form an alkyl nitrate radical. In the model these alkyl nitrates reacts with NO and thus will lead to ozone pro- duction at dawn through the photolysis of NO2. This contribution is, however, of mnor importance. There is a i Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 553 Figure 6. Average decadal concentration of isoprene (C5H8) in the lowest model layer in ppbV. Setup the same as in Figure 4. discussion in the literature of what will be the fate of these alkyl nitrates. They might self react, react with NO3 radical or with OH as discussed by [38]. Moreover, NO3 reacts also with NO2 to form N2O5 which hydrolyses heterogeneously with water to form nitric acid. This last reaction accounts for about the same removal as the reac- tion between OH and NO2 at mid latitudes. In this study it is difficult to isolate the exact impact of NO3, O3 and OH on isoprene because many other reac- tions also occur simultaneously in the simulations and most of the areas with a high emission load of isoprene also to some extent are influenced by anthropogenic sources. The main source of organic peroxy radicals is from AVOCs and BVOCs. The inorganic peroxy radical can be formed either from the simplest aldehyde (HCHO) or from various reaction of OH with inorganic oxides (O3, H2O2, SO2 and CO) and the photolysis of carbonyl con- taining compounds. HO2 is removed either from the re- action with NO or by its self reaction to form hydrogene peroxide (H2O2) and water. These two processes are competing reactions where reaction with NO dominates in NOx rich areas exposed to high emission from combus- tion processes and the HO2 self reaction is dominating in the free troposphere and in marine environments. The HO2 reaction with NO results in additional ozone in the atmospheric ozone budget, whereas the self-reaction leads to loss of odd oxygen and by that loss of O3. These features are well represented in the upper panel of Figures 4, 9 and 10, where the highest ozone concentration is coincident with the highest NO2 and NO concentrations. Figure 7 shows that the largest increase in hydroper- oxy radicals is found in the 21st century in the “semi-remote” areas, which has a high fraction of vegeta- tion and industry, and in the subtropical and tropical ar- eas. However, a significant increase is also found over most of the domain in the 22nd century. Only in the 20th century the hydroperoxy radical concentration is pro- jected to decrease over the North Atlantic, western Europe and parts of Siberia. The increased concentration of hy- droperoxy radicals in the regions with high emissions from vegetation and regions with high emissions from anthropogenic sources, is caused by the increased level of water vapour and isoprene emissions in the future decades. When the water vapour content increases, the OH concentration increases, due to photolysis of ozone. Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 554 Figure 7. Average decadal concentration of hydroperoxy radical (HO2) in the lowest model layer in ppbV. Setup the same as in 4. The hydroxyl radicals can then be converted to hydrop- eroxy radicals as described above. In Figure 8 the changes in organic peroxy radicals over the three centuries are shown. In the 20th century (1990s minus 1890s) the largest increase is found in the Tropics. Organic peroxy radicals are increasing in the 21st century in most of the domain, whereas the project- tions of the organic peroxy radicals are less significant in the 22nd but still increasing. An increase in organic per- oxy radicals can be explained by the same parameters as for hydroperoxy radicals. In Figure 9 the decadel average concentration in NO2 is shown for the four decades. The picture is mixed with respect to increase and decrease, but the most significant changes are again found in the 21st century (lower panel of Figure 9). Most of the increase is in both the 21st and 22nd century found over the parts of the North Atlantic Ocean, the Arctic Ocean and the northern part of the Pa- cific Ocean. In contrast NO2 mainly decrease over Europe, South America and Africa in all three centuries. Figure 10 displays the concentration of NO. Over the Northwestern part of Europe (Benelux and surroundings) there is an excess of NO. The Benelux area has the worlds largest NOx emission density. These high NOx emissions imply low O3 concentration in this area, since O3 is used to convert the emitted NO to NO2. 5. Discussion The changes in the ozone concentration is due to a com- bined effect of increased temperature, solar radiation, humidity, biogenic isoprene emissions and depends on the chemical regime. In a clean atmosphere, where the NOx and AVOC load is low, increased water vapour in the atmosphere acts to reduce the ozone concentration, which is reflected in the projected changes over the oceans in the 21st and 22nd centuries (center panel, Figure 4). However, in regions with moderate to high NOx levels, the interaction between the emitted NO and the formed isoprene peroxides from OH, increases the concentration of HO2 (Figure 7) and NO2 (Figure 10), which then en- hances the ozone formation. This can explain the higher ozone concentrations in Africa south of Sahara, Southeast Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 555 Figure 8. Average decadal concentration of organic peroxy radicals in the lowest model layer in ppbV. Setup the same as in 4. Figure 9. Average decadal concentration of nitrogen dioxide (NO2) in the lowest model layer in ppbV. Setup the same as in 4. Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 556 Figure 10. Average decadal concentration of (NO) in the lowest model layer in ppbV. Setup the same as in 4. Asia and South America (Figure 4). In addition, these areas are covered with a large proportion of tropical plants and tropical rainforest, which emits a large frac- tion of the ozone precursor, isoprene. Moreover, the iso- prene emission itself is increasing in these simula- tions due to changed climate conditions, which can fur- ther amplify the signal in the future ozone concentration. In the Arctic the ozone concentration is projected to increase throughout all three centuries. However, the change is most significant in the 21st century. From analysis of each season it is found most significant dur- ing the winter season (not shown). In the Arctic region there is no sunlight in the winter months, and hence not any ozone degradation due to photolysis. But since the length of Arctic winter does not change over the centu- ries with respect to incoming radiation and there is no significant change projected in the global radiation over the Arctic region, further analysis of the ozone related species cannot explain the change over the Arctic region. Over the Arctic land areas the ozone dry deposition increases (Figure 11), which contributes to the projected decrease in O3 in the air over land (Figure 4). Deposition to vegetative surfaces is much larger than to snow cov- ered surfaces. Hence the projected decrease in snow cover (not shown) can contribute to explain the projected changes in O3 in the 21st and 22nd century. Over the Arctic Ocean the temporal and spatial extent of sea ice decreases in the 21st century (not shown). Ozone does dry deposit to water surfaces in the model [26,27] and the deposition is larger for ice surfaces than for water, which results in a decrease in the dry deposi- tion as the sea ice melts over time. This is in good agreement with the results of the dry deposition (Figure 11), which decreases over the areas of the Arctic ocean, where sea ice extent is decreasing. Moreover, changes in horizontal transport from the source areas may also contribute to the observed increase in O3 over the Arctic Ocean. If this is the case more ozone is transported into the Arctic from the source ar- eas where increased ozone levels are projected. This ad- ditional ozone is compensated by increased deposition over land but amplifies the increase in concentration over the ice free ocean due to a decrease in dry deposition. Finally vertical downward transport could also in crease Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 557 Figure 11. Average decadel ozone dry deposition in mg/m–2/year. Setup as in Figure 4. the concentration of the surface ozone concentration. When entering the free troposphere, the trend in ozone is less confined to the surface and has a more zonal pattern with a highly significant increase within and north of the subtropics and a highly significant decrease in the tropics (Figure 12). In the free troposphere the concentration levels are less influenced by local gradients in emissions and therefore local features are less pronounced. Since the ozone concentrations in general are increasing at higher altitudes, this could contribute with additional ozone at the surface in the future. With the current model setup the stratosphere-troposphere exchange of ozone cannot be expected to be simulated in detail. First of all the model only extents to 100 hPa and secondly the ver- tical model resolution in the upper atmosphere is rela- tively poor. Nevertheless, the model includes a rough description of the ozone layer. It is possible that more stratospheric ozone can be produced in the model due to climate change and transported downwards increasing the surface ozone concentrations. Furthermore, the verti- cal transport patterns can change under changed climate conditions. Over Europe, East Asia and most of the U.S. (the south-eastern part) the ozone concentrations are esti- mated to increase due to impacts of climate change. This can be explained by the increased isoprene emission which results from increased temperature. The fact that the future biogenic isoprene emissions are the controlling factor for impacts of climate change on the future ozone concentrations is confirmed in several earlier studies [3,5,40]. In this study the IGAC-GEIA biogenic emission model [34] has been used to calculate the isoprene emission dynamically in the DEHM model. The domain-total an- nual isoprene emissions is 488 Tg/year for the baseline scenario, which is within the global range 460 - 770 Tg/year found in previous studies [37,41,42]. Since isoprene is the single most important NMVOC for ozone production [43], a correct description of the isoprene emissions is important. Nevertheless to fully describe the emission of isoprene is a complicated task. Since isoprene emissions originate from vegetation it depends on temperature and sunlight. However, investi- gations have shown that the emission rate of isoprene also depend on the ambient CO2 and O3 concentration, biomass, plant specie, leaf age, soil moisture, wind dam- Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 558 Figure 12. Changes in ozone concentration in ppbv between the 1990s to the 2090s and the significance of these changes the surface layer, layer 12 (~2100 m) and layer 15 (~4750 m). age etc. [43], but there are large uncertainties connected to each of these variables. For example some studies ar- gues that enhanced ambient CO2 levels lead to increased isoprene emission due to the resulting increase in bio- mass and controversially other studies have shown that increased CO2 levels impact the isoprene synthesis rate for some plant species and hence decrease the isoprene emissions [43]. However, [5] have recently carried out model experi- ments showing that dry deposition of ozone to vegetation might impact the future ozone concentrations even more. [5] did some sensitivity studies where they changed the description of dry deposition to vegetation to be depend- ent on several meteorological parameters. When plants are exposed to climate change they change their ozone uptake and here soil moisture is one of the key parame- ters. When already dry areas, dries out even more, the ozone uptake to vegetation stops and this decreases the dry deposition to vegetation. Particularly [5] found that in Spain this process amounts for 80% of the total change in ozone. In the current model setup the EMEP [25] parameteri- sation has been used and the dry deposition to vegetation depend on changes in meteorology. The dry deposition scheme does, however, not account for the soil moisture effect described by [5]. So far several studies have investigated the impact of climate change over specific regions in the US in the 21st century [2,44-46]. The study by [44] is the only global study and more over the only study including the entire 21st century. The projection of future ozone is very similar to the projections in this study. The studies by [2,45] only covers projections until year 2050, hence the results are not comparable. 6. Conclusions In this study a 340-year long climate simulation from the ECHAM5/MPI-OM model forced with the SRES A1B emission scenario has been used to drive the chemical transport model DEHM. Several DEHM simulations have been carried out with past, present and future mete- orology and constant year 2000 anthropogenic emissions in order to isolate the impacts from climate change on the air pollution levels in the Northern Hemisphere. Special emphasis has been put on the analysis of ozone and the related photochemistry in Europe and the Arctic. The present study illustrates that changes in the ozone concentration due to climate change mainly are driven by two competitive processes; ozone destruction due to in- creased water vapour in the atmosphere and ozone for- mation due to increased levels of ozone precursors. Since the anthropogenic emissions have been kept constant in these simulations, biogenic isoprene is the only directly emitted precursor that is free to vary according to the projected climate conditions. Moreover the increase in ozone concentration depends on the NOx availability in Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 559 the boundary layer and the future ozone concentration is projected to increase significantly in urban and semi- remote areas and to decrease significantly in the remote areas. In the free troposphere the effect from increased water vapour dominates in the tropics while the effect from increased isoprene and changes in transport domi- nates at high latitudes. These results show a close relation between the tem- perature change and the future ozone concentration. The two parameters are not directly connected but are related through a wide range of interacting processes. From the climate simulation it is known that the largest change in temperature is projected to happen in the 21st century and the same is true for ozone, isoprene, NOx and OH. In the 20th century the temperature change is relatively small and in some areas decreasing and other areas in- creasing. The same patterns are seen in most chemical species e.g. in the O3 concentration. In the 22nd century the trends are the same as in the 21st century, but the rate of change decreases for all parameters, which is in good agreement with the constant forcing in the applied cli- mate simulation (constant A1B year-2100 level emission throughout the 22nd century). Surface ozone is of great importance since high ozone concentrations can have impacts on both humans and nature. This study has shown that at the surface level the background ozone concentration will decrease during the 21st and the 22nd century. In contrast local ozone in the source areas of precursors (both anthropogenic and bio- genic) will increase significantly due to climate change alone. In the free troposphere the ozone concentration will in general increase, except in the tropics where a significant decrease is found. Over the Arctic Ocean a significant increase in surface ozone is found in the fu- ture, which can be explained by increased input from the source areas. Moreover, decreased dry deposition of ozone due to increased sea ice and increased import from higher levels can support the projected increase in future surface level ozone in the Arctic region. Ozone in the atmosphere affects not only the human health and nature. It is also a significant short-lived greenhouse gas in the atmosphere. Today most atmos- pheric climate models have lower resolution than ACTMs and a more simplified descriptions of the atmospheric physical and chemical processes and therefore account poorly for the feedback from atmospheric chemistry to the climate system. The increase in ozone concentrations in large parts of the upper troposphere could have sig- nificant radiative effect, which is not yet accounted for in climate models. To include this and similar chemical feedback effects in the atmosphere, online model or ex- tensive Earth system models are needed. Nevertheless, CTM studies like the present are still necessary in order to study in detail the individual chemical and physical processes in the atmosphere. 7. Acknowledgements This work was partly funded by the Centre for Energy, Environment and Health (CEEH), financed by The Dan- ish Strategic Research Program on Sustainable Energy under contract no 2104-06-0027 (www.ceeh.dk). REFERENCES [1] S. L. Wu, L. J. Mickley, D. J. Jacob, D. Rind and D. G. Streets, “Effects of 2000-2050 Changes in Climate and Emissions on Global Tropospheric Ozone and the Pol- icy-Relevant Background Ozone in the United States,” Journal of Geophysical Research, Vol. 113, 2008, Article ID: D18312. [2] P. N. Racherla and P. J. Adams, “The Response of Surface Ozone to Climate Change over Eastern United States,” Atmospheric Chemistry and Physics, Vol. 8, No. 4, 2008, pp. 871-885. doi:10.5194/acp-8-871-2008 [3] G. B. Hedegaard, J. Brandt, J. H. Christensen, L. M. Frohn, C. Geels, K. M. Hansen and M. Stendel, “Impacts of Climate Change on Air Pollution Levels in the North- ern Hemisphere with Special Focus on Europe and the Arctic,” Atmospheric Chemistry and Physics, Vol. 8, No. 12, 2008, pp. 3337-3367. doi:10.5194/acp-8-3337-2008 [4] E. Katragkou, P. Zanis, I. Tegoulias, D. Melas, I. Kiout- sioukis, B. C. Krger, P. Huszar, T. Halenka and S. Rauscher, “Decadal Regional Air Quality Simulations over Europe in Present Climate: Near Surface Ozone Sen- sitivity to External Meteorological Forcing,” Atmospheric Chemistry and Physics, Vol. 10, 2010, pp. 11805-11821. [5] C. Andersson and M. Engardt, “European Ozone in a Fu- ture Climate: Importance of Changes in Dry Deposition and Isoprene Emissions,” Journal of Geophysical Re- search, Vol. 115, No. D2, 2010, Article ID: D02303. doi:10.1029/2008JD011690 [6] J. Langner, M. Engardt, A. Baklanov, J. Christensen, M. Gauss, C. Geels, G. B. Hedegaard, R. Nuterman, D. Simpson, J. Soares, M. Sofiev, P. Wind and A. Zakey, “A Multi-Model Study of Impacts of Climate Change on Sur- face Ozone in Europe,” Atmospheric Chemistry and Phys- ics, Vol. 12, No. 2, 2012, pp. 4901-4939. doi:10.5194/acpd-12-4901-2012 [7] E. Roeckner, G. Bauml, L. Bonaventura, R. Brokopf, M. Esch, M. Giorgetta, S. Hagemann, I. Kirchner, L. Korn- blueh, E. Manzini, A. Rhodin, U. Schlese, U. Schul- zweida and A. Tompkins, “The Atmospheric General Circulation Model ECHAM5, Part I,” Max-Planck-Insti- tute für Meteorologie, Hamburg, 2003. [8] E. Roeckner, R. Brokopf, M. Esch, M. Giorgetta, S. Hagemann, E. Kornblueh, L. Manzini, U. Schlese and U. Schulzweida, “Sensitivity of Simulated Climate to Hori- zontal and Vertical Resolution in the ECHAM5 Atmos- phere Model,” Journal of Climate, Vol. 19, No. 16, 2006, pp. 3771-3791. doi:10.1175/JCLI3824.1 [9] S. J. Marsland, H. Haak, J. H. Jungclaus, M. Latif and F. Roske, “The Max-Planck-Institute Global Ocean/Sea Ice Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. 560 Model with Orthogonal Curvilinear Coordinates,” Ocean Modelling, Vol. 5, No. 2, 2003, pp. 91-127. doi:10.1016/S1463-5003(02)00015-X [10] W. May, “Climatic Changes Associated with a Global 2 Degrees C-Stabilization Scenario Simulated by the EC HAM5/MPI-OM Coupled Climate Model,” Climate Dy- namics, Vol. 31, No. 2-3, 2008, pp. 283-313. doi:10.1007/s00382-007-0352-8 [11] J. H. Jungclaus, N. Keenlyside, M. Botzet, H. Haak, J. J. Luo, M. Latif, J. Marotzke, U. Mikolajewicz and E. Roeckner, “Ocean Circulation and Tropical Variability in the Coupled Model ECHAM5/MPI-OM,” Journal of Cli- mate, Vol. 19, No. 16, 2006, pp. 3952-3972. doi:10.1175/JCLI3827.1 [12] N. Nakicenovic, J. Alcamo, G. Davis, B. de Vries, J. Fenhann, S. Gaffin, K. Gregory, A. Grbler, T. Jung, T. Kram, E. L. Rovere, L. Michaelis, S. Mori, T. Morita, W. Pepper, H. Pitcher, L. Price, K. Riahi, A. Roehrl, H.-H. Rogner, A. Sankovski, M. Schlesinger, P. Shukla, S. Smith, R. Swart, S. van Rooijen, N. Victor and Z. Dadi, “Special Report on Emission Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change,” Cambridge University Press, New York, 2000. [13] G. A. Meehl, T. F. Stocker, W. D. Collins, P. Friedling- stein, A. T. Gaye, J. M. Gregory, A. Kitoh, R. Knutti, J. M. Murphy, A. Noda, S. C. B. Raper, I. G. Watterson, A. J. Weaver and Z.-C. Zhao, “Climate Change 2007: The Physical Science Basis,” In: Contribution of Working Group I to the Fourth Assessment Report of the Intergov- ernmental Panel on Climate Change Global Climate Projections, Cambridge University Press, Cambridge and New York, 2007, pp. 747-846. [14] M. R. Spiegel, “Schaum’s Outline of Theory and Prob- lems of Statistics,” 2nd Edition, McGraw-Hill, New York, 1992. [15] H. Goosse, P. Y. Barriat, W. Lefebvre, M. F. Loutre and V. Zunz, “Introduction to Climate Dynamics and Climate Modelling,” 2009. http://stratus.astr.ucl.ac.be/textbook/ [16] G. B. Hedegaard, “Impacts of Climate Change on Air Pollution Levels in the Northern Hemisphere,” Technical Report 240, National Environmental Research Institute, Roskilde, 2007. www.dmu.dk [17] J. H. Christensen, C. Hewitt, A. Busuioc, A. Chen, X. Gao, I. Held, R. Jones, R. K. Kolli, W.-T. Kwon, R. La- prise, V. Magaa Rueda, L. Mearns, C. G. Menendez, J. Risnen, A. Rinke, A. Sarr and P. Whetton, “Climate Change 2007: The Physical Science Basis,” In: Contribu- tion of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Re- gional Climate Projections, Cambridge University Press, Cambridge, 2007, pp. 847-940. [18] R. Vautard and D. Hauglustaine, “Impact of Global Cli- mate Change on Regional Air Quality: Introduction to the Thematic Issue,” Comptes Rendus Geoscience, Vol. 339, No. 11-12, 2007, pp. 703-708. doi:10.1016/j.crte.2007.08.012 [19] P. A. Stott, D. A. Stone and M. R. Allen, “Human Con- tribution to the European Heatwave of 2003,” Nature, Vol. 432, 2004, pp. 610-614. doi:10.1038/nature03089 [20] J. H. Christensen, “The Danish Eulerian Hemispheric Model—A Three-Dimensional Air Pollution Model Used for the Arctic,” Atmospheric Environment, Vol. 31, No. 24, 1997, pp. 4169-4191. doi:10.1016/S1352-2310(97)00264-1 [21] L. M. Frohn, “A Study of Long-Term Highresolution Air Pollution Modelling,” Ph.D. Dissertation, University of Copenhagen and National Environmental Research Insti- tute, Copenhagen, 2004. [22] J. Brandt, J. D. Silver, L. M. Frohn, C. Geels, A. Gross, A. B. Hansen, K. M. Hansen, G. B. Hedegaard, C. A. Skjøth, H. Villadsen, A. Zare and J. H. Christensen, “An Inte- grated Model Study for Europe and North America Using the Danish Eulerian Hemispheric Model with Focus on Intercontinental Transport of Air Pollution,” Atmospheric Environment, Vol. 53, 2012, pp. 156-176. doi:10.1016/j.atmosenv.2012.01.011 [23] G. B. Hedegaard, “Future Air Pollution Levels in the Northern Hemisphere—Sensitivity to Climate Change and Projected Emissions,” Ph.D. Thesis, Aarhus Univer- sity, Aarhus, 2011. [24] J. A. Logan, “An Analysis of Ozonesonde Data for the Troposphere: Recommendations for Testing 3-D Models and Development of a Gridded Climatology for Tropo- spheric Ozone,” Journal of Geophysical Research, Vol. 104, No. D13, 1999, pp. 115-116. doi:10.1029/1998JD100096 [25] D. Simpson, H. Fagerli, J. Jonson, S. Tsyro, P. Wind and J.-P. Touvinen, “Transboundary Acidification, Eutrophi- cation and Ground Level Ozone in Europe, Part I,” Nor- wegian Meteorological Institute, Unified EMEP Model description EMEP MSC-W, Oslo, 2003. [26] W. A. H. Asman, L. L. Sensen, R. Berkowicz, K. Granby, H. Nielsen, B. Jensen, E. Runge, C. Lykkelund, S. E. Gryning and A. M. Sempreviva, “Dry Deposition Proc- esses,” Marine Research 35, Danish Environmental Pro- tection Agency, Copenhagen, 1994. [27] O. Hertel, J. Christensen, E. H. Runge, W. A. H. Asman, R. Berkowicz and M. F. Hovmand, “Development and Testing of a New Variable Scale Pollution Model— ACDEP,” Atmospheric Environment, Vol. 29, No. 11, 1995, pp. 1267-1290. doi:10.1016/1352-2310(95)00067-9 [28] L. M. Frohn, J. H. Christensen, J. Brandt and O. Hertel, “Development of a High Resolution Integrated Nested Model for Studying Air Pollution in Denmark,” Physics and Chemistry of the Earth, Vol. 26, Part B, 2001, pp. 769-774. [29] L. M. Frohn, J. H. Christensen and J. Brandt, “Develop- ment and Testing of Numerical Methods for Two-Way Nested Air Pollution Modelling,” Physics and Chemistry of the Earth, Vol. 27, No. 35, 2002, pp. 1487-1494. doi:10.1016/S1474-7065(02)00151-1 [30] L. M. Frohn, J. H. Christensen and J. Brandt, “Develop- ment of a High-Resolution Nested Air Pollution Model: The Numerical Approach,” Journal of Computational Physics, Vol. 179, No. 1, 2002, pp. 68-94. doi:10.1006/jcph.2002.7036 [31] T. Graedel, T. Bates, A. Bouwman, D. Cunnold, J. Dignon, Copyright © 2012 SciRes. ACS  G. B. HEDEGAARD ET AL. Copyright © 2012 SciRes. ACS 561 I. Fung, D. Jacob, B. Lamb, J. Logan, G. Marland, P. Middleton, J. Pacyna, M. Placet and C. Veldt, “A Com- pilation of Inventories of Emissions to the Atmosphere,” Global Biogeochemical Cycles, Vol. 7, No. 1, 1993, pp. 1-26. doi:10.1029/92GB02793 [32] J. Olivier, A. Bouwman, C. van der Maas, J. Berdowski, C. Veldt, J. Bloos, A. Visschedijk, P. Zandveld and J. Haverlag, “Description of EDGAR Version 2.0: A Set of Global Emission Inventories of Greenhouse Gases and Ozone-Depleting Substances for All Anthropogenic and Most Natural Sources on a Per Country Basis and on 1 Degree × 1 Degree Grid,” National Institute for Public Health and the Environment (RIVM), Bilthoven, 1996. [33] V. Vestreng, “Emission Data Reported to UNECE/ EMEP: Evaluation of the Spatial Distribution of Emis- sion,” MSC-W Status Report, Meteorological Synthesiz- ing Centre—West, Oslo, 2001. [34] A. Guenther, C. Hewitt, D. Erickson, R. Fall, C. Geron, T. Graedel, P. Harley, L. Klinger, M. Lerdau, W. McKay, T. Pierce, B. Scholes, R. Steinbrecher, R. Tallamraju, J. Taylor and P. Zimmerman, “A Global-Model of Natural Volatile Organic-Compound Emissions,” Journal of Geo- physical Research, Vol. 100, No. D5, 1995, pp. 8873- 8892. doi:10.1029/94JD02950 [35] A. H. Goldstein and I. E. Galbally, “Known and Unex- plored Organic Constituents in the Earths Atmosphere,” Environmental Science & Technology, Vol. 41, No. 5, 2007, pp. 1514-1521. doi:10.1021/es072476p [36] G. B. Hedegaard, “Air Pollution—From a Local to a Global Perspective,” Polyteknisk Forlag, Lyngby, 2009, pp. 163-174. [37] A. Guenther, T. Karl, P. Harley, C. Wiedinmyer, P. I. Palmer and C. Geron, “Estimates of Global Terrestrial Isoprene Emissions Using MEGAN (Model of Emissions of Gases and Aerosols from Nature),” Atmospheric Chemistry and Physics, Vol. 6, No. 11, 2006, pp. 3181- 3210. doi:10.5194/acp-6-3181-2006 [38] A. W. Rollins, A. Kiendler-Scharr, J. L. Fry, T. Brauers, S. S. Brown, H.-P. Dorn, W. P. Dube, H. Fuchs, A. Men- sah, T. F. Mentel, F. Rohrer, R. Tillmann, R. Wegener, P. J. Wooldridge and R. C. Cohen, “Isoprene Oxidation by Nitrate Radical: Alkyl Nitrate and Secondary Organic Aerosol Yields,” Atmospheric Chemistry and Physics, Vol. 9, No. 18, 2009, pp. 6685-6703. doi:10.5194/acp-9-6685-2009 [39] H. Skov, J. Hjorth, C. Lohse, N. R. Jensen and G. Restelli, “Products and Mechanisms of the Reactions of the Nitrate Radical (NO3) with Isoprene, 1,3-Butadiene and 2,3-Di- methyl-1,3-Butadiene in Air,” Atmospheric Environment: Part A. General Topics, Vol. 26, No. 15, 1992, pp. 2771- 2783. doi:10.1016/0960-1686(92)90015-D [40] Y. F. Lam, J. S. Fu and L. J. Mickley, “Impacts of Future Climate Change and Effects of Biogenic Emissions on Surface Ozone and Particulate Matter Concentrations in the United States,” Atmospheric Chemistry and Physics, Vol. 11, No. 10, 2011, pp. 4789-4806. doi:10.5194/acp-11-4789-2011 [41] A. Arneth, R. K. Monson, G. Schurgers, U. Niinemets and P. I. Palmer, “Why Are Estimates of Global Terres- trial Isoprene Emissions So Similar (and Why Is This Not so for Monoterpenes)?” Atmospheric Chemistry and Phy- sics, Vol. 8, No. 16, 2008, pp. 4605-4620. [42] K. Ashworth, O. Wild and C. N. Hewitt, “Sensitivity of Isoprene Emissions Estimated Using Megan to the Time Resolution of Input Climate Data,” Atmospheric Chemis- try and Physics, Vol. 10, No. 3, 2010, pp. 1193-1201. doi:10.5194/acp-10-1193-2010 [43] D. Fowler, M. Amann, R. Anderson, M. Ashmore, P. Cox, M. D. M, D. Derwent, P. Grennfelt, N. Hewitt, O. Hov, M. Jenkin, F. Kelly, P. Liss, M. Pilling, J. Pyle, J. Slingo and D. Stefenson, “Ground-Level Ozone in the 21st Century: Future Trends, Impacts and Policy Implications,” Science Policy Report 15/08, The Royal Society, London, 2008. [44] K. Murazaki and P. Hess, “How Does Climate Change Contribute to Surface Ozone Change over the United States,” Journal of Geophysical Research, Vol. 111, No. D05301, 2006, 16 pp. doi:10.1029/2005JD005873 [45] S. Wu, L. J. Mickley, E. M. Leibensperger, D. J. Jacob, D. Rind and D. G. Streets, “Effects of 2000-2050 Global Change on Ozone Air Quality in the United States,” Journal of Geophysical Research, Vol. 113, 2008, Article ID: D06302. [46] H. Liao, Y. Zhang, W.-T. Chen, F. Raes and J. H. Sein- feld, “Effect of Chemistry-Aerosol-Climate Coupling on Predictions of Future Climate and Future Levels of Tro- pospheric Ozone and Aerosols,” Journal of Geophysical Research, Vol. 114, 2009, Article ID: D10306.
|