 Open Journal of Pathology, 2012, 2, 127-132 Published Online October 2012 (http://www.SciRP.org/journal/ojpathology) http://dx.doi.org/10.4236/ojpathology.2012.24023 Copyright © 2012 SciRes. OJPathology 127 Plasma Cell Neoplasms, Clinicopathological Characteristics and Immunophenotype of 21 Patients Mónica Belinda Romero-Guadarrama1,2*, Cinthia Adriana Meza Medina3, Elvira Aguilar Martínez4 1Unidad de Patología del Hospital General de México, Mexico City, Mexico; 2Facultad de Medicina, Universidad Nacional Autónoma de México, Mexico City, Mexico; 3Facultad de Medicina, Universidad Autónoma de Nayarit, Tepic, Mexico; 4Unidad de Hematología del Hospital General de México, Mexico City, Mexico. Email: *monicaromero@att.net.mx Received August 9th, 2012; revised September 9th, 2012; accepted September 19th, 2012 ABSTRACT Introduction: Plasma cell neoplasms are monoclonal proliferations characterized by the secretion of an immunoglobu- lin product known as component “M” or monoclonal. The World Health Organization (WHO 2008) defines as plasma cell neoplasms the following: plasma cell myeloma, plasmacytoma and those syndromes defined by immunoglobulin deposits and primary amyloidosis, The objective of the present work was to correlate their clinical, morphological and phenotype characteristics in 21 patients. Material and Methods: A 2-year retrospective review was performed of the files of the surgical pathology laboratory and of the hematology service of the General Hospital of Mexico, searching for patients with a diagnosis of plasma cell neoplasm. We analyzed the following variables: age, gender, clinical symp- toms, evolution, localization, laboratory tests, morphology, and expression of immunohistochemical markers. Of the 21 patients, 12 (57.1%) corresponded to plasma cell myelomas and 9 (42.8%) were plasmacytomas (seven extraosseous and two solitary bone plasmacytoma); women predominated with 61.4% and ag e ranged b etween 22 and 84 years. Mass and epistaxis were observed in the patients with plasmacyto mas, and sympto ms of me dulla ry compr ession and anemia were observed in those patients with plasma cell myeloma. The time of symptomatology varied from 3 to 12 months. Laboratory tests revealed that lactate dehydrogenase (LDH), beta 2 microglobulin, C-reactive protein were altered and that hypercalcemia and anemia were present more in the systemic form of the disease. Treatment depended on the clinical staging and laboratory data. Mature fo rms predominated morphologically. Immunohistochemical stain revealed a constant expression for CD 138, six patients expressed CD 56, and expression of the Kappa and Lambda light chains was while. Keywords: Plasma Cell Neoplasms 1. Introduction Clonal proliferations of plasmatic cells and their precur- sors constitute a spectrum of diseases previously known as: “plasma cell dyscrasias”. This group includes mono- clonal gammopathy of indeterminate significance, which is characterized by the presence of low levels of para- protein in the peripheral blood, with a variable time of evolution, and later on development of a plasma cells myeloma; this phenomenon is considered as a precursor lesion. Other immunoglobulin secreting neoplasms that affect lymphoid and plasmatic cells lie within the current clas- sification of WHO-2008 regarding hematolymphoid neo- plasms, such as the lymphoplasmacytic lymphoma, W ald- enström’s macroglobulinemia, and heavy chain diseases [1]. A myeloma is a disease of adults with an average age of 62 years, it manifests by bone pain, lithic injuries in several bones, anemia, and recurring infections. It was first described in 1844 by Dr. Samuel Solly, who as- signed the name Mollities ossium to the condition [2]. Dr Bence Jones studied urine samples and described the proteins that bear his name (BJ) [3]. It was in 1873 that Rustizky described and used for the first time the term “multiple myeloma” to point out the variety of bone le- sions [4]. The solitary bone plasmacytoma affects the axial skeleton and the thoracic vertebras, it is manifested by localized bone pain and medullary compression symp- toms; 70% of patients present systemic symptoms after 2 to 4 years of evolution [5]. The extramedullary plas- macytoma is localized in the head and neck, gastrointes- tinal tract, central nervous system, skin, and other organs. In general, it does not produce detectable serum parapro- *Corresponding a uthor. 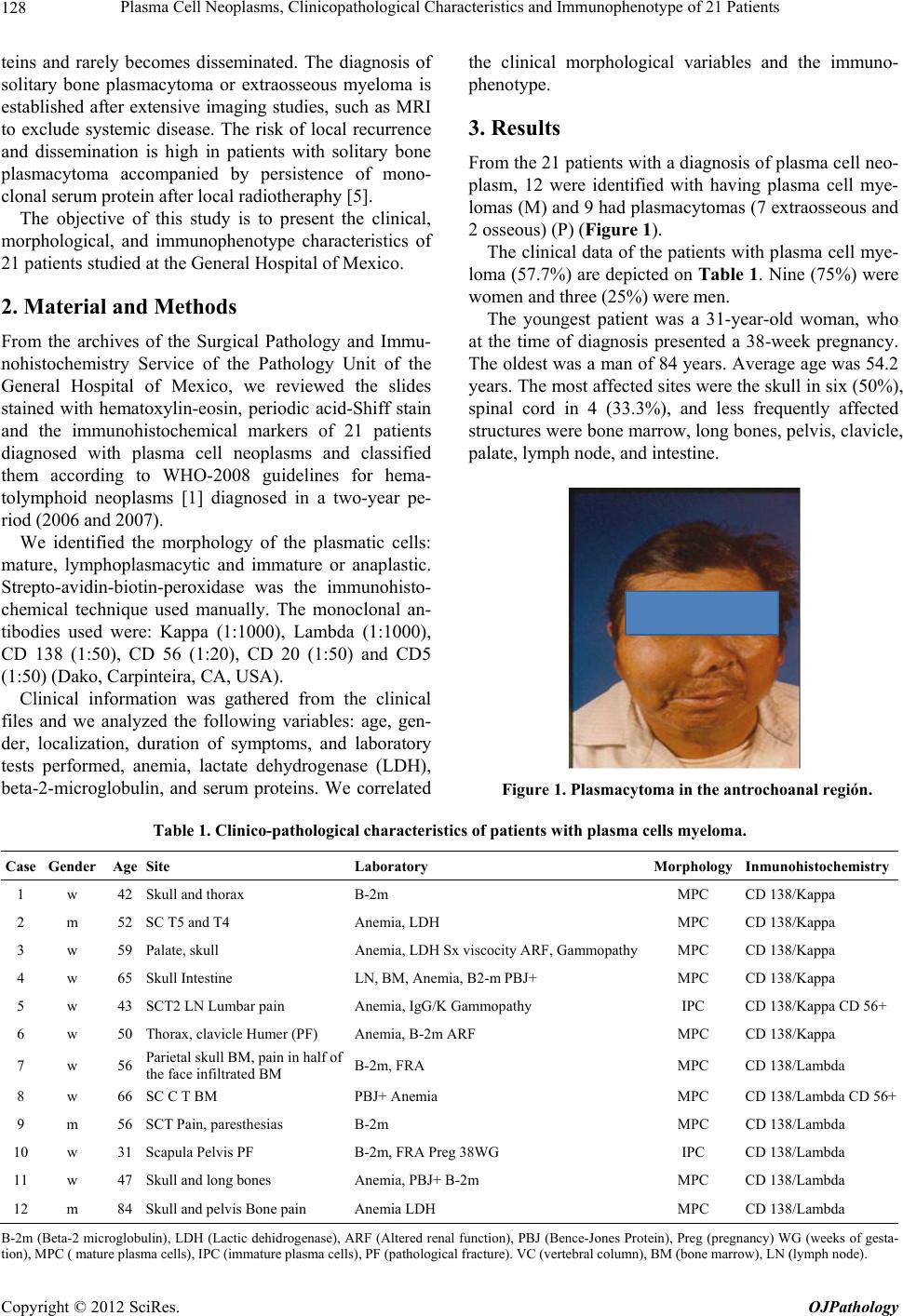 Plasma Cell Neoplasms, Clinicopathological Characteristics and Immunophenotype of 21 Patients 128 teins and rarely becomes disseminated. The diagnosis of solitary bone plasmacytoma or extraosseous myeloma is established after extensive imaging studies, such as MRI to exclude systemic disease. The risk of local recurrence and dissemination is high in patients with solitary bone plasmacytoma accompanied by persistence of mono- clonal serum protein after local radiothera phy [5]. The objective of this study is to present the clinical, morphological, and immunophenotype characteristics of 21 patients studied at the General Hospital of Mexico. 2. Material and Methods From the archives of the Surgical Pathology and Immu- nohistochemistry Service of the Pathology Unit of the General Hospital of Mexico, we reviewed the slides stained with hematoxylin-eosin, periodic acid-Shiff stain and the immunohistochemical markers of 21 patients diagnosed with plasma cell neoplasms and classified them according to WHO-2008 guidelines for hema- tolymphoid neoplasms [1] diagnosed in a two-year pe- riod (2006 and 2007). We identified the morphology of the plasmatic cells: mature, lymphoplasmacytic and immature or anaplastic. Strepto-avidin-biotin-peroxidase was the immunohisto- chemical technique used manually. The monoclonal an- tibodies used were: Kappa (1:1000), Lambda (1:1000), CD 138 (1:50), CD 56 (1:20), CD 20 (1:50) and CD5 (1:50) (D ako, Carpi nt e ir a, CA, USA). Clinical information was gathered from the clinical files and we analyzed the following variables: age, gen- der, localization, duration of symptoms, and laboratory tests performed, anemia, lactate dehydrogenase (LDH), beta-2-microglobulin, and serum proteins. We correlated the clinical morphological variables and the immuno- phenotype. 3. Results From the 21 patients with a diagnosis of plasma cell neo- plasm, 12 were identified with having plasma cell mye- lomas (M) and 9 had plasmacytomas (7 extraosseous and 2 osseous ) (P) (Figure 1). The clinical data of the patients with plasma cell mye- loma (57.7%) are depicted on Table 1 . Nine (75%) were women and three (25%) were men. The youngest patient was a 31-year-old woman, who at the time of diagnosis presented a 38-week pregnancy. The oldest was a man of 84 years. Average age was 54.2 years. The most affected sites were the skull in six (50%), spinal cord in 4 (33.3%), and less frequently affected structures were bone marrow, long bones, pelvis, clavicle, palate, lymph node, and intestine. Figure 1. Plasmacytoma in the antrochoanal región. Table 1. Clinico-pathological characteristics of patients with plasma cells myeloma. Case Gender Age Site Laboratory Morphology Inmunohistochemistry 1 w 42 Skull and thorax B-2m MPC CD 138/Kappa 2 m 52 SC T5 and T4 Anemia, LDH MPC CD 138/Kappa 3 w 59 Palate, skull Anemia, LDH Sx viscocity ARF, GammopathyMPC CD 138/Kappa 4 w 65 Skull Intestine LN, BM, Anemia, B2-m PB J+ MPC CD 138/Kappa 5 w 43 SCT2 LN Lumbar pain Anemia, IgG/K Gammopathy IPC CD 138/Kappa CD 56+ 6 w 50 Thorax, clavicle Humer (PF) Anemia, B-2m ARF MPC CD 138/Kappa 7 w 56 Parietal skull BM, pain in half of the face infiltrated BM B-2m, FRA MPC CD 138/Lambda 8 w 66 SC C T BM PBJ+ Anemia MPC CD 138/Lambda CD 56+ 9 m 56 SCT Pain, paresthesias B-2m MPC CD 138/Lambda 10 w 31 Scapula Pelvis PF B-2m, FRA Preg 38WG IPC CD 138/Lamb da 11 w 47 Skull and long bones Anemia, PB J+ B-2m MPC CD 138/Lambda 12 m 84 Skull and pelvis Bone pain Anemia LDH MPC CD 138/Lambda B-2m (Beta-2 microglobulin), LDH (Lactic dehidrogenase), ARF (Altered renal function), PBJ (Bence-Jones Protein), Preg (pregnancy) WG (weeks of gesta- tion), MPC ( mature plasma cells), IPC (immature plasma cells), PF (pathological fracture). VC (vertebral column), BM (bone marrow), LN (lymph node ). Copyright © 2012 SciRes. OJPathology  Plasma Cell Neoplasms, Clinicopathological Characteristics and Immunophenotype of 21 Patients 129 Seven patients (58.3%) presented anemia, in six (50%) 2-beta-microglobulin of more than 3.5 mg/L was found, three patients presented monoclonal gammopathy: IgG > 3.5 g/dL, IgA > 2 g/dL, and two patients had altered re- nal function, one coursed with hypercalcemia and ele- vated LDH. Morphologically, the neoplastic cells corresponded to mature plasmatic cells in 10 patients (83.3%) (Figure 2) and two showed immature and pleomorphic plasmatic cells (Figure 3). The determined immunohistochemical markers are summarized in Table 1, in which expression for CD138 was observed (Figure 4); 6 patients (50%) expressed light Kappa chain and 50% Lambda (Figures 5 and 6 respectively). Two patients (16%) expressed CD Figure 2. Mature plasma cells neoplasm (myeloma), in which cells depict and eosinophil cytoplasm and an eccen- tric nucleus. Some cells show cytoplasmic immunoglobulins (H-E). Figure 3. Plasma cells neoplasm with immature morphology. Note the pleomorphism of cells (H-E). Figure 4. Inmunohistoche mistry, note CD 138 expression in the cytoplasms of neoplastic plasma cells. Figure 5. Lambda expression can be observed in most neo- plastic cells, Immunohistochemi c al r eaction. Figure 6. Note the lack of Kappa expression. Immunohisto- chemical reaction. Copyright © 2012 SciRes. OJPathology  Plasma Cell Neoplasms, Clinicopathological Characteristics and Immunophenotype of 21 Patients 130 56, which morphologically corresponded to mature plas- ma cells and the other presented immature cells (Table 1), The others markers such CD 20 and CD 5 were nega- tive. The Table 2 depicts the clinical data of patients with plasmacytomas (9 cases, 42.8%). Seven were extraosse- ous (77.7%) and two were bone (33.3%). Four patients were men and five women, the youngest patient was a 22-year-old man without HIV antecedents and the loca- tion was in a lymph node associated to Castleman’s dis- ease, hyaline vascular type. The oldest patient was a 79-ye ar-old patien t, and the av erage ag e was of 52 years. The sites of the extraosseous plasmacytomas were: two in the nasal region and two more in the palate, an d one in the following sites, anthrocoanal, gum, and cervical lymph node. The bone plasmacytomas were found in cervical vertebra and the sternum. Morphologically, seven were constituted by mature plasma cells and two re- vealed the presence of lymphocytes and plasmatic cells (lymphoplasmacytic variant). Immuno histochemical pat- terns in five expressed Kappa and two Lambda; expres- sion for CD 56 was observed in four (44.4%). The others markers such CD 20 and CD 5 were negative (Table 2). 4. Discussion According to the compiled Histopathological Registry of 1997, plasma cell tumors occupied the 15th place and corresponded to less than 1% (0.4%) of the total malign- nant neoplasms in our country [6]. At the Instituto Nacional de Cancerología [National Cancer Institute] in Mexico, 193 new cases of plasma cells myeloma were diagnosed between 2000-2004; of these 92 were men and 101 were women, and repre- sented 1% of all the neop lasms diagnosed at this Institute [7]. At the General Hospital of Mexico, 21 patients were diagnosed with plasma cells neoplasm in a 2-year period, of which 12 were myelomas and 9 were plasmacytomas. In the USA, plasma cells myeloma corresponds to 1% of the total of all malignant tumors and to 10% to 15% of the hematopoietic neoplasms [8,9]. Bone and extraosseous plasmacytomas comprise 3% to 5% of plasmatic cell neoplasms in the USA [10]. The etiology is still unknown; however, they have been related with chronic antigenic stimulation due to infection, or with the exposure to specific toxic sub st a n c es or radiation [11]. Ries et al. observed that plasma cells myeloma, bone and extraosseous plasmacytoma are more common in men th an in women [9]. Accordin g to Rizo et al. [7] from the Instituto Nacional de Cancerología, and our results women predominate, as can be observed in Tables 1 and 2. The ave rag e age fo r myel o mas was of 54 years, and 52 years for plasmacytomas. In the Western literature the average age for myeloma patients is 70 years, and 55 years for plasmacytomas [1]. The clinical presentation of plasma cells myeloma varies [12] and occasionally the symptomatic diagnosis is delayed several months; the main bone symptoms are lumbar pain or fractures of the affected bones. In the present work, pain was the predominant symptom in myeloma (Table 1), renal failure results from the tubu lar damage caused by the proteinuria of the monoclonal light chain proteins. One patient presented altered renal func- tion and gammopathy, and in another it was not associ- ated with gammopathy. Fractures were found in two pa- tients. Among the altered laboratory tests, in the systemic form of the disease, we found anemia, hypercalcemia (>12 mg/dL), hyperviscocity, monoclonal peak as re- vealed by protein electrophoresis, protein C reactive, and elevation of LDH. Anemia was the most frequent altera- tion found in both the study performed by Kyle in the Mayo Clinic with 1027 patients (67% of patients with anemia) and in this study with a similar percentage of affectation (66.6%). The presence of anemia in patients Table 2. Clinicopathological characteristics of extraosseus and osseus plasmacytomas. Case Gender Age Site Morphology Inmunohistochemistry 1 m 49 gum/tumor MPC CD 138/Kappa 2 m 54 nasal/epistaxis MPC CD 138/Kappa CD 56 3 w 75 palate/tumor MPC CD 138/Lambda 4 w 63 nasal/epistaxis MPC CD 138/Kappa CD 56 5 m 84 antrochoanal/tumor MPC CD 138/Kappa 6 w 79 palate/lymphoplasmatic infiltration MPC CD 138/Lambda 7 w 57 sternun/tumor MPC CD 138/Kappa CD 56 8 w 57 SC MPC CD 138/Kappa CD 56 9 m 22 GL MPC CD 138/Lambda MPC (mature plasmatic cells). No laboratory tests alterat io ns were observed in a ny patient. CD 20 and CD 5 were ne g ative in neoplastic plasma cells. Copyright © 2012 SciRes. OJPathology  Plasma Cell Neoplasms, Clinicopathological Characteristics and Immunophenotype of 21 Patients 131 results first by the replacement of bone marrow by neo- plastic plasma cells and/or because of renal damage re- sulting from the loss of erythropoietin [13]. The other laboratory alteration found in this series was the eleva- tion of beta-2-micro globulin in serum, which is us ed as a survival marker, if it is higher than 5.5 mg/dL, patient’s survival is reduced to only 28 months; therefore, it is an important prognostic laboratory marker [14]. Clinical variants of plasma cells myeloma include the smoldering plasma cells myeloma, in which the patients course with stable disease for long periods of time, it presents with solitary plasmacytoma with bone ab nor ma l i - ties detected only through Magnetic Resonance Image (MRI) and only 10% - 20% of plasmatic cells are seen in the bone marrow [1]. Another variant is the non-secreting myeloma that corresponds to 3% of plasmatic cells myeloma. There is lack of protein M as assessed through fixation el ectr oph o- resis. [10] Leukemia of plasmatic cells is characterized by an increase in the number of clonal plasmatic cells in peripheral blood and exceeds 2 × 10/L, (2 × 109/L,) or when the neoplastic plasma cells are encountered in ex- tra-medullary sites, such as the spleen, liv er, pleural fluid, ascites, and cerebrospinal fluid; it can associate second- darily to a plasmatic cells myeloma during the course of the disease, [1] we did not identify any such case in our study. In 2006, 10 patien ts of the Institu to Naciona l de la Nutrición y Ciencias Médicas [National Institute of Nu- trition and Medical Sciences] were reported with a ful- minant clinical condition, the average age of the patients was 58 years and again women predom i na t e d [ 1 5 ]. By definition, extraosseous plas macytomas are located in tissues outside the bone; in the present study, the most affected site was the upper respiratory tract including the oropharynx. The plasmacytoma in the lymph node oc- curred adjacent to hyaline-vesicular type Castleman’s disease. Bone plasmacytoma was found one in the spinal cord and the other in the sternum. Histologically, the mature plasma cells predominated in the myelomas, their arrangement in the bone marrow is in groups interstitially, and when the bone marrow is not completely substituted normal hematopoietic elements can be observed; hence, it is important to use markers such as CD 138 or CD 79a, and light immunoglobulins to discard the disease. It is important to consider the percentage of plasmatic cells in the bone marrow that should be at least of 30%; another observed change is osteoclastic activity [16]. Histologically, we observed an almost complete sub- stitution of the normal bone marrow elements by abun- dant plasmatic cells and CD 138 expression was almost 100%, expression for Kappa and Lambda light chains was the same. CD 56 is expressed aberrantly en plasma cells myeloma up to 70% [17]. In the pre sent r evie w, th is marker was only expressed in two cases, one with a morphology of mature plasmatic cells and the other with immature p lasma cells. Aside from CD 56 other markers have been expressed, such as CD 117, CD 20, and CD 10; in contrast to plas- matic cells myeloma, expression of CD 56 is negative in plasmatic cells leukemia [18]. In plasmacytomas, mature morphology was observed in seven patients, and two showed lymphoplasmacytic morphology; expression of CD 56 was observed in four cases, three with mature morphology and one with lym- phoplasmacytic morphology. Expression of CD 20 favors the diagnosis of infiltration due to lymphoma, hence it must be taken into account for the differential diagnosis. The immunophenotype of this form of plasmatic cells neoplasm has not been studied extensively, but apparently it is similar to the myeloma phenotype. Expression of CD 56 was observed more frequently in this form, however, more studies have to be made, because probably this last marker could be used as a prognostic marker in the future. Although diverse genetic anomalies have been ob- served through f luorescence in situ hybr idization (FISH), such as in (13q14), hyperdiploidia, t (11; 14), among others; however, the most frequent translocation in plas- matic cells myeloma is the one affecting chromosome 14q23 with a 70% frequency [19]. It is noteworthy, that patients with myeloma associated to translocation t (11; 14) (q13; q32) are usually positive to cyclin D-1 and are associated with lymphoplasmacytic morphology with extensive infiltration to the bone marrow. In this work, only two cases presented this morphology and positivity to cyclin D-1 in the cytoplasm o f the cells and not in the nucleus [20,21]. In plasmacytomas, the expression of Kappa and Lambda is very important to demonstrate clonality and to confirm the diagnosis. There are reports in the literature on the frequency of CD 56 expression and the prognostic of the patients with myeloma, which, as well known, is a marker of NK cells and of some T cell subpopulations [22]. In conclusion, we observed that the myeloma and the localized form, the extramedullary plasmacytoma, are the most frequent. The average age of patients for the mye- loma group was of 54.2 year in this series and, in patients studied in two other national institutions, the age was below 60 years, that is, 15 years younger than that re- ported for American patients. Myeloma and another plasma cell neoplasms are not found in children and is rare in adults younger than 35 years. In our milieu, women are more affected. Morphologically, the presence of mature plasmatic cells predominated in both types of disease, and CD 56 expression was observed in the localized form of the disease. Copyright © 2012 SciRes. OJPathology  Plasma Cell Neoplasms, Clinicopathological Characteristics and Immunophenotype of 21 Patients 132 REFERENCES [1] S. H. Swerdlow, E. Campo, N. L. Harris, E. S. Jaffe, S. A. Pileri, H. Stein, J. Thiele and J. W. Vardiman, “WHO Classification of Tumours of Haematopoietic and Lym- phoid Tissues,” International Agency for Research on Cancer (IARC), Lyon, 2008. [2] S. Solly, “Remarks on the Pathology of Mollities Ossium: With Cases,” Medico-Chirurgical Transactions, Vol. 27, 1844, pp. 435-461. [3] H. B. Jones, “On a New Substance Occurring in the Urine of a Patient with Mollities and Fragilitas Ossium,” Phi- losophical Transactions of the Royal Society of London, Vol. 1, 1848, p. 673. [4] V. Rusitzky, “Multiples Myelom,” Dtsch Z Chir, Vol. 3, 1873, pp. 162-172. [5] R. Soutar, H. Lucraft, G. Jackson, et al., “Guidelines on the Diagnosis and Management of Solitary Plasmacytoma of Bone and Solitary Extramedullary Plasmacytoma,” British Journal of Haematology, Vol. 124, No. 6, 2004, pp. 717-726. [6] R. Tapia-Conyer, P. Kuri-Morales, C. Macías Martínez, et al., “Registro Histopatológico de Neoplasias en Mé- xico,” 2012. http//www.dgepi.salud.go.mx/infoepi/Manual/Man18-Ne oplasia/Man18.htm [7] R. P. Rizo, M. I. Sierra, P. G. Vázquez, et al., “Registro Hospitalario de Cáncer: Compendio de Cáncer 2000- 2004,” Cancerología, Vol. 2, No. 3, 2007, pp. 203-287. [8] A. Jemal, R. Siege l, E. Ward, T. Murray, J. Xu and M. J. Thum, “Cancer Statistics,” Cancer Journal for Clinicians, Vol. 57, No. 1, 2007, pp. 43-66. [9] L. A. Ries, et al, Eds., “SEER Cancer Statistics Review 1975-2001,” National Cancer Institute, Bethesda, 2004. [10] Annon, “Criteria for the Classification of Monoclonal Gammopathies, Multiple Myeloma and Related Disorders: A Report of the International Myeloma Working Group,” British Journal of Haematology, Vol. 121, No. 5, 2003, pp. 749-757. [11] M. S. Linet, S. D. Harlow and J. K. McLaughlin, “A Case-Control Study of Multiple Myeloma in Whites; Chronic Antigenic Stimulation, Occupation and Drug Use,” Cancer Research, Vol. 47, No. 11, 1987, pp. 2978- 2981. [12] J. Malpas, D. Bergsagel, R. Kyle and K. Anderson, Eds., “Myeloma, Biology and Management Saunders: Philadel- phia,” 3rd Edition, 2004, pp. 121-143. [13] R. A. Kyle, M. A. Gertz, T. E. Witzig, J. A. Lust, M. Q. Lacy, A. Dispenzieri, R. Fonseca, et al., “Review of 1027 Patients with Newly Diagnosed Multiple Myeloma,” Mayo Clinic Proceedings, Vol. 78, No. 1, 2003, pp. 21-33. [14] E. E. C. Ceballos, J. E. C. Franco, J. R. E. Zamora and J. R. L. Méndez, “Oncologists Clinical of Iberoamerica. Hemato-Oncology. Part I,” Planeation and Development Editorial, S.A. of C.V., 2009, pp. 82-84. [15] V. H. J. Zepeda, V. J. Domínguez, “Plasma Cell Leu- kemia: A Rare Condition,” Annals of Hematology, Vol. 85, No. 4, 2006, pp. 263-267. doi:10.1007/s00277-005-0054-4 [16] R. D. Brunning and R. W. McKenna, “Plasma Cell Dys- crasias and Related Disorders in: Atlas or Tumor Pathol- ogy,” Armed Forces Institute of Pathology, Washington, 1994 [17] P. Lin, R. Owens, G. Tricot and C. S. Wilson, “Flow Cytometric Immunophenotypic Analysis of 306 Cases of Multiple Myeloma,” American Journal of Clinical Pa- thology, Vol. 121, No. 4, 2004, pp. 482-488. [18] N. Sahara, A. Takeshita, K. Shigeno, S. Fujisawa, K. Takeshita, et al., “Clinicopathological and Prognostic Characteristics of CD56 Negative Multiple Myeloma,” British Journal of Hematology, Vol. 117, No. 4, 2002, pp. 882-885. [19] R. Königsberg, N. Zojer, J. Ackerman, E. Krömer, H. Kittler, E. Fritz, et al., “Predictive Role of Interphase Cy- togenetics for Survival of Patients with Multiple My- eloma,” Journal of Clinical Oncology, Vol. 18, No. 4, 2000, pp. 804-812. [20] R. Fonseca, E. A. Blood, M. M. Oken, R. A. Kyle, G. W. Dewald, R. J. Bailey et al., “Myeloma and the t(11; 14) (q13; q32); Evidence for a Biologically Defined Unique Subset of Patients,” Blood, Vol. 99, No. 10, 2002, pp. 3735-3741. [21] J. D. Hoyer, C. A. Hanson, R. Fonseca, P. R. Greipp, G. Dewald and P. J. Kurtin, “The (11; 14) (q13; q32) Trans- location in Multiple Myeloma,” American Journal of Clinical Pathology, Vol. 113, No. 6, 2000, pp. 831-837. [22] H. Chang, S. Samiee and Q. L. Yi, “Prognostic Relevance of CD56 Expression in Multiple Myeloma: A Study In- cluding 107 Cases Treated with High-Dose Melphalan- Based Chemotherapy and Autologous Stem Cell Trans- plant,” Leukemia & Lymphoma, Vol. 47, No. 1, 2006, pp. 43-47. doi:10.1080/10428190500272549. Abbreviations WHO = World Health Organization. MRI = Magnetic Resonance Imagen B-2m = Beta-2 microglobulin LDH = Lactic Dehydrogenase ARF = Altered Renal Function PBJ = Bence-Jones Protein P reg = Pregnancy WG = Weeks of Gestation MPC = Mature Plasma Cells IPC = Immature Plasma Cells PF = Pathological Fracture VC = Vertebral Column BM = Bone Marrow LN = Lymph Node Copyright © 2012 SciRes. OJPathology
|