Paper Menu >>
Journal Menu >>
 Pharmacology & Pharmacy, 2012, 3, 439-446 http://dx.doi.org/10.4236/pp.2012.34059 Published Online October 2012 (http://www.SciRP.org/journal/pp) 1 Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy Juan Francisco Rodríguez-Landa1,2*, Fabiola Hernández-López1,2, Margarita Saavedra1,2 1Institute of Neuroethology, Universidad Veracruzana, Av. Dr. Luis Castelazo s/n, Col. Industrial Las Ánimas, Veracruz, Mexico; 2Fac- ulty of Biological Pharmaceutical Chemistry, Universidad Veracruzana, Veracruz, Mexico. Email: *juarodriguez@uv.mx Received July 30th, 2012; revised August 28th, 2012; accepted September 10th, 2012 ABSTRACT Phytoestrogens are natural compounds found in some vegetables, and they replicate many of the physiochemical and physiological properties of estrogens, including the regulation of mood. The phytoestrogen genistein exerts anxiolytic- like effects in rats with a chronic absence of ovarian hormones, but the mechanism involved in this effect remains to be explored. The present study explored the participation of estrogen receptor-β in the anxiolytic-like effect of genistein (1.0 mg/kg, i.p., for 4 days) in Wistar rats with 12-week postovariectomy, considered as experimental model of postsur- gical menopause. In the light/dark test, a useful tool for anxiety study and for the screening of anxiolytic drugs, gen- istein reduced the latency to enter and increased the time spent in the light compartment and significantly increased the frequency and time spent exploring the light compartment compared with the control group, which is considered as an anxiolytic-like effect at experimental level. All behavioral effects produced by genistein in the light/dark test were blocked by previous tamoxifen administration (5.0 mg/kg, s.c., for 6 days), a non selective antagonist for estrogen re- ceptor-β. The effects produced by genistein or tamoxifen in this test were not related to significant changes in general motor activity evaluated in the open field test. In conclusion, the specific contribution of present investigation was iden- tify that estrogen receptor-β is involved in the anxiolytic-like effect produced by phytoestrogen genistein in rats with a long-term absence of ovarian hormones; supporting the hypothesis that estrogen receptor-β participates in the regulation of anxiety associated with low concentration of ovarian hormones and in the anxiolytic-like effects produced by natural estrogenic compounds such as phytoestrogens. Keywords: Anxiety; Genistein; Phytoestrogen; Estrogen Receptor- ; Light/Dark Test; Tamoxifen 1. Introduction Phytoestrogens are natural chemical compounds abun- dantly found in fruits, vegetables, legumes, whole grains, and especially flaxseed, clover, and soy products, and they replicate some of the physiochemical and physio- logical properties of estrogens [1-3]. The phytoestrogens genistein and daidzein, among others, are thought to ex- ert the most potent estrogenic activity at the preclinical and clinical levels [1,4]. These previous studies suggest that phytoestrogens may be utilized in the treatment of physiological and emotional alterations related to low concentrations of ovarian hormones (i.e. 17β-estradiol and progesterone), which occur in natural or surgical menopause [5,6]. Some clinical trials suggested that a phytoestrogen-rich diet protects women against age-re- lated diseases, certain types of cancers, and postmeno- pausal symptoms, such as osteoporosis, hot flashes, and mood swings [7-10], demonstrating the potential thera- peutic effect of phytoestrogen in the management of physical and emotional alterations related to dysregula- tion of ovarian hormone function. In preclinical trials, controversy exists with regard to the effects of phytoestrogens on mood. Some reports showed a significant reduction in anxiety-like behavior in rats fed a phytoestrogen-rich diet [11,12], whereas rats fed a low-phytoestrogen diet exhibited increased anxiety- like behavior [13]. Apparently, low phytoestrogen con- centrations inhibit aromatase, which blocks the conver- sion of testosterone to 17β-estradiol, whereas high phy- toestrogen concentrations produced estrogen-like actions [14], which would explain the anxiolytic-like effect of phytoestrogen at higher doses. *Corresponding author. Copyright © 2012 SciRes. PP  Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy 440 Administration of genistein (10 mg/kg/14days) in ova- riectomized rats exerted antidepressant-like effects in the forced swim test [15,16]. Additionally, administration of genistein (0.5 and 1.0 mg/kg/4days) in rats with a chronic absence of ovarian hormones exerted anxiolytic-like ef- fects in the light/dark test [17], a common behavioral test used for assessing anxiolytic compounds at the experi- mental level [18]. Considering evidence that phytoestro- gens [19] and particularly genistein [20-22] have a par- ticularly high affinity for estrogen receptor-β, is sug- gested that this substrate-receptor interaction may have a causal role in the anxiolytic-like effect seen. Selective modulators of this receptor (e.g., 17β-estradiol, diaryl- propionitrile, and dihydrocoumes, among others) exert anxiolytic-like effects at the preclinical level [23,24]. Nonetheless, the participation of estrogen receptor-β in the anxiolytic-like effect of genistein in rats with a chronic absence of ovarian hormones produced after 12-week postovariectomy remains to be explored. In the present study, we hypothesized that the anxio- lytic-like effect of genistein in ovariectomized rats is established by an interaction with estrogen receptor-β. Therefore, we assessed the effect of pretreatment with tamoxifen, a non selective estrogen receptor-β antagonist that readily crosses the blood-brain barrier [25], on the anxiolytic-like effect of genistein in the light/dark test in rats with 12-week postovariectomy. 2. Materials and Methods 2.1. Animals Thirty-two ovariectomized adult female Wistar rats, weighing 200 - 250 g at the beginning of the experiments, were used. The rats were housed in Plexiglas cages (eight rats per cage) under a 12/12 h light/dark cycle (light on at 6:00 AM) at an average temperature of 25˚C (±1˚C) with ad libitum access to purified water and food (Teklad lab animal diets, Harlan Co.). All of the experimental pro- cedures were performed according to the Guide for the care and use of laboratory animals published by the Na- tional Institutes of Health [26] and the Norma Oficial Mexicana para el Cuidado y Uso de Animales de Labo- ratorio [27]. All efforts were made to reduce the number of animal and suffering during experiments. 2.2. Ovariectomy Ovariectomy was performed according to previous stud- ies [17,28]. After the surgery and to ensure the long-term absence of ovarian hormones and high levels of anxiety behavior, the rats were returned to the housing facilities for 12 weeks [29]. After this time period, the rats were randomly assigned to each of the experimental groups and subjected to the treatments and behavioral tests. 2.3. Experimental Groups and Treatments The rats at 12 weeks postovariectomy were assigned to four independent groups (eight rats per group). The treat- ment conditions included four combinations: vehicle-ve- hicle, tamoxifen-vehicle, vehicle-genistein, and tamoxifen- genistein. The first treatment, tamoxifen (5.0 mg/kg) or its vehicle (corn oil), was administered subcutaneously for 6 consecutive days before the behavioral tests that began 11 weeks postovariectomy. The second treatment, genistein (1.0 mg/kg) or its vehicle (35% 2-hidroxy- propyl- -cyclodextrin solution), was administered intrap- eritoneally for 4 consecutive days before the behavioral tests, which began simultaneously on day 3 of tamoxifen or vehicle treatment. All treatments were administered every 24 h (volume injected: 1.0 mL/kg). Thirty minutes after the last administration of genistein or its vehicle, the rats were evaluated in the light/dark test and subse- quently in the open field test. Genistein (minimum 98% HPLC), tamoxifen (minimum 99%), and 2-hidroxy-pro- pyl- -cyclodextrin were acquired from Sigma-Aldrich Co. (St. Louis, Missouri, USA). Corn oil was acquired from Fábrica de aceites La central S.A de C.V. (Guadalajara, Jalisco, México). The treatment schedule, route of administration, and dose of genistein utilized in this study were based on the findings of Rodríguez-Landa et al. 2009 [17] in which anxiolytic-like effects were observed in rats 12 weeks postovariectomy in the light/dark test. The treatment schedule, route of administration, and dose of tamoxifen utilized in this study were based on the findings of Walf and Frye, 2005 [24], and Gutiérrez-García et al. 2009 [30]. These authors found that a single injection of ta- moxifen (10.0 mg/kg, s.c.) blocked the anxiolytic-like effects produced by a single injection of estrogen recep- tor-β selective modulators, and treatment with tamoxi- fen (1.0 mg/kg, s.c.) for 6 consecutive days reduced the anxiolytic-like effects produced by estrogen receptor-β stimulation after a single injection of testosterone. There- fore, we adjusted the schedule and doses of tamoxifen to use intermediate doses compared with the aforemen- tioned studies. 2.4. Behavioral Tests 2.4.1. Light/Dark Test In the present study, the light/dark test described by Zu- luaga et al. 2005 [31] was used. The dimensions of the apparatus were 80 40 40 cm. The box was further divided into two equal chambers (40 40 40 cm) by a barrier that had a doorway (10 10 cm) that allowed the rats to cross freely from one chamber to the other. The dark compartment was not illuminated, whereas the light compartment was illuminated by a 40 W white light. A Copyright © 2012 SciRes. PP  Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy 441 video camera (Sony, DCR-SR42, 40 optical zoom, Carl Zeiss lens) was installed above the illuminated compart- ment to record the rat activity in the box. Later, two in- dependent observers measured the behavioral variables until reaching a coincidence higher than 95% in meas- urement. On the test day, the rats were brought to the experi- mental room at 6:00 PM (which was the beginning of the dark phase) and left for 1 h to acclimatize them to the novel surroundings. The light/dark test was initiated at 7:00 PM. After this time period, the rats were individu- ally placed in the middle of the dark compartment facing the doorway, and behavioral activity was measured for 5 min. The evaluated variables were 1) frequency of en- tries into the light compartment (i.e., total number of entries into the light compartment); 2) latency to the first entry into the light compartment (i.e., the time taken after initial placement of the rat into the dark compartment until it crossed completely into the light compartment); 3) time spent in the light compartment (i.e., the total time spent in the light compartment); and 4) the frequency, latency, and time spent exploring the light compartment (i.e., exploration was assumed when the rat was leaning into the light compartment until its head and half of its body were in the light compartment without completely crossing into the light compartment). These variables were selected because they had been previously shown to provide a reliable measure of experimental anxiety [17, 31,32]. After the light/dark test, the rat was immediately subjected to the open field test. 2.4.2. Open Field Test In this study, general motor activity was evaluated to discard the possibility of hypoactivity, hyperactivity, or no changes in activity associated with the treatments, which could otherwise interfere with the interpretation of behavioral activity in the light/dark test [17,31]. No other measures (e.g., total number of central entries in the open field test, grooming, or rearing) were evaluated. To evalu- ate the effect of the treatments on spontaneous motor activity, the rats were individually subjected to a 5 mins period in the open field test. An opaque Plexiglas cage (44 33 cm) with walls 20 cm high was used. The floor was divided into 12 squares (11 11 cm). A video cam- era (Sony, DCR-SR42, 40 optical zoom, Carl Zeiss lens) was installed above the cage to record the activity of the rat. Later, two independent observers measured the be- havioral variables until reaching a coincidence higher than 95% in measurement. At the beginning of the test, the rat was gently placed in one of the corners of the cage. The dependent variable was the number of squares crossed by the rat (i.e., cross- ings). A crossing was assuming when the animal passed from one square to another with its rear legs. After each test session, the light/dark test and open field test cage were carefully cleaned with a cleaning solution (30% ethanol) to remove the scent of the previ- ously evaluated rat, which could otherwise modify the spontaneous behavior of the subsequent rat [33]. 2.5. Statistical Analysis The data from the light/dark test and open field test were analyzed using one-way analysis of variance (ANOVA) with independent groups and the Student-Newman-Keuls post hoc test when the p values reached ≤0.05. Results are expressed as mean ± standard error. 3. Results 3.1. Light/Dark Test The template is Frequency, latency, and time spent in the light compartment. The one-way ANOVA failed to show significant differences (F3,28 = 0.675, p = 0.57) in the frequency of entries into the light compartment among the different treatments (vehicle, 3.00 0.46; tamoxifen, 2.75 0.59; genistein, 3.62 0.75; tamoxifen + genistein, 2.50 0.51). The one-way ANOVA showed significant differences (F3,28 = 4.260, p < 0.013) in the latency to the first entry into the light compartment. The post hoc test revealed that genistein treatment significantly (p < 0.05) reduced the latency to the first entry into the light compartment compared with the control group, an effect blocked by tamoxifen pretreatment (Figure 1(a)). A tendency to- ward a reduction in the latency to the first entry into the light compartment was detected in the tamoxifen group, but no significant differences were found compared with the control group, and the differences were only attained compared with the genistein-treated group. The analysis of the time spent in the light compartment revealed significant differences (F3,28 = 33.633, p < 0.001) among treatments. The post hoc test showed that rats treated with genistein spent significantly (p < 0.05) more time in the light compartment compared with the control group, an effect blocked by tamoxifen pretreatment (Fig- ure 1(b)). Frequency, latency, and time spent exploring the light compartment. The one-way ANOVA showed significant differences (F3,28 = 13.250, p < 0.001) in the frequency exploring the light compartment among the different treatments. The post hoc test revealed that genistein sig- nificantly (p < 0.05) increased the frequency exploring the light compartment compared with the control group, an effect blocked by tamoxifen pretreatment (Figure 2(a)). Copyright © 2012 SciRes. PP 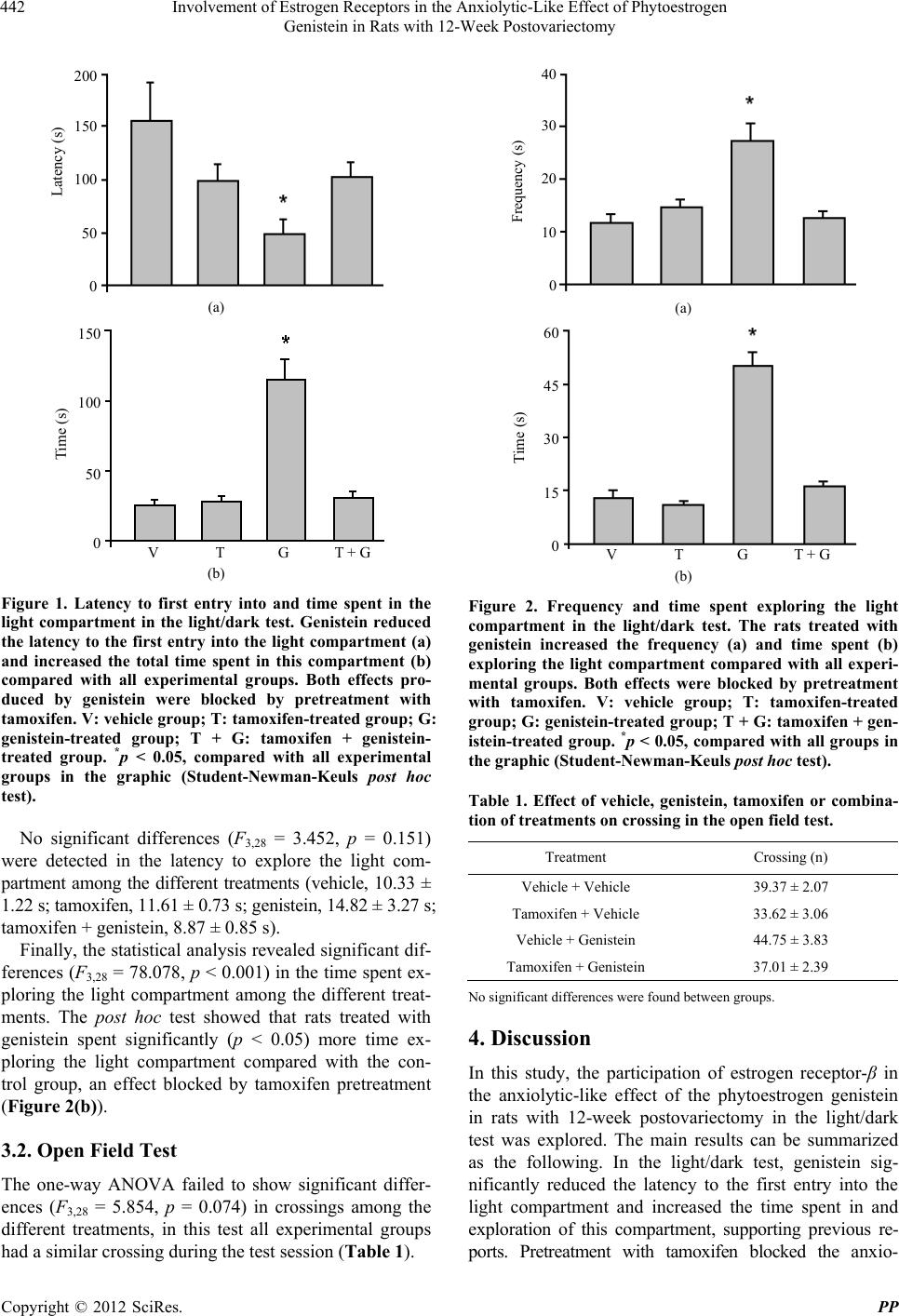 Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy 442 200 150 100 50 0 Latency (s) (a) 150 100 50 0 Time (s) V T G T + G (b) Figure 1. Latency to first entry into and time spent in the light compartment in the light/dark test. Genistein reduced the latency to the first entry into the light compartment (a) and increased the total time spent in this compartment (b) compared with all experimental groups. Both effects pro- duced by genistein were blocked by pretreatment with tamoxifen. V: vehicle group; T: tamoxifen-treated group; G: genistein-treated group; T + G: tamoxifen + genistein- treated group. *p < 0.05, compared with all experimental groups in the graphic (Student-Newman-Keuls post hoc test). No significant differences (F3,28 = 3.452, p = 0.151) were detected in the latency to explore the light com- partment among the different treatments (vehicle, 10.33 ± 1.22 s; tamoxifen, 11.61 ± 0.73 s; genistein, 14.82 ± 3.27 s; tamoxifen + genistein, 8.87 ± 0.85 s). Finally, the statistical analysis revealed significant dif- ferences (F3,28 = 78.078, p < 0.001) in the time spent ex- ploring the light compartment among the different treat- ments. The post hoc test showed that rats treated with genistein spent significantly (p < 0.05) more time ex- ploring the light compartment compared with the con- trol group, an effect blocked by tamoxifen pretreatment (Figure 2(b)). 3.2. Open Field Test The one-way ANOVA failed to show significant differ- ences (F3,28 = 5.854, p = 0.074) in crossings among the different treatments, in this test all experimental groups had a similar crossing during the test session (Table 1). 40 30 20 10 0 Frequency (s) (a) 60 45 30 15 0 Time (s) V T G T + G (b) Figure 2. Frequency and time spent exploring the light compartment in the light/dark test. The rats treated with genistein increased the frequency (a) and time spent (b) exploring the light compartment compared with all experi- mental groups. Both effects were blocked by pretreatment with tamoxifen. V: vehicle group; T: tamoxifen-treated group; G: genistein-treated group; T + G: tamoxifen + gen- istein-treated group. *p < 0.05, compared with all groups in the graphic (Student-Newman-Keuls post hoc test). Table 1. Effect of vehicle, genistein, tamoxifen or combina- tion of treatments on crossing in the open field test. Treatment Crossing (n) Vehicle + Vehicle 39.37 ± 2.07 Tamoxifen + Vehicle 33.62 ± 3.06 Vehicle + Genistein 44.75 ± 3.83 Tamoxifen + Genistein 37.01 ± 2.39 No significant differences were found between groups. 4. Discussion In this study, the participation of estrogen receptor-β in the anxiolytic-like effect of the phytoestrogen genistein in rats with 12-week postovariectomy in the light/dark test was explored. The main results can be summarized as the following. In the light/dark test, genistein sig- nificantly reduced the latency to the first entry into the light compartment and increased the time spent in and exploration of this compartment, supporting previous re- ports. Pretreatment with tamoxifen blocked the anxio- Copyright © 2012 SciRes. PP  Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy 443 lytic-like effects of genistein in the light/dark test in rats subjected to an experimental model of surgical post- menopause. In the open field test, no significant changes in crossings associated with the treatments were found. Altogether, these findings indicate that estrogen receptor- β is involved in the anxiolytic-like effects produced by genistein in rats with 12-week postovariectomy in the light/dark test. The light/dark test has been useful for the screening of substances with anxiolytic or anxiogenic potential [18, 34,35], including ovarian hormones, such as estradiol and progesterone [36,37]. In this test, an “anxious” animal exhibits an increase in the latency to the first entry into the light compartment and reduces the time spent in this compartment [18,38,39]. In contrast, animals treated with anxiolytic drugs (e.g., diazepam, alprazolam, and buspirone) spend more time in the light compartment [35,38,40], indicating an anxiolytic-like effect at experimental level. In the present study, the rats treated with phytoestrogen genistein exhibited a short latency to the first entry into the light compartment and spent more time in it compared with the control group, indicating an anxiolytic-like effect. Furthermore, genistein administration increased the frequency and time spent in exploration toward the light compartment, which is considered an additional indicator of an anxiolytic-like effect in the light/dark test, considering that diazepam and other compounds with anxiolytic effects exert similar effects [17,41]. Interestingly, the anxiolytic-like effect of genistein was blocked by pretreatment with tamoxifen, a nonspecific estrogen re- ceptor-β antagonist [42,43]. These data support the hypo- thesis that genistein exerts agonistic effects at estrogen receptor-β [21,22,42] producing anxiolytic-like effects as occur with other positive modulators of estrogen re- ceptor-β at the experimental level [24]. Additionally, present results further indicate that estrogen receptor-β would participates in the expression of anxiety-like behavior associated to low concentration of ovarian hormones pro- duced by ovariectomy. It is necessary point out that tamoxifen is not a pure estrogen receptor antagonist. At some dosages, tamoxifen may have agonist actions by interacting with estrogen receptor- , whereas the antagonistic properties may be attributable to actions at estrogen receptor-β [44], which occurs with others antagonists of this receptor, such as raloxifene [42,43]. In our study, no significant effects were found on the variables evaluated in the light/dark test in the tamoxifen-vehicle-treated group, discarding the possible nonspecific effects on estrogen receptor α or β produced by the tamoxifen dose used. Altogether, our data suggest that the tamoxifen dose used in the present study acted as an estrogen receptor-β antagonist as re- ported by other authors [24,30]. Finally, in the light/dark test, detecting false anxiolytic- like effects is possible when drugs increase general motor activity [18,38]. In this study, the rats treated with geni- stein, tamoxifen or combination of treatments did not exhibit an increase in general motor activity in the open field test, reflected by the number of crossings. Therefore, the effects produced by genistein in the light/dark test indicate an anxiolytic-like effect at the experimental level, as occur with clinically effective anxiolytic drugs, such as diazepam, which increase the time spent in the white compartment in the light/dark test, without producing sig- nificant changes in locomotion in the open field test [31, 45,46]. In conclusion, the results of this study provide a modest evidence that estrogen receptor-β participates in the anxiolytic-like effect of the phytoestrogen genistein in the light/dark test in rats with a long-term absence of ovarian hormones. These data support the hypothesis that phytoestrogens interact with estrogen receptor-β and pro- duce similar effects as estrogens on mood, which could be considered in future investigations that focus on find- ing new therapeutic alternatives to ameliorate anxiety or depression associated with low concentrations of ovarian hormones produced by surgical or natural menopause. Further studies are also necessary to explore the possible side effects associated with long-term administration of this phytoestrogen, which occurs with other estrogenic compounds [47-50]. 5. Acknowledgements The authors wish to thank Dr. Michael Arends for revis- ing and editing the English of this manuscript and QFB. Maximino García and QFB. Ivan Alarcón for technical assistance. This study was partially supported by a grant from PROMEP (103.5/05/1955, UVER-PTC-155), Sis- tema Nacional de Investigadores (SNI, Exp. 32753 and 19190), and Cuerpo Académico UVE-CA-202. The sec- ond author received a fellowship from CONACyT Reg. 223529. REFERENCES [1] E. D. Lephart, T. W. West, K. S. Weber, R. W. Rhees, K. D. R. Setchell, H. Adlercreutz and D. T. Lund, “Neuro- behavioral Effects of Dietary Soy Phytoestrogens,” Neu- rotoxicology Teratology, Vol. 24, No. 1, 2002, pp. 5-16. doi:10.1016/S0892-0362(01)00197-0 [2] T. Oseni, R. Patel, J. Pyle and V. C. Jordan, “Selective Estrogen Receptor Modulators and Phytoestrogens,” Planta Medica, Vol. 74, No. 13, 2008, pp. 1656-1665. doi:10.1055/s-0028-1088304 [3] L. Pilšáková, I. Riečanský and F. Jagla, “The Physiological Actions of Isoflavone Phytoestrogens,” Physiological Re- search, Vol. 59, No. 5, 2010, pp. 651-664. Copyright © 2012 SciRes. PP  Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy 444 [4] P. Moutsatsou, “The Spectrum of Phytoestrogens in Nature: Our Knowledge Is Expanding,” Hormones (Athens), Vol. 6, No. 3, 2007, pp. 173-193. [5] G. Pisani, L. Facioni, F. Fiorani and G. Pisan, “Psycho- sexual Problems in Menopause,” Minerva Ginecologica, Vol. 50, No. 3, 1998, pp. 77-81. [6] A. M. Paoletti, S. Floris, M. Mannias, M. Orru, D. Crippa, R. Orlandi, M. M. Del Zompo and G. B. Melis, “Evi- dence That Cyproterone Acetate Improves Psychological Symptoms and Enhances the Activity of the Dopaminergic System in Postmenopause,” Journal of Clinical Endocri- nology and Metabolism, Vol. 86, No. 2, 2001, pp. 608- 612. doi:10.1210/jc.86.2.608 [7] T. M. K. Schult, K. E. Ensrud, T. Blackwell, B. Ettinger, R. Wallace and J. A. Tice, “Effect of Isoflavones on Lipid and Bone Turnover Markers in Menopausal Women,” Maturitas, Vol. 48, No. 3, 2004, pp. 209-218. doi:10.1016/j.maturitas.2003.09.027 [8] R. D’Anna, M. L. Cannata, M. Atteritano, F. Cancellieri, F. Corrado, G. Baviera, O. Triolo, F. Antico, A. Gaudio, N. Frisina, A. Bitto, F. Polito, L. Minutoli, D. Altavilla, H. Marini and F. Squadrito, “Effects of the Phytoestrogen Genistein on Hot Flushes, Endometrium, and Vaginal Epithelium in Postmenopausal Women: A 1-Year Ran- domized, Double-Blind, Placebo-Controlled Study,” Meno- pause, Vol. 14, No. 4, 2007, pp. 648-655. doi:10.1097/01.gme.0000248708.60698.98 [9] M. Atteritano, F. Pernice, S. Mazzaferro, S. Mantuano, A. Frisina, R. D’Anna, M. L. Cannata, A. Bitto, F. Squadrito, N. Frisina and M. Buemi, “Effects of Phytoestrogen Gen- istein on Cytogenetic Biomarkers in Postmenopausal Women: 1 Year Randomized, Placebo-Controlled Study,”. European Journal of Pharmacology, Vol. 589, No. 1-3, 2008, pp. 22-26. doi:10.1016/j.ejphar.2008.04.049 [10] A. L. Murkies, C. Lombard, B. J. G. Strauss, G. Wilcox, H. G. Burger and M. S. Morton, “Dietary Flour Supple- mentation Decreases Post-Menopausal Hot Flushes: Ef- fect of Soy and Wheat,” Maturitas, Vol. 21, No. 3, 1995, pp.189-195. doi:10.1016/0378-5122(95)00899-V [11] T. D. Lund and E. D. Lephart, “Dietary Soy Phytoestro- gen Produce Anxiolytic Effects in the Elevated Plus- Maze,” Brain Research, Vol. 913, No. 2, 2001, pp. 180- 184. doi:10.1016/S0006-8993(01)02793-7 [12] E. D. Lephart, K. D. R. Setchell, R. J. Handa and T. D. Lund, “Behavioral Effects of Endocrine-Disrupting Sub- stances: Phytoestrogens,” Institute of Laboratory Animals Resources Journal, Vol. 45, No. 4, 2004, pp. 443-454. [13] D. E. Hartley, J. E. Edwards, C. E. Spiller, N. Alom, S. Tucci, P. Seth, M. L. Forsling and S. E. File, “The Soya Isoflavone Content of Rat Diet Can Increase Anxiety and Stress Hormone Release in the Male Rat,” Psychophar- macology, Vol. 167, No. 1, 2003, pp. 46-53. doi: 10.1007/s00213-002-1369-7 [14] K. Almstrup, M. F. Fernández, J. H. Petersen, N. Oleam, N. E. Skakkebaek and H. Leffers, “Dual Effects of Phy- toestrogens Result in U-Shaped Dose-Response Curves,” Environmental Health Perspectives, Vol. 110, No. 8, 2002, pp. 743-748. doi:10.1289/ehp.02110743 [15] N. S. Sapronov and S. B. Kasakova, “Effects of Synthetic and Plant-Derived Selective Modulators of Estrogen Re- ceptors on Depression-Like Behavior of Female Rats,” Bulletin of Experimental Biology and Medicine, Vol. 146, No. 1, 2008, pp. 73-76. doi:10.1007/s10517-008-0210-7 [16] A. Kageyama, H. Sakakibara, W. Zhou, M. Yoshioka, M. Ohsumi, K. Shimoi and H. Yokogoshi, “Genistein Regu- lated Serotonergic Activity in the Hippocampus of Ova- riectomized Rats under Forced Swim Stress,” Bioscience Biotechnology and Biochemistry, Vol. 74, No. 10, 2010, pp. 2005-2010. [17] J. F. Rodríguez-Landa, J. D. Hernández-Figueroa, B. C. Hernández-Calderón and M. Saavedra, “Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with Long- Term Absence of Ovarian Hormones in the Black and White Model,” Progress in Neuro-Psychopharmacology and Biological Psychiatry, Vol. 33, No. 2, 2009, pp. 367- 372. doi:10.1016/j.pnpbp.2008.12.024 [18] M. Bourin and M. Hascoët, “The Mouse Light/Dark Box Test,” European Journal of Pharmacology, Vol. 463, No. 1-3, 2003, pp. 55-65. doi:10.1016/S0014-2999(03)01274-3, [19] G. G. J. M. Kuiper, J. G. Lemmen, B. Carlsson, J. C. Cor- ton, S. H. Safe, P. T. van der Saag, B. van der Burg and J. A. Gustafsson, “Interaction of Estrogenic Chemical and Phytoestrogens with Estrogen Receptor ,” Endocrinology, Vol. 139, No. 10, 1998, pp. 4252-4263. doi:10.1210/en.139.10.4252 [20] G. G. J. M. Kuiper, B. Carlsson, K. Grandien, E. Enmark, J. Häggblad, S. Nilsson and J. A. Gustafsson, “Compari- son of the Ligand Binding Specificity and Transcript Tissue Distribution of the Estrogen Receptors α and β”. Endocrinology, Vol. 138, No. 3, 1997, pp. 863-870. doi:10.1210/en.138.3.863 [21] A. C. W. Pike, A. M. Brzozowski, R. E. Hubbard, T. Bonn, A. G. Thorsell, O. Engström, J. Ljunggren, J. A. Gustafsson and M. Carlquist, “Structure of the Ligand-Binding Do- main of Oestrogen Receptor Beta in the Presence of A Partial Agonist and Full Antagonist,” The EMBO Journal, Vol. 18, No. 17, 1999, pp. 4608-4618. doi:10.1093/emboj/18.17.4608 [22] A. C. W. Pike, A. M. Brzozowski, J. Walton, R. E. Hub- bard, T. Bonn, J. A. Gustafsson and M. Carlquist, “Struc- tural Aspects of Agonism and Antagonism in the Oestro- gen Receptor,” Biochemical Society Transactions, Vol. 28, No. 4, 2000, pp. 396-400. [23] T. D. Lund, T. Rovis, W. C. J. Chung and R. J. Handa, “Novel Actions of Estrogen Receptor- on Anxiety-Re- lated Behaviors,” Endocrinology, Vol. 146, No. 2, 2005, pp. 797-807. doi:10.1210/en.2004-1158 [24] A. A. Walf and C. A. Frye, “ERβ-Selective Estrogen Re- ceptor Modulators Produce Antianxiety Behavior When Administered Systemically to Ovariectomized Rats,” Neu- ropsychopharmacology, Vol. 30, No. 9, 2005, pp. 1598- 1609. doi:10.1038/sj.npp.1300713 [25] A. A. Walf and C. A. Frye, “Estradiol Reduces Anxiety- and Depression-Like Behavior of Aged Female Mice,” Physiology and Behavior, Vol. 99, No. 2, 2010, 169-174. Copyright © 2012 SciRes. PP  Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy 445 doi:10.1016/j.physbeh.2009.09.017 [26] National Research Council, “Guide for the Care and Use of Laboratory Animals: A Report of the Institute of Laboratory Animal Resource Committee on the Care and Use of Laboratory Animals,” NIH Publication No. 85-23, revised, Department of Health and Human Services, Washington DC, 1996. [27] Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, “Norma Official Mexicana Nom- 062-Zoo-1999, Especificaciones Técnicas Para La Pro- ducción, Cuidado Y Uso De Los Animales De Labo- ratorio,” México, DF, 1999. [28] L. Martínez-Mota, C. M. Contreras and M. Saavedra, “Progesterone Reduces Immobility in Rats Forced to Swim,” Archives of Medical Research, Vol. 30, No. 4, 1999, pp. 286-289. doi:10.1016/S0188-0128(99)00024-X, [29] O. Picazo, E. Estrada-Camarena and A. Hernández-Aragón, “Influence of the Post-Ovariectomy Time Frame on the Experimental Anxiety and the Behavioural Actions of Some Anxiolytic Agents,” European Journal of Pharma- cology, Vol. 530, No. 1-2, 2006, pp. 88-94. doi:10.1016/j.ejphar.2005.11.024 [30] A. G. Gutiérrez-García, C. M. Contreras. D. I. Vásquez- Hernández, T. Molina-Jiménez and E. Jácome-Jácome, “Testosterone Reduces Cumulative Burying in Female Wistar Rats with Minimal Participation of Estradiol,” Phar- macology, Biochemistry, and Behavior, Vol. 93, No. 4, 2009, pp. 406-412. doi:10.1016/j.pbb.2009.06.002 [31] M. J. Zuluaga, D. Agrati, M. Pereira, N. Uriarte, A. Fernández-Guasti and A. Ferreira, “Experimental Anxiety in the Black and White Model in Cycling, Pregnant and Lactating Rats,” Physiology and Behavior, Vol. 84, No. 2, 2005, pp. 279-286. doi:10.1016/j.physbeh.2004.12.004 [32] B. Costall, B. J. Jones, M. E. Kelly, R. J. Naylor and D. M. Tomkins, “Exploration of Mice in A Black and White Test Box: Validation as A Model of Anxiety,” Pharma- cology, Biochemistry, and Behavior, Vol. 32, No. 3, 1989, pp. 777-785. doi:10.1016/0091-3057(89)90033-6, [33] A. G. Gutiérrez-García, C. M. Contreras, M. R. Mendoza- López, S. Cruz-Sánchez, O. García-Barradas, J. F. Ro- dríguez-Landa and B. Bernal-Morales, “A Single Session of Emotional Stress Produces Anxiety in Wistar Rats,” Behavioral Brain Research, Vol. 167, No. 1, 2006, pp. 30- 35. doi:10.1016/j.bbr.2005.08.011 [34] E. S. Onaivi and B. R. Martin, “Neuropharmacological and Physiological Validation of a Computer-Controlled Two-Compartment Black and White Box for the Assess- ment of Anxiety,” Progress in Neuro-Psychopharmacol- ogy and Biological Psychiatry, Vol. 13, No. 6, 1989, pp. 963-976. doi:10.1016/0278-5846(89)90047-X [35] M. Imaizumi, S. Miyazaki and K. Onodera, “Effects of Xanthine Derivatives in a Light/Dark Test in Mice and the Contribution of Adenosine Receptors,” Methods and Findings in Experimental and Clinical Pharmacology, Vol. 16, No. 9, 1994, pp. 639-644. [36] C. J. Auger and R. M. Forber-Lorman, “Progestin Re- ceptor-Mediated Reduction of Anxiety-Like Behavior in Male Rats,” PLoS One, Vol. 3, No. 11, 2008, p. e3606. doi:10.1371/journal.pone.0003606 [37] A. A. Walf and C. A. Frye, “Estradiol Reduces Anxiety- and Depression-Like Behavior of Aged Female Mice,” Physiology and Behavior, Vol. 99, No. 2, 2010, pp. 169- 174. doi:10.1016/j.physbeh.2009.09.017, [38] T. Shimada, K. Matsumoto, M. Osanai, H. Matsuda, K. Terasawa and H. Watanabe, “The Modified Light/Dark Transition Test in Mice: Evaluation of Classic and Puta- tive Anxiolytic and Anxiogenic Drugs,” General Phar- macology, Vol. 26, No. 1, 1995, pp. 205-210. doi:10.1016/0306-3623(94)00148-G, [39] M. A. Birkett, N. M. Shinday, E. J. Kessler, J. S. Meyer, S. Ritchie and J. K. Rowlett, “Acute Anxiogenic-Like Effects of Selective Serotonin Reuptake Inhibitors Are Attenuated by the Benzodiazepine Diazepam in Balb/C Mice,” Pharmacology, Biochemistry, and Behavior, Vol. 98, No. 4, 2011, pp. 544-551. doi:10.1016/j.pbb.2011.03.006, [40] E. T. Venâncio, N. F. Rocha, E. R. Rios, M. L. Feitosa, M. I. Linhares, F. H. Melo, M. S. Matias, F. M. Fonseca, F. C. Sousa, L. K. Leal and M. M. Fonteles, “Anxiolytic- Like Effect of Standardized Extract of Justicia Pectoralis (Sejp) in Mice: Involvement of Gaba/Benzodiazepine Re- ceptor,” Phytotherapy Research, Vol. 25, No. 3, 2001, pp. 444-450. doi:10.1002/ptr.3274 [41] B. F. Bradley, N. J. Starkey, S. L. Brown and R. W. Lea, “The Effects of Prolonged Rose Odor Inhalation in Two Animal Models of Anxiety,” Physiology and Behavior, Vol. 92, No. 5, 2007, pp. 931-938. doi:10.1016/j.physbeh.2007.06.023, [42] T. Barkhem, B. Carlsson, Y. Nilsson, E. Enmark, J. Gustafsson and S. Nilsson, “Differential Response of Es- trogen Receptor and Estrogen Receptor to Partial Es- trogen Agonist/Antagonists,” Molecular Pharmacology, Vol. 54, No. 1, 1998, pp. 105-112. [43] E. M. McInerney, K. E. Weis, J. Sun, S. Mosselman and B. S. Katzenellenbogen, “Transcription Activation By the Human Estrogen Receptor Subtype β (ERβ) Studied with ERβ and ER Receptor Chimeras,” Endocrinology, Vol. 139, No. 11, 1998,pp. 4513-4522. doi:10.1210/en.139.11.4513 [44] T. Watanabe, S. Inoue, S. Ogawa, Y. Ishii, H. Hiroi, K. Ikeda, A. Orimo and M. Muramatsu, “Agonistic Effect of Tamoxifen Is Dependent on Cell Type, Ere-Promoter Context, and Estrogen Receptor Subtype: Functional Dif- ference between Estrogen Receptors α and β,” Biochemi- cal and Biophysical Research Communication, Vol. 236, No. 1, 1997, pp. 140-145. doi:10.1006/bbrc.1997.6915 [45] F. Clénet, M. Hascoët, G. Fillion, H. Galons and M Bourin, “Anxiolytic Profile of Hg1, A 5-Ht-Moduline Antagonist, in Three Mouse Models of Anxiety,” Euro- pean Neuropsychopharmacology, Vol. 14, No. 6, 2004, pp. 449-456. doi:10.1016/j.euroneuro.2003.12.004 [46] W. H. Peng, C. R. Wu, C. S. Chen, C. F. Chen, Z. C. Leu and M. T. Hsieh, “Anxiolytic Effect of Berberine on Ex- ploratory Activity of the Mouse in Two Experimental Anxiety Models: Interaction with Drugs Acting At 5-Ht Receptors,” Life Sciences, Vol. 75, No. 20, 2004, pp. 2451- Copyright © 2012 SciRes. PP  Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Week Postovariectomy Copyright © 2012 SciRes. PP 446 2462. doi:10.1016/j.lfs.2004.04.032 [47] M. Canonico and P. Y. Scarabin, “Hormone Therapy and Risk of Venous Thromboembolism among Postmeno- pausal Women,” Climateric, Vol. 12, No. 1, 2009, pp. 76- 80. doi:10.1080/13697130903006837 [48] L. J. Martin, S. Minkin and N. F. Boyd, “Hormone Ther- apy, Mammographic Density, and Breast Cancer Risk,” Maturitas, Vol. 64, No. 1, 2009, pp. 20-26. doi:10.1016/j.maturitas.2009.07.009 [49] J. L. Shifren and I. Schiff, “Role of Hormone Therapy in the Management of Menopause,” Obstetrics Gynecology, Vol. 115, No. 4, 2010, pp. 839-855. doi:10.1097/AOG.0b013e3181d41191 [50] M. P. Warren, “Hormone Therapy for Menopausal Symp- toms: Putting Benefits and Risks into Perspective,” Jour- nal of Family Practice, Vol. 59, No. 12, 2010, pp. E1-E7. |

