Food and Nutrition Sciences
Vol.4 No.9A(2013), Article ID:37069,11 pages DOI:10.4236/fns.2013.49A1024
Effects of Genetics and Environment on Fatty Acid Stability in Soybean Seed*
![]()
1USDA-ARS, Crop Genetics Research Unit, Stoneville, USA; 2USDA-ARS, Crop Genetics Research Unit, Jackson, USA; 3Plant Genomics and Biotechnology Lab, Department of Biological Sciences, Fayetteville State University, Fayetteville, USA.
Email: nacer.bellaloui@ars.usda.gov
Copyright © 2013 Nacer Bellaloui et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 24th, 2013; revised June 24th, 2013; accepted July 2nd, 2013
Keywords: Fatty Acids; Oil Quality; Oil Stability; Seed Composition; Soybean Seed
ABSTRACT
Although seed oil production and composition are genetically controlled, changes of oil level and oil composition across genotypes and environments such as drought and temperature were observed. The mechanisms of how genotypes interact with environment, affecting oil production and composition, are still not well understood. The objective of this research was to investigate the effect of drought/water stress and temperature on soybean genotypes. Two soybean genotypes of maturity group (MG) II (PI 597411 B and PI 597408) and two of MG VI (Arksoy and PI 437726) were used. A repeated greenhouse experiment to study the effect of water stress and a repeated growth chamber experiment to study the effect of temperature were conducted. The results showed that both water stress and high temperature altered seed oil composition by increasing oleic acid and decreasing linoleic and linolenic acid concentrations. Severe water stress (soil water potential between −150 to −200 kPa) or high temperature (40/33˚C, day/night) resulted in higher palmitic acid and lower stearic acid. Genotypes differed in their responses to water stress or temperature. Analyses of seed carbohydrates (glucose, fructose, sucrose, raffinose, and stachyose) showed a significant decline of glucose, fructose, and sucrose and a significant increase of stachyose concentration by water stress and high temperature. Analyses of natural abundance of δ15N and δ13C isotopes showed changes in sources of nitrogen and carbon fixation, possibly affecting nitrogen and carbon metabolism pathways. The research demonstrated that both water stress and high temperature altered oil production and composition, and this could be partially related to limited availability and movement of carbohydrates from leaves to seed. Further research to investigate the enzymes controlling fatty acids conversion and nitrogen and carbon metabolism is needed.
1. Introduction
Soybean oil composition determines the oil quality. Soybean oil is composed of saturated and unsaturated fatty acids. Saturated fatty acids are triglycerides (glycerol with three molecules of fatty acids). In saturated fatty acids, all carbon atoms are occupied by hydrogen with no double bonds between the carbon atoms. However, in unsaturated fatty acid, at least one double bond exists between carbon atoms. Major saturated fatty acids are palmitic (C16:0; 10 to 12%) and stearic (C18:0, 3 to 5%), and major unsaturated fatty acids are oleic (C18:1; 24%), linoleic (C18:2; 54%), and linolenic (C18:3, 8.0%). In fatty acid triglycerides the hydroxyl groups (HO-) of the glycerol join the carboxyl groups (-COOH) to form ester as follows: HOCH2CH(OH)CH2OH + RCO2H + R'CO2H + R''CO2H ® RCO2CH2CH(O2CR')CH2CO2R'' + 3H2O (R, R', and R'' refer to fatty acid 1, 2, and 3, respectively) [1]. Soybeans with higher levels of monounsaturated fatty acids such as oleic acid or polyunsaturated fatty acids such as linoleic or linolenic are more desirable for human consumption than saturated fatty acids. However, from soybean processing perspective, higher levels of polyunsaturated fatty acids contribute to oil oxidative instability, short shelf life, and rancidity. Therefore, soybean seed with higher levels of oleic acids and lower levels of linoleic or linolenic acids are desirable to minimize hydrogenation of the oil. The hydrogenation process was reported to have undesirable health effects by increasing the risk of coronary heart disease and resulting in higher LDL-cholesterol and lower HDL-cholesterol [2]. During the partial hydrogenation polyunsaturated fatty acids such as linolenic acid are converted to oleic and stearic acids, reducing polyunsaturated fatty acids to about 18% and linolenic acid to below 2% [3]. The hydrogenation process led to increased demand for lowlinolenic soybean oil, allowing food processors to reduce the need for hydrogenation, thus reducing trans fatty acids in foods. The health benefits of soybean oil were previously reported [4-6].
The amount of oil and oil composition (individual fatty acids) in soybean was reported to be affected by genetics (cultivars, genotypes within cultivars, and maturity genes) and environment, especially drought and temperature/heat, and their interactions. Although the mechanism of how the genetics and environment affect the stability of oil level and its composition is not yet understood, alteration of oil level and its composition was observed under these conditions. It was reported that seed developed under higher temperature had lower linoleic and linolenic acid concentrations, but higher oleic acid concentrations [7,8]. Other researchers, using 17 normal and modified fatty acid genotypes across 10 environments, investigated the effects of average temperature over the final 30 days of the reproductive period on oleic and linolenic acids [9]. They found that the concentration of oleic acid in modified mid-oleic genotypes were less stable than in those of reduced oleic acid, and mid-oleic (50% - 60% oleic acid) lines, N98-4445A and N97-3363-4, and the elevated oleic acid line M23 (up to 80% oleic acid) was the most stable genotype. Reduced linolenic acid genotype IA 3017 (1% linolenic acid) showed higher stability of linolenic concentration across environments, but higher linolenic acid genotypes showed less stability [9]. They concluded that soybean lines with stable oleic acid and linolenic acid across environments could be used as a source for germplasm development and crops with desirable fatty acids composition. Studying the effect of planting date on normal (Century cultivar) and low-linolenic soybean genotypes (C1640 and 9509: genotypes differ in linolenic acid due to alleles at the fan locus), it was found that palmitic acid concentration decreased and stearic acid increased with later planting [10], and linolenic acid concentration was more sensitive than other fatty acids which was due to temperature changes coinciding with late planting [10]. In a greenhouse experiment, the concentration of linolenic acid decreased from 105 to 66 g/kg as daytime temperatures in the greenhouse increased from 21˚C to 29˚C [7], concentrations of linolenic acid decreased from 164 to 50 g/kg, linoleic acid decreased from 558 to 403 g/kg, and oleic acid increased from 131 to 387 g/kg as day/night temperature increased from 18/13˚C to 33/28˚C [11]. There were no changes in palmitic and stearic acids with temperature changes [11]. Investigating five low-linolenic acid lines at day/night temperatures ranging from 15/12˚C to 40/30˚C, linoleic and linolenic acids concentration decreased, oleic acid concentration increased, and palmitic and stearic acid concentrations remained relatively stable in the five lines [12].
Drought is another environmental stress factor that affects seed oil stability and deposition, especially during seed-fill stages (R5-R6). Research on drought and water stress effects on oil changes was previously reported. For example, it was reported that severe drought stress during seed-fill stage can lead to up to 12.4% oil decrease [8], and drought stress can increase stearic acid and decrease oleic acid [8]. However, the increase or decrease depended on the severity of drought [13]. In a field experiment investigating the effect of irrigation on oleic and linolenic fatty acids in elevated modified oleic acid and/or reduced modified linolenic acid genotypes, it was found that irrigation did not affect unsaturated fatty acid concentration [14]. However, oleic acid tended to increase and linolenic acid tended to decrease in elevated oleic acid and reduced linolenic acid genotypes, respectively, indicating the significance of optimum irrigation for maintaining optimum fatty acid levels in seed [14]. It was reported that seed-fill period is a critical stage for the plant, and exposure of the plant to water stress during this stage would affect the inverse relationship between oil and protein with temperature [14,15]. It was concluded that drought affects total oil levels [8], alters fatty acid composition [16], and affects oil stability and oil processing. It was observed that soybean seed damaged in the field and exposed to frost, heat, and moisture resulted in low refined oil quality and oxidative stability [17]. Drought also led to shrinking and cracking [17], shriveling and seed coat wrinkling [18], resulting in poor oil quality and processing concerns [17,18].
Therefore, development of drought tolerant soybeans with stable high oleic and low linolenic acid genes across geographical locations and under environmental stress factors such as drought and high temperature is critical to maintain the stability of oil production and desirable fatty acid composition. To achieve this goal, it was suggested that the instability of conventional cultivars and midoleic acid germplasm across environment was mainly due to temperature changes, affecting fatty acid enzymes [10,19] such as oleate and linoleate desaturases, decreasing oleyl and linoleyl desaturase activities at 35˚C [20]. Also, it was found that ω-6 desaturase enzyme, encoded by the FAD2-1A gene, was degraded at high growth temperatures of 30˚C [21]. Research on transcript level of the functional GmFAD2 isoforms, FAD2-1A, FAD2- 1B, FAD2-2B and FAD2-2C, showed that high level of expression in FAD2-2C was observed when soybean was grown at temperature 18/12˚C day/night during pod development stage [22]. Other researchers investigated the level of expression of omega-6 desaturase GmFAD2 genes and genes controlling seed oleic acid concentration in modified mid-oleic acid soybean mutant M23, and found lower expression of GmFATB1a, GmFAD2-1A, GmFAD2-1B, GmFAD2-2, and GmFAD2-3, but higher expression of the GmSACPD-C.
Recently, it was found through a breeding program that the mutant FAD2-1B alleles were associated with high oleic acid concentration, but the FAD2-1B mutant alleles alone could not produce high oleic unless FAD2- 1A mutations were combined with the novel mutant FAD2-1B alleles [23]. The resulted combined mutation produced 80% oleic acid, and this was recovered for lines that were only homozygous for both mutant alleles. The stability of these lines with 80% oleic acid was tested in different environments and stability of this trait across tested environments was shown [24]. In spite of the tremendous research efforts in developing such modified high oleic germplasm and its stability with temperature changes, more research is needed to show that production components and other seed quality traits in nonGMO or GMO soybeans with desirable fatty acid composition are not compromised across wider environments and geographic locations, especially under environmental stress factors of drought and high heat. The objective of this research was to further evaluate the responses of soybean genotypes of different maturities to moderate and severe water stress and to warm and high temperatures. Since sugars, nitrogen, and carbon metabolism are associated with oil and fatty acids metabolism, and in order to explain possible mechanisms accompany oil and fatty acid changes under these stress conditions, seed sugars (monosaccharaides: glucose and fructose; disaccharides sucrose; trisaccharides, raffinose; tetrasaccharides, stachyose), and nitrogen and carbon fixation using natural abundance δ15N and δ13C isotopes were also analyzed.
2. Materials and Methods
Greenhouse and growth chamber experiment were conducted, and each experiment was repeated twice. Four soybean genotypes were used, two soybean genotypes of MG II (PI 597411 B and PI 597408) and two of MG VI (Arksoy and PI 437726). Soybean plants were divided in different sets, and the treatments were well watered (soil water potential between −15 to −20 kPa) (water field capacity) (W), water stressed (WS) (soil water potential between −90 and −100 kPa), and severely water stressed (SWS) (soil water potential between −150 to −200 kPa). For the high temperature experiment, soybean genotypes were grown under growth chamber conditions with photon flux density of about 1000 μmol·m−2·s−1, supplied with a combination of 10,400 W high pressure sodium and metal halide lights. Temperatures were 25/20˚C (normal), 36/28˚C (warm), and 40/33˚C (high). Plants were grown in clay soil with 8% sand, 31.6% silt, and 60.4% clay, and contained adequate macroand micronutrients. Uniform size seedlings at about V1 stage were transplanted into 9.45 L size pots filled with soil. Water stress treatment in the greenhouse and temperature stress treatment in growth chambers were applied at seed-fill stage (R5-R6). Soil water potential was monitored daily using Soil Moisture Meter (WaterMark Company, Inc., Wisconsin, USA). Seed composition analysis was conducted at harvest maturity (R8 stage).
2.1. Fatty Acid Analysis
Seed composition analysis was conducted at harvest maturity (R8 stage). Briefly, 25 g of seed from each plot was ground using a Laboratory Mill 3600 (Perten, Springfield, IL). Palmitic, stearic, oleic, linoleic, and linolenic fatty acids were analyzed using near-infrared (NIR) reflectance [25,26], diode array feed analyzer, Perten. The calibration was developed by the University of Wisconsin, USA using Perten’s Thermo Galactic Grams PLS IQ software. The calibration was developed for unique samples using AOAC methods [27,28]. The fatty acid were analyzed and expressed on an oil basis.
2.2. Seed Analysis for Sucrose, Raffinose, and Stachyose
Seeds collected at harvest maturity (R8 stage) were analyzed for sucrose, raffinose, and stachyose concentrations according to previous methods [25,29]. Analyses were conducted by near infrared reflectance (NIR), using an AD 7200 array feed analyzer (Perten, Springfield, IL), as previously described [29]. Briefly about 25 g of seed from each plot were ground using a Laboratory Mill 3600 (Perten, Springfield, IL). Sugar analyses were performed based on a seed dry matter basis [25,29,30].
2.3. Seed Glucose Determination
Seed glucose was determined according to the enzymatic reaction using Glucose (HK) Assay Kit from Sigma, USA, Product Code GAHK-20. Glucose is phosphorylated by adenosine triphosphate (ATP) catalyzed by hexokinase. During this reaction glucose-6-phosphate (G6P) is formed, then oxidized to 6-phosphogluconate in the presence of oxidized nicotinamide adenine dinucleotide (NAD) in a reaction catalyzed by glucose-6-phosphate dehydrogenase (G6PDH). During this oxidation reaction, an equimolar amount of NAD is reduced to NADH. The consequent increase in absorbance at 340 nm is directly proportional to glucose concentration in the sample. The Glucose (HK) Assay Reagent was reconstituted according to the manufacturers’ instructions (Sigma, USA) in 20 ml deionized water. Mature seed samples were ground using a Laboratory Mill 3600 (Perten, Springfield, IL) to obtain uniform particles. A random ground sample of 0.1 mg was extracted with deionized water. After the extract was diluted, a sample of 100 μl was added to 1ml of the Glucose Assay Reagent in a cuvette and incubated at room temperature for 15 min. A sample blank and a reagent blank were also prepared. The absorbance was read at 340 nm after 15 min, using a Beckman Coulter DU 800 spectrophotometer (Fullerton, CA). The concentration of glucose was expressed as mg·g·dwt−1.
2.4. Seed Fructose Determination
Seed fructose was determined by an enzymatic reaction according to Fructose Assay Kit from Sigma, USA, Product Code FA-20. Fructose is phosphorylated by ATP in a reaction catalyzed by hexokinase. After the conversion of fructose 6-phosphate to G6P by phosphoglucose isomerase (PGI), the oxidation of G6P to 6-phosphogluconate takes place in the presence of NAD in the reaction catalyzed by glucose-6-phosphate dehydrogenase (G6PDH). During this oxidation reaction, an equimolar amount of NAD is reduced to NADH. The increase in absorbance at 340 nm is directly proportional to fructose concentration in a sample. Mature seed samples were ground using a Laboratory Mill 3600 (Perten, Springfield, IL). A random sample of 0.1 mg was extracted with deionized water, and the sample solution was heated to aid extraction. The extract was diluted, and a sample of 100 μl was added to 2 ml of the Glucose Assay Reagent and 0.02 ml PGI in a cuvette and incubated at room temperature for 15 min. A sample blank and a sample of Glucose Assay Reagent blank and PGI blank were also prepared as recommended by the manufacturer. Samples were read at absorbance 340 nm after 15 min, using a Beckman Coulter DU 800 spectrophotometer (Fullerton, CA). The concentration of fructose was expressed as mg·g·dwt−1.
2.5. Analysis of Natural Abundance δ15N and δ13C Isotopes
Seed natural abundance of δ15N and δ13C isotopes was conducted on about 0.9 mg of ground seeds as previously described [31-34]. Isotopic analysis was performed using a Thermo FinniGlyn Delta Plus Advantage Mass Spectrometer with a FinniGlyn ConFlo III, and Isomass Elemental Analyzer (Bremen, Germany). Isodat software version 2.38 was used to calculate Delta values. The elemental combustion system was Costech ECS 4010 with an autosampler (Bremen, Germany).
2.6. Experimental Design and Statistical Analysis
Water stress experiment was a split plot design with irrigation as the main plot and genotype as subplot. Four replicates were used. For the temperature experiment, a randomized complete block design was used. Three sets were grown under different temperatures. One set was grown under 25/20˚C, another set at 36/28˚C, and another at 40/33˚C. Statistical analyses were conducted with Proc Mixed in SAS [35], and means were separated by Fisher’s least significant difference test at 5% probability. Since there were no interactions between experiment one and two, the data were pooled and combined.
3. Results and Discussion
Analysis of variance showed that water stress and genotype had a significant effect on seed composition in both maturity groups (Tables 1 and 2). Similar observation was recorded for the effect of temperature on seed composition (Tables 3 and 4). No interactions between experiment (E) and other parameters were observed, indicating similar effects of both water stress and temperature on fatty acids. Oleic, linoleic, and linolenic acid concentrations were the main constituents that were affected by water stress, and palmitic, stearic, and to some extent total oil concentrations were the least affected by water stress.
3.1. Effect of Water Stress
In MG II, water stress resulted in a significant decrease in oil, linoleic and linolenic concentrations compared with seed in well watered (W) plants (Table 5). However, the opposite trend for oleic acid concentration was observed in WS plants. In severe water stressed plants (SWS), seed oil, linoleic, and linolenic acids decreased compared with W plants. Water stress resulted in higher oleic acid concentrations in PI 597408 than PI 597411 B (Table 5), reflecting genotype differences. Genotypes showed differences in the concentration of oil and fatty acids, and these differences were bigger under water stress conditions. In MG VI, water stress did not result in a significant decrease in total oil in Arksoy but resulted in a significant decrease in linoleic and linolenic acids (Table 6). Oleic acid trended to increase under water stress, opposite the trend for linoleic and linolenic acids. Severe water stress (SWS) resulted in lower oil and fatty acid concentrations compared with WS or W plants. Oil and fatty acid concentrations were different between PI 437726 and Arksoy. Total oil did not have a clear pattern between MG II and MG VI genotypes. Saturated fatty acids (palmitic and stearic acids) were the least sensitive
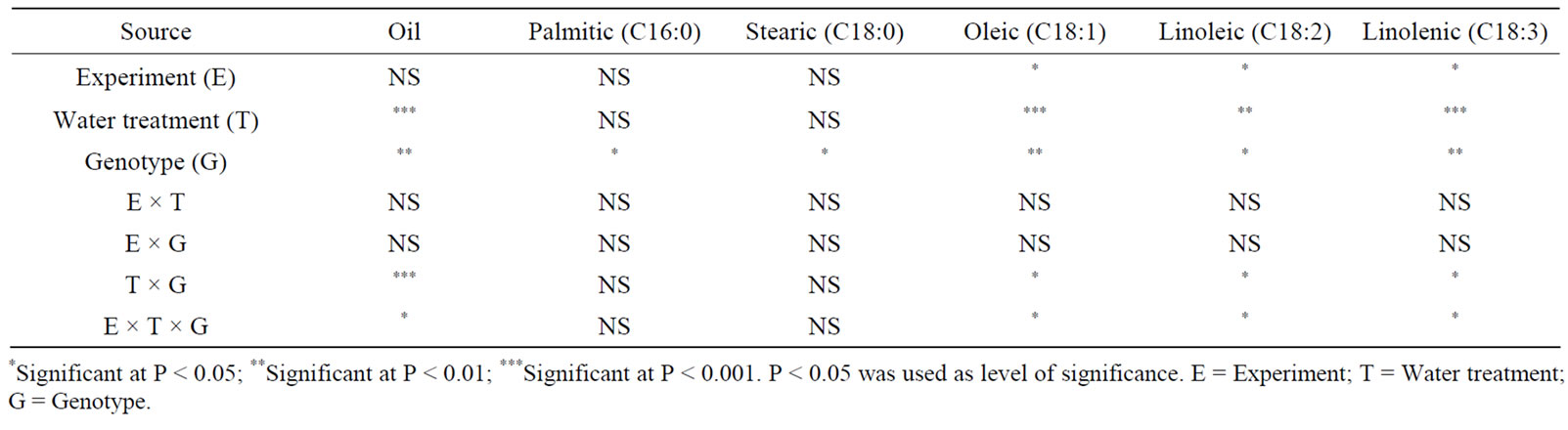
Table 1. Analysis of variance of the effect of water stress and genotype on fatty acid concentrations (g/kg) in soybean genotypes of maturity group II*.
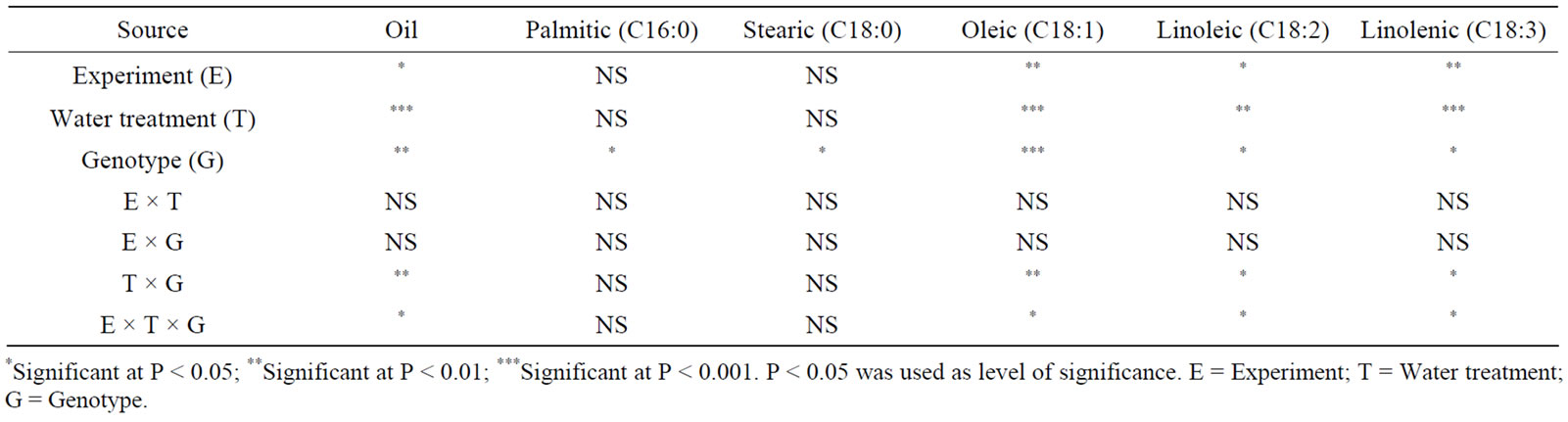
Table 2. Analysis of variance of the effect of water stress and genotype on fatty acid concentrations (g/kg) in soybean genotypes of maturity group VI*.

Table 3. Analysis of variance of the effect of temperature and genotype on fatty acid concentration (g/kg) in soybean genotypes of maturity group II*.
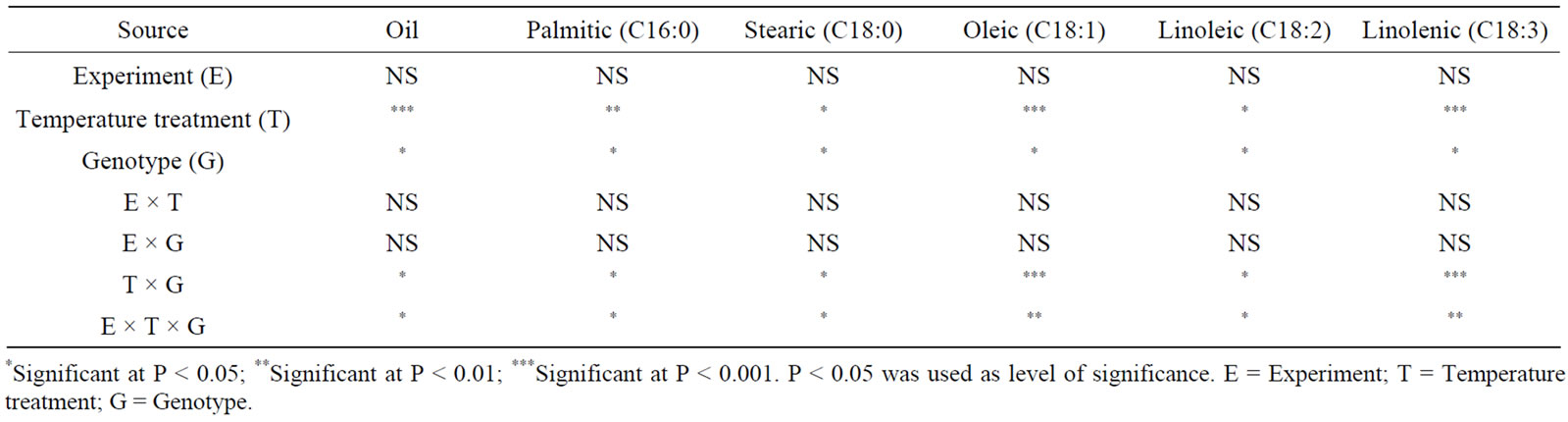
Table 4. Analysis of variance of the effect of temperature and genotype on fatty acid concentration (g/kg) in soybean genotypes of maturity group VI*.

Table 5. Response (mean values) of seed fatty acid concentrations (g/kg), sugars (non-structural sugars) (mg/g), and δ15N and δ13C isotopes to well watered (W) and water stressed (WS and SWS) soybean genotypes of maturity group (MG) II under greenhouse conditions*.

Table 6. Response (mean values) of seed fatty acid concentrations (g/kg), sugars (non-structural sugars) (mg/g), and δ15N and δ13C isotopes to well watered (W) and water stressed (WS and SWS) soybean genotypes of maturity group (MG) VI under greenhouse conditions*.
to water stress, in spite of the significant increase in palmitic and decrease in stearic acid concentrations in all cultivars under severe water stress.
The increase of total oil and oleic acid concentrations and decrease of linoleic and linolenic acid concentrations may be due to water stress altering the rate of oil and fatty acids accumulation by, possibly, affecting fatty acid desaturases (enzymes controlling fatty acid conversion). Effect of water stress or drought was previously reported, but mechanisms of how these effects occur are still not completely known. For example, it was reported that drought stress during seed-fill (R5-R6) stage can alter fatty acid composition in soybean seed, and severe drought resulted in a decrease of total oil up to 12.4%. Other researchers reported that the increase or decrease of seed oleic, linoleic, and linolenic fatty acids depends on the level of drought [13]. Effect of irrigation under field conditions was investigated [14]. They reported that when they compared irrigated with non-irrigated soybean, oleic acid tended to increase and linolenic acid tended to decrease in genotypes with high oleic acid and low linolenic acid. It was concluded that water stress during seedfill stage is critical because of the inverse relationship between oil and protein with temperature [36]. The effect of 11 environments on modified low saturated fatty acid, combined with low (1%) linolenic and elevated oleic acid was studied in Missouri and Iowa, USA. They found that the lines with the highest oleic acid concentration generally had the most variation across environments [37]. Oleic acid and linoleic acid contents were significantly affected by seasonal precipitation, but palmitic, stearic and linolenic concentrations were the least sensitive to seasonal changes [16]. It can be concluded that drought decreases total oil [8], alters fatty acid composition [16], and impacts oil stability [18]. The effect of drought on seed composition gives an opportunity to breeder and biotechnology researchers to develop drought tolerant soybeans with stable desirable fatty acid traits.
The different responses between genotypes in each MG to drought signify the importance of soybean genotype selection for optimum seed fatty acid composition, especially under stress environments such as drought. It was reported that differences in seed protein, oil, and fatty acids could be due to cultivar/genotype differences [38,39], maturity group [40], or irrigation management [41,42], and the increase or decrease of oil may depend on the severity of water stress (drought) [13], genotype [38,39], maturity time [40], and interactions between seed constituents, genotypes, and environments [25].
3.2. Effect of Temperature
In MG II genotypes, increasing the temperature from 25/20˚C to 36/28˚C resulted in higher oleic acid and lower linoleic and linolenic, but did not change total oil in both genotypes (Table 7). In high temperature (40/ 33˚C), however, total oil decreased as well. In MG VI genotypes, temperature increase resulted in higher oil and oleic acid, and lower linoleic and linolenic acid (Table 8). Maturity group VI responded differently to temperature where PI 597411 B had higher oleic acid and lower linoleic and linolenic acid concentrations than Arksoy. Palmitic and stearic concentrations were the least sensitive to temperature changes, although changes at (40/33˚C) were also observed.
Previous research indicated that higher temperature increased oleic acid concentration but decreased linoleic and linolenic acids concentrations [7,8]. Other researchers explained that effect of temperature on fatty acids could be due to the effect of changing temperature on enzymes controlling the accumulation and conversion of fatty acid (desaturases) [10,19,43] and ω-6 desaturase enzyme degradation at high growth temperatures of 30˚C [21].
Our results indicated that the increase of oleic acid and decrease of linoleic and linolenic acid at 36/28˚C and 40/33˚C compared with 25/20˚C may be due to a shift in nitrogen and carbon fixation, impacting nitrogen and carbon metabolism pathways. Analyses of seed carbohydrates (glucose, fructose, sucrose, raffinose, and stachyose) showed a significant decline of glucose, fructose, and sucrose and a significant increase of stachyose concentration with water stress and high temperature in all genotypes (Tables 5-8). Also, analyses of natural abundance of δ15N and δ13C isotopes (Tables 5-8) indicated that changes in sources of nitrogen and carbon fixation occurred, indicating possible changes in nitrogen and carbon metabolism pathways. The biological functions of sugar fractions such as raffinose and stachyose in soybean seed are still not completely understood [44], although relationships between sugar fractions were previously reported. For example, a positive correlation between total sugar and sucrose and raffinose was reported, but no significant correlation was found for stachyose [45-47]. Other researchers found a positive correlation between sucrose and raffinose, but a negative correlation was found between sucrose and stachyose [48]. Our results showed that more conversion of sucrose, glucose, and fructose took place to cope against temperature stress [49,50]. It has been reported that oligosaccharides including sucrose and raffinose are required during seed development and maturation, and may be involved in the acquisition of desiccation tolerance and protection of seeds against seed dehydration [51].
High temperature does not only affect fatty acids in conventional soybean only, but also affect fatty acids in modified soybean genotypes. For example, when the effect of average temperature (30 days before the reproductive period) on modified fatty acid genotypes was studied across 10 environments, significant differences were found for oleic acid stability among mid-oleic acid genotypes, and mid-oleic acid genotypes N98-4445A and N97-3363-4 were the most unstable among the 17 genotypes studied [9]. Elevated oleic acid line (M23) (up to 80% oleic acid) were the most stable genotype for oleic and linolenic acids [9]. Linolenic concentration in re

Table 7. Response (mean values) of seed fatty acid concentrations (g/kg) and sugars (non-structural sugars) (mg/g) to normal temperature (25/20˚C, day/night) and higher temperatures (36/28˚C, day/night; 40/33˚C, day/night) in soybean genotypes of maturity group (MG) II under growth chamber conditions*.
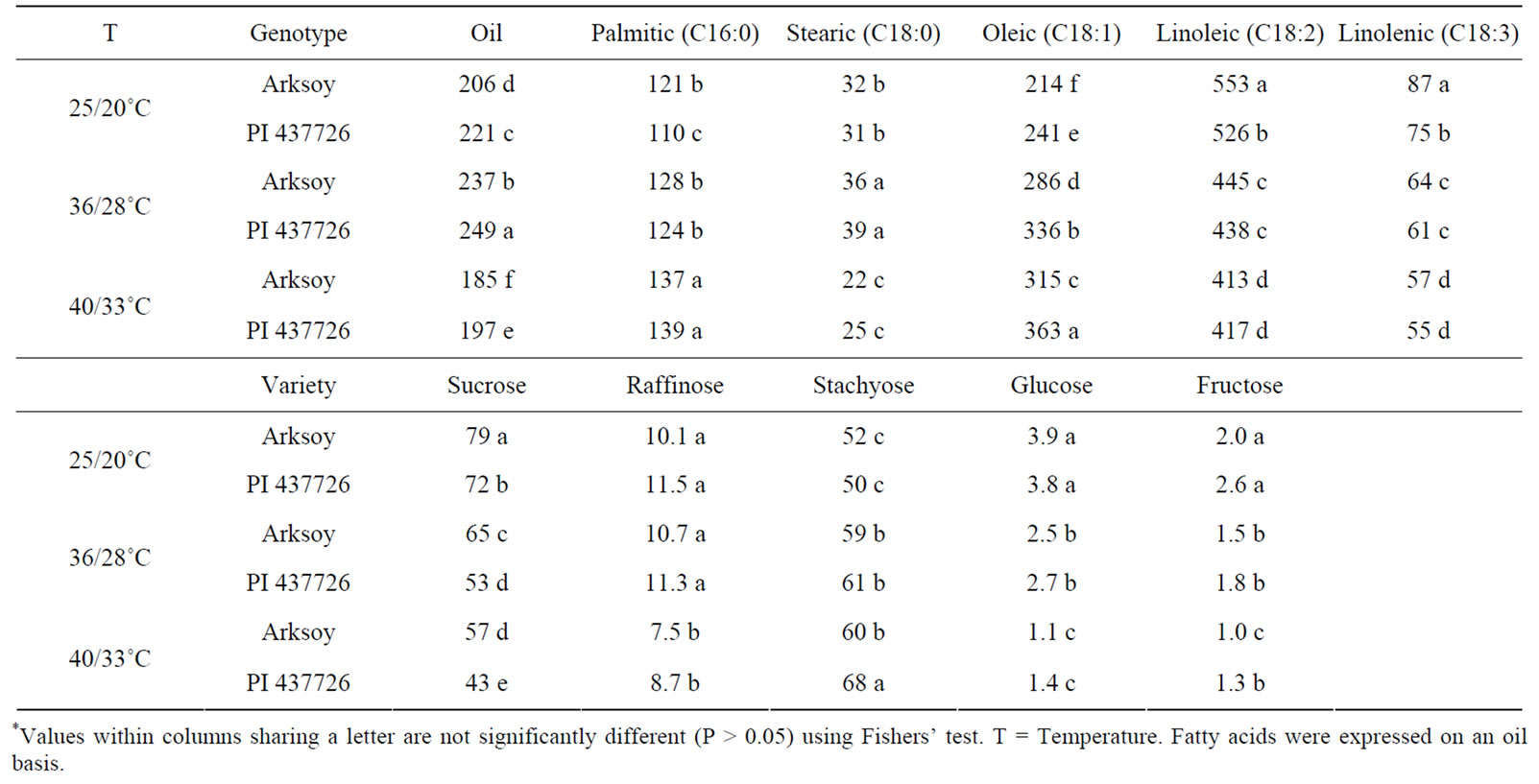
Table 8. Response (mean value) of seed fatty acid concentrations (g/kg) and sugars (non-structural sugars) (mg/g) to normal temperature (25/20˚C, day/night) and higher temperatures (36/28˚C, day/night; 40/33˚C, day/night) in soybean genotypes of maturity group (MG) VI under growth chamber conditions*.
duced linolenic acid genotype IA 3017 had higher stability across environments, but higher linolenic acid genotypes had less stability [9]. Greenhouse experiments showed that the concentration of linolenic acid decreased from 105 to 66 g/kg as daytime temperatures in the greenhouse increased from 21˚C to 29˚C [7], and concentrations of linolenic acid decreased from 164 to 50 g/kg, linoleic acid decreased from 558 to 403 g/kg, and oleic acid increased from 131 to 387 g/kg as day/night temperature increased from 18/13˚C to 33/28˚C [11]. Our results showed that oleic increased and linoleic and linolenic decreased, but total oil can increase with the increase of temperature until it reaches a certain temperature, then the total oil decreases with a higher temperature increase (40˚C). The stability of palmitic and stearic with the temperature changes was previously reported [11,12].
The recent development of modified soybean with desirable fatty acid traits (80% oleic acid, 1% linolenic acid, low or high saturated fatty acids) through conventional soybean breeding or biotechnology is a significant achievement [52-55]. However, the instability of conventional cultivars and mid-oleic acid germplasm across environment created a challenge, mainly due to temperature changes that influence the enzymes controlling biosynthesis of soybean seed fatty acids, especially at seedfill stage [10,19]. Temperature may affect oleate and linoleate desaturases [53] and decrease oleyl and linoleyl desaturase activities at 35˚C [20]. The ω-6 desaturase enzyme, encoded by the FAD2-1A gene, was degraded at high growth temperatures of 30˚C [21]. Because of the complexity of the effect of temperature on oleic acid stability, it was suggested that several soybean genomic loci known or suspected may be responsible for oleic acid phenotype, and some may be involved in oleate regulation [56]. Through the development of high oleic (80%) using combined mutations [23], it was possible to obtain a stable high oleic acid trait across two production environments in the Midsouth USA [24]. In spite of the breeding programs and biotechnology efforts to obtain stable fatty acid traits across environment, further research may be needed to establish that other seed quality component are not compromised in developing nonGMO or GMO soybeans with desirable fatty acid composition under wider environments and across geographic locations.
4. Conclusion
Our research demonstrated that both water stress and high temperature altered fatty acid composition by increasing oleic acid and decreasing linoleic and linolenic acids. The decrease of glucose, fructose, and sucrose (non-structural carbohydrates) indicated that the alteration of fatty acids may be due to limited availability of sugars in the plants and translocation of sugars from leaves to seed during seed-fill. The changes in δ15N and δ13C isotopes indicated a shift in sources of nitrogen and carbon fixation, impacting nitrogen and carbon metabolism pathways and enzymes controlling fatty acid conversion. Further research is needed to investigate the effects of water stress and temperature on enzymes controlling fatty acid accumulation and conversion.
5. Acknowledgements
The authors are thankful to Sandra Mosley for seed composition analysis and Les Price for nitrogen and carbon isotope analysis.
The US Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program (Not all prohibited bases apply to all programs). Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
REFERENCES
- Wikipedia, “Triglyceride,” 2013. http://en.wikipedia.org/wiki/Triglycerides
- Federal Register-68 FR 41433 (2003), “Food Labeling: Trans Fatty Acids in Nutrition Labeling, Consumer Research to Consider Nutrient Content and Health Claims and Possible Footnote or Disclosure Statements; Final Rule and Proposed Rule,” 2013. https://www.federalregister.gov/articles/2003/07/11/03-17526/food-labeling-trans
- T. E. Clemente and E. B. Cahoon, “Soybean Oil: Genetic Approaches or Modification of Functionality and Total Content,” Plant Physiology, Vol. 151, No. 3, 2009, pp. 1030-1040. doi:10.1104/pp.109.146282
- G. Sakthivelu, M. K. A. Devi, P. Giridhar, T. Rajasekaran, G. A. Ravishankar, M. T. Nikolova, G. B. Angelov, R. M. Todorova and G. P. Kosturkova, “Isoflavone Composition, Phenol Content, and Antioxidant Activity of Soybean Seeds from India and Bulgaria,” Journal of Agriculture and Food Chemistry, Vol. 56, 2008, pp. 2090-2095.
- M. J. Messina, V. Persky, K. D. R. Setchell and S. Barnes, “Soy Intake and Cancer Risks: A Review of the in Vitro and in Vivo Data,” Nutrition and Cancer, Vol. 21, No. 2, 1994, pp. 113-131. doi:10.1080/01635589409514310
- S. M. Potter, J. A. Baum, H. Y. Teng, R. J. Stillman, N. F. Shay and J. W. Erdman, “Soy Protein and Isoflavones: Their Effects on Blood Lipids and Bone Density in Postmenopausal Women,” American Journal of Clinical Nutrition, Vol. 68,1998, pp. 1375s-1379s.
- R. W. Howell and F. I. Collins, “Factors Affecting Linolenic and Linoleic Acid Content of Soybean Oil,” Agronomy Journal, Vol. 49, No. 11, 1957, pp. 593-597. doi:10.2134/agronj1957.00021962004900110007x
- D. L. Dornbos Jr. and R. E. Mullen, “Soybean Seed Protein and Oil Contents and Fatty-Acid Composition Adjustments by Drought and Temperature,” Journal of the American Oil Chemists’ Society, Vol. 69, 1992, pp. 228- 231.
- M. L. Oliva, J. G. Shannon, D. A. Sleper, M. R. Ellersieck, A. J. Cardinal, R. L. Paris and J. D. Lee, “Stability of Fatty Acid Profile in Soybean Genotypes with Modified Seed Oil Composition,” Crop Science, Vol. 46, No. 5, 2006, pp. 2069-2075. doi:10.2135/cropsci2005.12.0474
- J. R. Wilcox and J. F. Cavins, “Normal and Low Linolenic Acid Soybean Strains: Response to Planting Date,” Crop Science, Vol. 32, No. 5, 1992, pp. 1248-1251. doi:10.2135/cropsci1992.0011183X003200050037x
- R. B. Wolf, J. F. Cavins, R. Kleiman and L. T. Black, “Effect of Temperature on Soybean Seed Constituents: Oil, Protein, Moisture, Fatty Acids, Amino Acids and Sugars,” Journal of the American Oil Chemists’ Society, Vol. 59, 1982, pp. 230-232.
- B. D. Rennie and I. W. Tanner, “Fatty Acid Composition of Oil from Soybean Seeds Grown at Extreme Temperatures,” Journal of the American Oil Chemists’ Society, Vol. 66, 1989, pp. 1622-1624.
- J. E. Specht, K. Chase, M. Macrander, G. L. Graef, J. Chung, J. P. Markwell, H. H. Orf and K. G. Lark, “Soybean Response to Water: A QTL Analysis of Drought Tolerance,” Crop Science, Vol. 41, No. 2, 2001, pp. 493- 509. doi:10.2135/cropsci2001.412493x
- J. D. Lee, M. L. Oliva, D. A. Sleper and J. G. Shannon, “Irrigation Has Little Effect on Unsaturated Fatty Acid Content in Soya Bean Seed Oil within Genotypes Differing in Fatty Acid Profile,” Journal of Agronomy and Crop Science, Vol. 194, No. 4, 2008, pp. 320-324. doi:10.1111/j.1439-037X.2008.00315.x
- N. Bellaloui, “Effect of Water Stress and Foliar Boron Application on Seed Protein Oil Fatty Acids and Nitrogen Metabolism in Soybean,” American Journal of Plant Sciences, Vol. 2, No. 5, 2011, pp. 692-701. doi:10.4236/ajps.2011.25084
- J. Gao, X. Hao, K. D. Thelen and G. P. Robertson, “Agronomic Management System and Precipitation Effects on Soybean Oil and Fatty Acid Profiles,” Crop Science, Vol. 49, No. 3, 2009, pp. 1049-1057. doi:10.2135/cropsci2008.08.0497
- G. R. List, D. R. Erickson, E. H. Pryde, O. L. Brekke, T. L. Mounts and R. A. Falb, “Handbook of Soy Oil Processing and Utilization,” AOCS Press, Champaign, 1980, pp. 267-354.
- T. J. Brumm, C. R. Hurburgh and L. A. Johnson, “Cracking and Dehulling Shriveled and Wrinkled Soybeans,” Journal of the American Oil Chemists’ Society, Vol. 67, 1990, pp. 750-756.
- E. Bachlava and A. J. Cardinal, “Correlation between Temperature and Oleic Acid Seed Content in Three Segregating Soybean Populations,” Crop Science, Vol. 49, No. 4, 2009, pp. 1328-1335. doi:10.2135/cropsci2008.11.0660
- T. M. Cheesbrough, “Changes in the Enzymes for Fatty Acid Synthesis and Desaturation During Acclimation of Developing Soybean Seeds to Altered Growth Temperature,” Plant Physiology, Vol. 90, No. 2, 1989, pp. 760- 764. doi:10.1104/pp.90.2.760
- G. Q. Tang, W. P. Novitzky, H. C. Griffin, S. C. Huber and R. E. Dewey, “Oleate Desaturase Enzymes of Soybean: Evidence of Regulation through Differential Stability and Phosphorylation,” Plant Journal, Vol. 44, No. 3, 2005. pp. 433-446. doi:10.1111/j.1365-313X.2005.02535.x
- J. A. Schlueter, I. F. Vasylenko-Sanders, S. Deshpande, J. Yi, M. Siegfried, B. A. Roe, S. D. Schlueter, B. E. Scheffler and R. C. Shoemaker, “The FAD2 Gene Family of Soybean: Insights into the Structural and Functional Divergence of a Paleopolyploid Genome,” Crop Science, Vol. 47, 2007, pp. S14-S26.
- T. Pham, J. D. Lee, J. G. Shannon and K. D. Bilyeu, “Mutant Alleles of FAD2-1A and FAD2-1B Combine to Produce Soybeans with the High Oleic Acid Seed Oil Trait,” BMC Plant Biology, Vol. 10, 2010, p. 195. doi:10.1186/1471-2229-10-195
- J. D. Lee, K. D. Bilyeu, V. R. Pantalone, M. G. Gillen, Y. S. So and J. G. Shannon, “Environmental Stability of Oleic Acid Concentration in Seed Oil for Soybean Lines with FAD2-1A and FAD2-1B Mutant Genes,” Crop Science, Vol. 52, No. 3, 2012, pp. 1290-1297. doi:10.2135/cropsci2011.07.0345
- J. R. Wilcox and R. M. Shibles, “Interrelationships among Seed Quality Attributes in Soybean,” Crop Science, Vol. 41, No. 1, 2001, pp. 11-14. doi:10.2135/cropsci2001.41111x
- N. Bellaloui, J. R. Smith, J. D. Ray and A. M. Gillen, “Effect of Maturity on Seed Composition in the Early Soybean Production System as Measured on Near-Isogenic Soybean Lines,” Crop Science, Vol. 49, No. 2, 2009, pp. 608-620. doi:10.2135/cropsci2008.04.0192
- AOAC, “Method 988.05,” In: K. Helrich, Ed., Official Methods of Analysis, 15th Edition, The Association of Official Analytical Chemists, Inc., Arlington, 1990, p. 70.
- AOAC, “Method 920.39,” In: K. Helrich, Ed., Official Methods of Analysis, 15th Edition, The Association of Official Analytical Chemists, Inc., Arlington, 1990, p.79.
- N. Bellaloui, J. R. Smith, A. M. Gillen and J. D. Ray, “Effect of Maturity on Seed Sugars as Measured on Near-Isogenic Soybean (Glycine Max) Lines,” Crop Science, Vol. 50, No. 5, 2010, pp. 1978-1987. doi:10.2135/cropsci2009.10.0596
- E. Boydak, M. Alpaslan, M. Hayta, S. Gercek and M. Simsek, “Seed Composition of Soybeans Grown in the Harran Region of Turkey as Affected by Row Spacing and Irrigation,” Journal of Agriculture and Food Chemistry, Vol. 50, No. 16, 2002, pp. 4718-4720. doi:10.1021/jf0255331
- C. C. Delwiche and P. L. Steyn, “Nitrogen Isotope Fractionation in Soils and Microbial Reactions,” Environmental Science and Technology, Vol. 4, No. 11, 1970, pp. 929-935. doi:10.1021/es60046a004
- M. B. Peoples and D. F. Herridge, “Nitrogen Fixation by Legumes in Tropical and Subtropical Agriculture,” Advances in Agronomy, Vol. 44, 1990, pp. 155-223. doi:10.1016/S0065-2113(08)60822-6
- G. Shearer and D. H. Kohl, “N2 Fixation in Field Setting: Estimation Based on Natural 15N Abundance,” Australian Journal of Plant Physiology, Vol. 13, 1986, pp. 699-756.
- N. Bellaloui, A. Mengistu and R. L. Paris, “Soybean Seed Composition in Cultivars Differing in Resistance to Charcoal Rot (Macrophomina phaseolina)” Journal of Agriculture Science, Vol. 146, No. 6, 2008, pp. 667-675. doi:10.1017/S0021859608007971
- SAS Institute, “SAS 9.1 TS Level 1M3, Windows Version 5.1.2600,” SAS Institute, Cary, 2001.
- C. Carrera, M. J. Martínez, J. Dardanelli and M. Balzarini, “Water Deficit Effect on the Relationship between Temperatures during the Seed Fill Period and Soybean Seed Oil and Protein Concentrations,” Crop Science, Vol. 49, No. 3, 2009, pp. 990-998. doi:10.2135/cropsci2008.06.0361
- C. W. Scherder, W. R. Fehr and J. G. Shannon, “Stability of Oleate Content in Soybean Lines Derived from M23,” Crop Science, Vol. 48, No. 5, 2008, pp. 1749- 1754. doi:10.2135/cropsci2008.01.0018
- D. M. Maestri, D. O. Labuckas, J. M. Meriles, A. L. Lamarques, J. A. Zygadlo and C. A. Guzman, “Seed Composition of Soybean Cultivars Evaluated in Different Environmental Regions,” Journal of the Science of Food and Agriculture, Vol. 77, No. 4, 1998, pp. 494-498. doi:10.1002/(SICI)1097-0010(199808)77:4<494::AID-JSFA69>3.0.CO;2-B
- E. L. Piper and K. J. Boote, “Temperature and Cultivar Effects on Soybean Seed Oil and Protein Concentrations,” Journal of the American Oil Chemists’ Society, Vol. 76, 1999, pp. 1233-1242.
- M. Zhang, M. S. Kang, P. F. Reese and H. L. Bhardwaj, “Soybean Cultivar Evaluation via GGE Biplot Analysis,” Journal of New Seeds, Vol. 7, No. 4, 2005, pp. 37-50. doi:10.1300/J153v07n04_03
- N. Bellaloui and A. Mengistu, “Seed Composition Is Influenced by Irrigation Regimes and Cultivar Differences in Soybean,” Irrigation Science, Vol. 26, No. 3, 2008, pp. 261-268. doi:10.1007/s00271-007-0091-y
- N. Bellaloui, A. Mengistu, D. K. Fisher and C. A. Abel, “Soybean Seed Composition as Affected by Drought and Phomopsis in Phomopsis Susceptible and Resistant Genotypes,” Journal of Crop Improvement, Vol. 26, No. 3, 2012, pp. 428-453. doi:10.1080/15427528.2011.651774
- J. W. Burton, “Recent Developments in Breeding Soybeans for Improved Oil Quality,” Fat Science Technology, Vol. 93, 1991, pp. 121-128.
- C. Ren, K. D. Bilyeu and P. R. Beuselinck, “Composition, Vigor, and Proteome of Mature Soybean Seeds Developed under High Temperature,” Crop Science, Vol. 49, No. 3, 2009, pp. 1010-1022. doi:10.2135/cropsci2008.05.0247
- T. Hymowitz and F. I. Collins, “Variability of Sugar Content of Seed of Glycine max (L.) Merr. and G. soja Serb. and Zucco,” Agronomy Journal, Vol. 66, No. 2, 1974, pp. 239-240. doi:10.2134/agronj1974.00021962006600020017x
- U. A. Hartwig, C. A. Maxwell, C. M. Joseph and D. A. Phillips, “Chrysoeriol and Luteolin Released from Alfalfa Seeds Induce Nod Genes in Rhizobium meliloti,” Plant Physiology, Vol. 92, No. 1, 1990, pp. 116-122. doi:10.1104/pp.92.1.116
- A. Hou, P. Chen, J. Alloatti, D. Li, L. Mozzoni, B. Zhang and A. Shi, “Genetic Variability of Seed Sugar Content in Worldwide Soybean Germplasm Collections,” Crop Science, Vol. 49, No. 3, 2009, pp. 903-912. doi:10.2135/cropsci2008.05.0256
- A. S. Malik, O. Boyko, N. Atkar and W. F. Young, “A Comparative Study of MR Imaging Profile of Titanium Pedicle Screws,” Acta Radiologica, Vol. 42, No. 3, 2001, pp. 291-293. doi:10.1080/028418501127346846
- J. H Thorne, “Temperature and Oxygen Effects on C-Photosynthate Unloading and Accumulation in Developing Soybean Seeds,” Plant Physiology, Vol. 69, No. 1, 1982, pp. 48-53. doi:10.1104/pp.69.1.48
- J. M. G. Thomas, K. J. Boote, L. H. Allen Jr., M. GalloMeagher and J. M. Davis, “Elevated Temperature and Carbon Dioxide Effects on Soybean Seed Composition and Transcript Abundance,” Crop Science, Vol. 43, No. 4, 2003, pp. 1548-1557. doi:10.2135/cropsci2003.1548
- R. L. Obendorf, M. Horbowicz, A. M. Dickerman, P. Brenac and M. E. Smith, “Soluble Oligosaccharides and Galactosyl Cyclitols in Maturing Soybean Seeds in Planta and in Vitro,” Crop Science, Vol. 38, No. 1, 1998, pp. 78-84. doi:10.2135/cropsci1998.0011183X003800010014x
- Pioneer, “Plenish™ High Oleic Soybeans,” 2012. http://www.pioneer.com/home/site/about/products/product-traits-technology/plenish/
- K. Warner and W. Fehr, “Mid-Oleic/Ultra Low Linolenic Acid Soybean Oil: A Healthful New Alternative to Hydrogenated Oil for Frying,” Journal of the American Oil Chemists’ Society, Vol. 85, 2008, pp. 945-951.
- “Six New Soybean Varieties Highlight Progress in Developing Healthier Oils at Iowa State University (ISU),” Healthier Soybean Oils, Iowa State University, 2008. http://www.notrans.iastate.edu/
- Monsanto Company, “Soybean Seeds,” 2012. http://www.monsanto.com/products/Pages/soybean-seeds.aspx
- R. G. Upchurch and M. E. Ramirez, “Gene Expression Profiles of Soybeans with Mid-Oleic Acid Seed Phenotype,” Journal of the American Oil Chemists’ Society, Vol. 87, 2010, pp. 857-864.
NOTES
*Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.

