 Pharmacology & Pharmacy, 2012, 3, 388-396 http://dx.doi.org/10.4236/pp.2012.34052 Published Online October 2012 (http://www.SciRP.org/journal/pp) 1 Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels Ighodaro Igbe1*, Eric K. I. Omogbai1, Adebayo O. Oyekan2 1Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria; 2Center for Cardio- vascular Diseases, College of Pharmacy and Health Sciences, Texas Southern University, Houston, USA. Email: *igbe.ighodaro@uniben.edu Received May 22nd, 2012; revised June 24th, 2012; accepted July 15th, 2012 ABSTRACT Many agents are known to cause qualitative and quantitative differences in intrarenal blood flow. This study tested the hypothesis that angiotensin II (AII) evokes a differential effect on cortical (CBF) and medullary blood flow (MBF) and that AT2 receptor mediates AII-induced increase in renal MBF by mechanisms related to nitric oxide (NO) and prostanoids. AII (100, 300 and 1000 µg/kg/min) increased mean arterial blood pressure (MABP) by 24% ± 7% (p < 0.05); decreased CBF by 30% ± 2% (p < 0.05); but increased MBF by 21% ± 8% (p < 0.05). Indomethacin (5 mg/kg), enhanced AII effects on MABP by 154% ± 26% (p < 0.05), MBF by 141% ± 46% but decreased CBF by 74% ± 54% (p < 0.05) indicating the involvement of dilator prostanoids in the systemic and medullary circulation but constrictor prostanoids in the cortex. NG nitro-L-arginine (L-NNA), an inhibitor of NO synthase (100 mg/L in drinking water) en- hanced AII effects on MABP (169 ± 75, p < 0.05) and decreased CBF (107% ± 50%, p < 0.05) but blunted the effects of AII on MBF (150% ± 21%, p < 0.05). 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ; 2 mg/kg), a guanylyl cyclase inhibitor, enhanced AII effects on MABP (118% ± 32% , p < 0.05) and decreased CBF(85% ± 47% , p < 0.05) but blunted the effects of AII on MBF (96% ± 15%, p < 0.05). However, glibenclamide (20 µg/kg), a KATP channel blocker, did not affect intra-renal hemodynamics elicited by AII. Blockade of AT2 receptors with PD123319 (50 µg/kg/min) did not change basal or AII-induced changes MABP or CBF but blunted AII-induced increase in MBF by 60% ± 11% (p < 0.05). CGP42112 (10 µg/kg/min), an AT2 receptor agonist, elicited a reduction in MABP and increases in CBF and MBF that were abolished or attenuated by PD123319. These findings demonstrate that AII elicited differen- tial changes in intrarenal blood flow; an AT1-mediated reduction in CBF but an AT2-mediated increase in MBF. The AT2 receptor-mediated increase in MBF involves guanylase cyclase, NO and dilator prostanoids but not KATP channels. Keywords: Angiotensin II; Hemodynamics; Medullary Blood Flow; AT2 Receptors; Prostanoids 1. Introduction The intrarenal vasculature can respond to neural and a variety of humoral stimuli with vasodilatation or vaso- constriction, resulting in increased or decreased perfusion of renal tissue, respectively [1]. Such responses may have more serious functional consequences within the medulla than in the cortex. This is of major physiological and pathophysiological importance as the medulla is widely viewed as having a crucial role in maintaining body fluid homeostasis and in the control of arterial pres- sure [2]. The renin-angiotensin system is a coordinated hormonal cascade important to the regulation of renal sodium ex- cretion and blood pressure. The major effector peptide, angiotensin II (AII), binds to two major receptors; AT1 and AT2. While the majority of AII actions are mediated via the AT1 receptor, evidence has accumulated that the AT2 receptor opposes the AT1 receptor, especially by inducing vasodilation instead of vasoconstriction and may be important in the regulation of blood pressure and renal function by counterbalancing the vasoconstrictor and antinatriuretic actions of AT1 receptors [3]. However, the roles of AT1 and AT2 receptors in regulating regional kidney perfusion remain unclear. In rats and rabbits, infusions of AII reduced total renal blood flow (RBF) and cortical blood flow but have a lesser effect on medullary blood flow [4,5]. AII can even increase MBF, especially when administered as a bolus [1,6]. Many studies have demonstrated that the medullary vasculature was poorly sensitive to the vasoconstrictor effects of AII *Corresponding author. Copyright © 2012 SciRes. PP  Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels 389 compared with the cortical circulation [4,7,8]. A study [9] has shown that AII induced a potent vasoconstriction of isolated medullary vasa recta in Sprague-Dawley rats, a response also observed in conscious rats [10]. Conversely, other studies have shown that the systemic infusion of AII increased papillary blood flow in young Sprague- Dawley and Wistar rats [11] by increasing local medullary synthesis of vasodilator agents such as prostaglandins, nitric oxide (NO), or kinins. Nitric oxide (NO) synthase and/or cyclooxygenase (COX) blockade can enhance AII-induced reductions in medullary blood flow (MBF) and abolish AII-induced increases in MBF, both of which are chiefly AT1 mediated [12-14]. However, the contributions of AT2 receptors to these effects have received little attention, even though they are expressed in vessels that might contribute to MBF control (e.g., afferent arterioles and vasa recta). In a routine experiment to address the effects of AII in the rat, we noticed a differential effect on CBF and MBF and this led us to characterize these effects. We hypothesized that AII evokes a differential effect on intrarenal hemo- dynamics by an AT1-mediated cortical vasoconstriction but AT2 receptor-mediated increase in renal medullary blood flow. To test this hypothesis, mean arterial blood pressure (MABP), MBF and CBF responses to graded doses of AII were determined in the presence of indo- methacin, Nω-nitro-L-arginine, ODQ (1H-[1,2,4] oxadia- zolo[4,3,-a]quinoxalin-1-one), or glibenclamide. In addi- tion, we characterized the increase in MBF using CGP42112, a highly selective AT2 agonist, and PD123319, an AT2 antagonist. 2. Materials and Methods 2.1. Drugs and Chemicals Nω-nitro-L-arginine (L-NNA;Sigma-Aldrich, St. Louis, MO) and indomethacin (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.1 M NaHCO3, and pH was adjusted to 7.0 - 7.2. Glibenclamide (Sigma-Aldrich, St. Louis, MO) and 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ; Sigma-Aldrich, St. Louis, MO) were prepared in dimethylsulfoxide (DMSO) as stock solutions of 0.1 M, from which aliquots were diluted in normal saline for intravenous administration. Angiotensin II (Sigma-Aldrich), CGP42112 (21st Century Biochemicals, USA) and PD123319 (a gift from Park Davis, USA) were dissolved in normal saline (0.9% NaCl). All agents were kept on ice during the experiments. Female Sprague-Dawley rats (230 - 290 g body wt; Harlan Sprague Dawley, Houston, TX) were maintained on standard rat food (Purina Chow; Purina, St Louis, MO) and allowed ad libitum access to water and food until the beginning of the experiments. The study protocol was approved by the Animal Care and Use Committee of Texas Southern University. 2.2. Surgical Preparation Animals were anesthetised with thiobutabarbital (Inactin), 100 mg/kg ip (Sigma-Aldrich) and placed on a heated surgical table to maintain body temperature at 37˚C. The tail vein was cannulated with a 25-gauge butterfly needle (Vacutainer, Becton and Dickson) for infusion or administration of drugs. The trachea was isolated and a polyethylene catheter (PE-250) was placed in the tra- chea for spontaneous ventilation. A polyethylene catheter (PE-50) was placed in the left carotid artery to monitor the blood pressure. Mean arterial blood pressure (MABP) was measured with a pressure transducer (model BLPR2, World Precision Instruments, Sarasota, FL) to a signal manifold (Transbridge, model TBM-4, World Precision Instrument, Sarasota, FL) and recorded on a data acqui- sition system (model DI720, DataQ Instruments, Akron, OH). The left kidney was exposed by an abdominal in- cision, intrarenal blood flow was measured simulta- neously by laser-Doppler (LD) flowmeter (system 5000, version 1.20, Periflux, Stockholm, Sweden) via a surface probe (model PF 407) to measure CBF or an optical fiber LD probe (model PF 402) fixed to a micromanipulator and placed in the medulla (5 mm below the kidney sur- face) to measure MBF. CBF and MBF were recorded as perfusion units (PU). 2.3. Experimental Protocol After surgery and placing of probes for recording regional blood flows, a 30- to 45-min equilibration period was allowed. AII was administered by an infusion pump (Model 100, SP 100i syringe pump, WPI, USA) at graded doses of 100, 300 and 1000 ng/kg/min. These graded doses were administered cumulatively. The effects on MABP, CBF and MBF were determined in the presence of indomethacin, a COX inhibitor (10 mg/kg iv; n = 6) [15], L-NNA, Nω-nitro-L-arginine, a NO synthase in- hibitor (100 mg/L in drinking water for 2 days; n = 6) [16], ODQ, 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1- one, a guanylase cyclase inhibitor (2 mg/kg iv; n = 5) [17], glibenclamide, a KATP channel blocker (20 µg/kg, iv) [18]; or their respective vehicles: 0.1 M NaHCO3 for L-NNA and indomethacin, 5% DMSO for glibenclamide and ODQ and normal saline for AII. Data obtained from rats treated with 5% DMSO and 0.1 M NaHCO3 were not different from those obtained from rats treated with normal saline; hence, data from both groups were pooled to represent control data for all the treatment groups. Another set of experiments to characterize the possible mechanisms involved in AII-induced increase in MBF Copyright © 2012 SciRes. PP 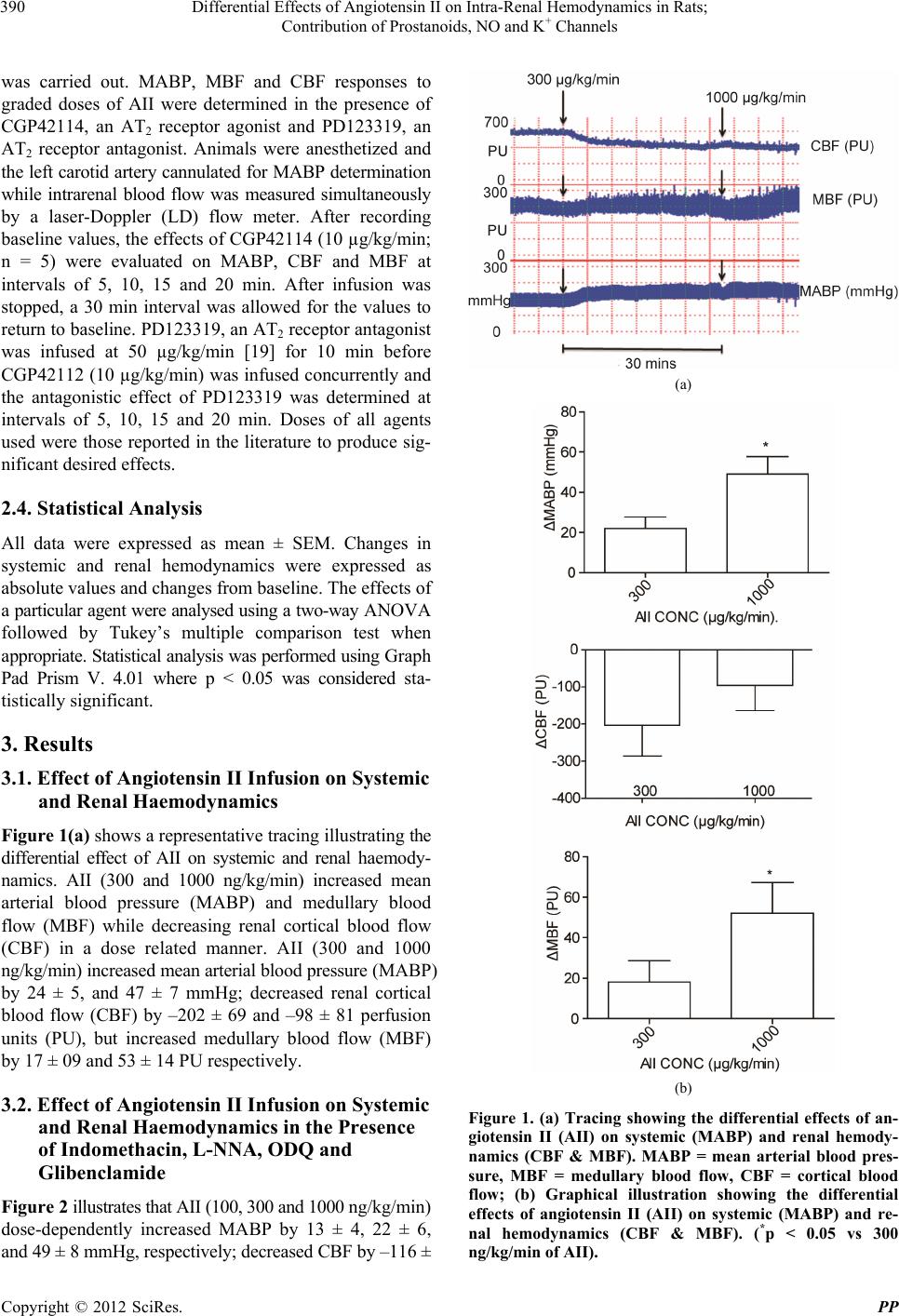 Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels 390 was carried out. MABP, MBF and CBF responses to graded doses of AII were determined in the presence of CGP42114, an AT2 receptor agonist and PD123319, an AT2 receptor antagonist. Animals were anesthetized and the left carotid artery cannulated for MABP determination while intrarenal blood flow was measured simultaneously by a laser-Doppler (LD) flow meter. After recording baseline values, the effects of CGP42114 (10 µg/kg/min; n = 5) were evaluated on MABP, CBF and MBF at intervals of 5, 10, 15 and 20 min. After infusion was stopped, a 30 min interval was allowed for the values to return to baseline. PD123319, an AT2 receptor antagonist was infused at 50 µg/kg/min [19] for 10 min before CGP42112 (10 µg/kg/min) was infused concurrently and the antagonistic effect of PD123319 was determined at intervals of 5, 10, 15 and 20 min. Doses of all agents used were those reported in the literature to produce sig- nificant desired effects. 2.4. Statistical Analysis All data were expressed as mean ± SEM. Changes in systemic and renal hemodynamics were expressed as absolute values and changes from baseline. The effects of a particular agent were analysed using a two-way ANOVA followed by Tukey’s multiple comparison test when appropriate. Statistical analysis was performed using Graph Pad Prism V. 4.01 where p < 0.05 was considered sta- tistically significant. 3. Results 3.1. Effect of Angiotensin II Infusion on Systemic and Renal Haemodynamics Figure 1(a) shows a representative tracing illustrating the differential effect of AII on systemic and renal haemody- namics. AII (300 and 1000 ng/kg/min) increased mean arterial blood pressure (MABP) and medullary blood flow (MBF) while decreasing renal cortical blood flow (CBF) in a dose related manner. AII (300 and 1000 ng/kg/min) increased mean arterial blood pressure (MABP) by 24 ± 5, and 47 ± 7 mmHg; decreased renal cortical blood flow (CBF) by –202 ± 69 and –98 ± 81 perfusion units (PU), but increased medullary blood flow (MBF) by 17 ± 09 and 53 ± 14 PU respectively. 3.2. Effect of Angiotensin II Infusion on Systemic and Renal Haemodynamics in the Presence of Indomethacin, L-NNA, ODQ and Glibenclamide Figure 2 illustrates that AII (100, 300 and 1000 ng/kg/min) dose-dependently increased MABP by 13 ± 4, 22 ± 6, and 49 ± 8 mmHg, respectively; decreased CBF by –116 ± (a) (b) Figure 1. (a) Tracing showing the differential effects of an- giotensin II (AII) on systemic (MABP) and renal hemody- namics (CBF & MBF). MABP = mean arterial blood pres- sure, MBF = medullary blood flow, CBF = cortical blood flow; (b) Graphical illustration showing the differential effects of angiotensin II (AII) on systemic (MABP) and re- nal hemodynamics (CBF & MBF). (*p < 0.05 vs 300 ng/kg/min of AII). Copyright © 2012 SciRes. PP 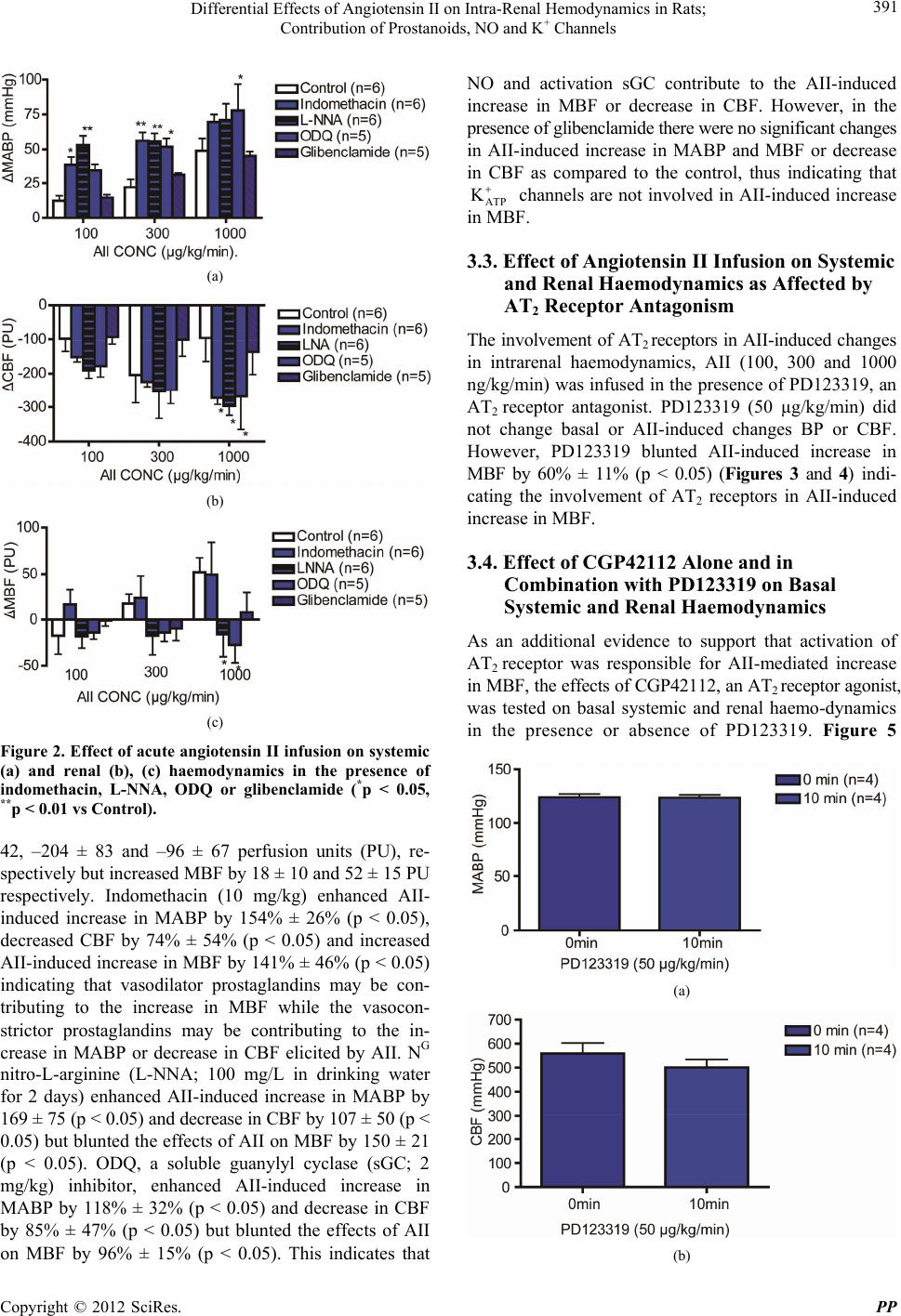 Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels 391 (a) (b) (c) Figure 2. Effect of acute angiotensin II infusion on systemic (a) and renal (b), (c) haemodynamics in the presence of indomethacin, L-NNA, ODQ or glibenclamide (*p < 0.05, **p < 0.01 vs Control). 42, –204 ± 83 and –96 ± 67 perfusion units (PU), re- spectively but increased MBF by 18 ± 10 and 52 ± 15 PU respectively. Indomethacin (10 mg/kg) enhanced AII- induced increase in MABP by 154% ± 26% (p < 0.05), decreased CBF by 74% ± 54% (p < 0.05) and increased AII-induced increase in MBF by 141% ± 46% (p < 0.05) indicating that vasodilator prostaglandins may be con- tributing to the increase in MBF while the vasocon- strictor prostaglandins may be contributing to the in- crease in MABP or decrease in CBF elicited by AII. NG nitro-L-arginine (L-NNA; 100 mg/L in drinking water for 2 days) enhanced AII-induced increase in MABP by 169 ± 75 (p < 0.05) and decrease in CBF by 107 ± 50 (p < 0.05) but blunted the effects of AII on MBF by 150 ± 21 (p < 0.05). ODQ, a soluble guanylyl cyclase (sGC; 2 mg/kg) inhibitor, enhanced AII-induced increase in MABP by 118% ± 32% (p < 0.05) and decrease in CBF by 85% ± 47% (p < 0.05) but blunted the effects of AII on MBF by 96% ± 15% (p < 0.05). This indicates that NO and activation sGC contribute to the AII-induced increase in MBF or decrease in CBF. However, in the presence of glibenclamide there were no significant changes in AII-induced increase in MABP and MBF or decrease in CBF as compared to the control, thus indicating that ATP channels are not involved in AII-induced increase in MBF. K 3.3. Effect of Angiotensin II Infusion on Systemic and Renal Haemodynamics as Affected by AT2 Receptor Antagonism The involvement of AT2 receptors in AII-induced changes in intrarenal haemodynamics, AII (100, 300 and 1000 ng/kg/min) was infused in the presence of PD123319, an AT2 receptor antagonist. PD123319 (50 µg/kg/min) did not change basal or AII-induced changes BP or CBF. However, PD123319 blunted AII-induced increase in MBF by 60% ± 11% (p < 0.05) (Figures 3 and 4) indi- cating the involvement of AT2 receptors in AII-induced increase in MBF. 3.4. Effect of CGP42112 Alone and in Combination with PD123319 on Basal Systemic and Renal Haemodynamics As an additional evidence to support that activation of AT2 receptor was responsible for AII-mediated increase in MBF, the effects of CGP42112, an AT2 receptor agonist, was tested on basal systemic and renal haemo-dynamics in the presence or absence of PD123319. Figure 5 (a) (b) Copyright © 2012 SciRes. PP 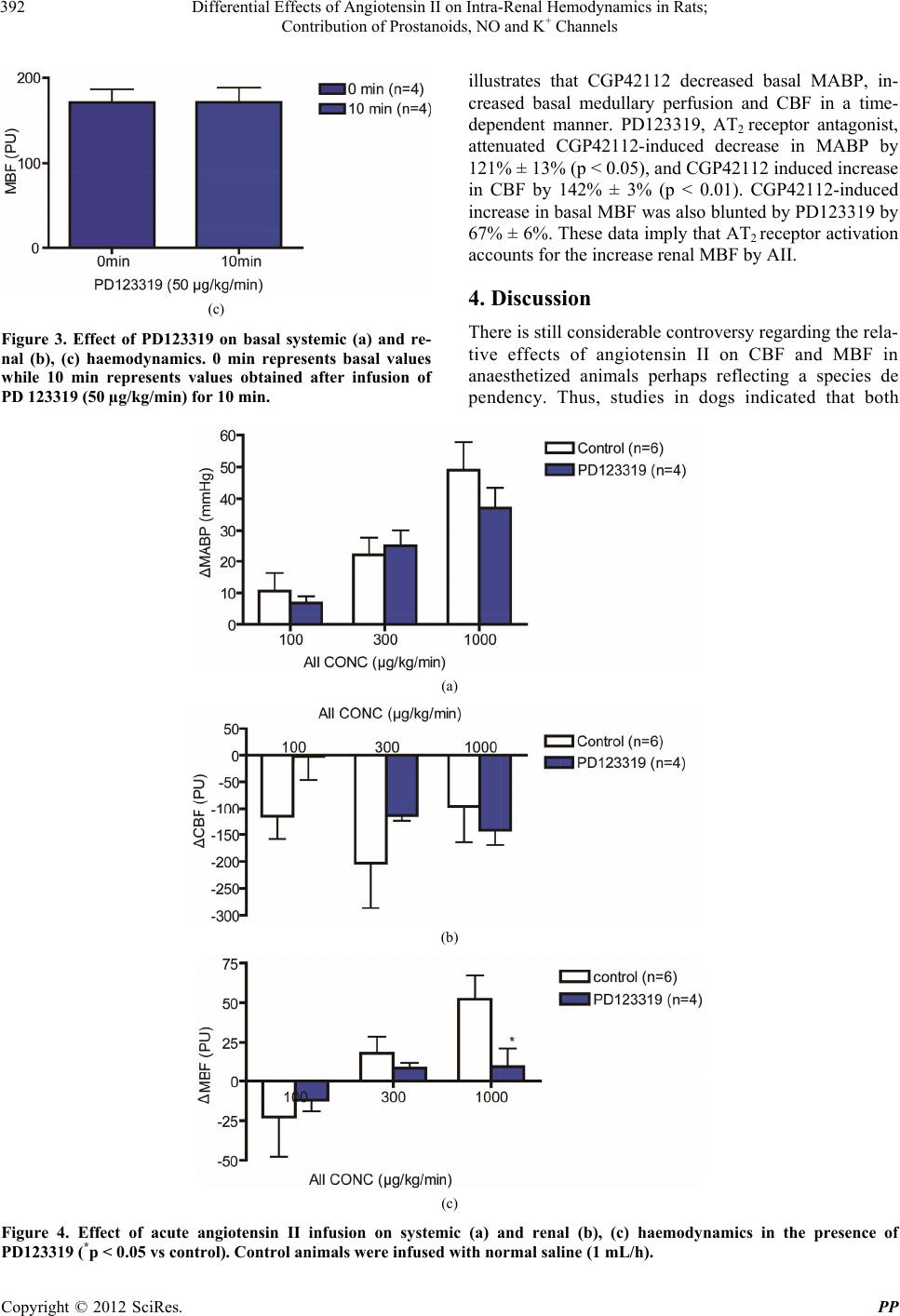 Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels Copyright © 2012 SciRes. PP 392 illustrates that CGP42112 decreased basal MABP, in- creased basal medullary perfusion and CBF in a time- dependent manner. PD123319, AT2 receptor antagonist, attenuated CGP42112-induced decrease in MABP by 121% ± 13% (p < 0.05), and CGP42112 induced increase in CBF by 142% ± 3% (p < 0.01). CGP42112-induced increase in basal MBF was also blunted by PD123319 by 67% ± 6%. These data imply that AT2 receptor activation accounts for the increase renal MBF by AII. 4. Discussion (c) There is still considerable controversy regarding the rela- tive effects of angiotensin II on CBF and MBF in anaesthetized animals perhaps reflecting a species de pendency. Thus, studies in dogs indicated that both Figure 3. Effect of PD123319 on basal systemic (a) and re- nal (b), (c) haemodynamics. 0 min represents basal values while 10 min represents values obtained after infusion of PD 123319 (50 µg/kg/min) for 10 min. (a) (b) (c) Figure 4. Effect of acute angiotensin II infusion on systemic (a) and renal (b), (c) haemodynamics in the presence of PD123319 (*p < 0.05 vs control). Control animals were infused with normal saline (1 mL/h). 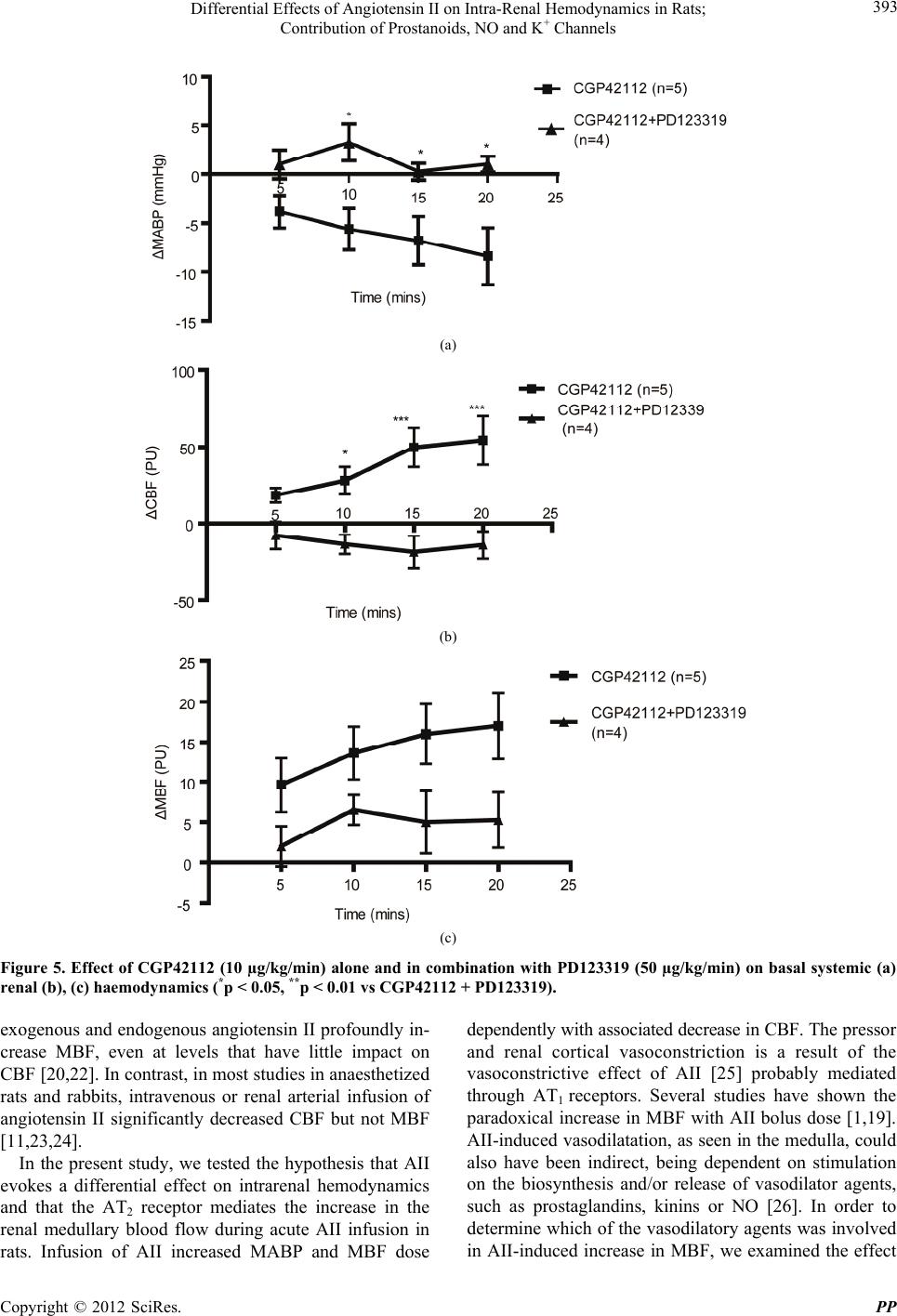 Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels 393 (a) (b) (c) Figure 5. Effect of CGP42112 (10 µg/kg/min) alone and in combination with PD123319 (50 µg/kg/min) on basal systemic (a) renal (b), (c) haemodynamics (*p < 0.05, **p < 0.01 vs CGP42112 + PD123319). exogenous and endogenous angiotensin II profoundly in- crease MBF, even at levels that have little impact on CBF [20,22]. In contrast, in most studies in anaesthetized rats and rabbits, intravenous or renal arterial infusion of angiotensin II significantly decreased CBF but not MBF [11,23,24]. In the present study, we tested the hypothesis that AII evokes a differential effect on intrarenal hemodynamics and that the AT2 receptor mediates the increase in the renal medullary blood flow during acute AII infusion in rats. Infusion of AII increased MABP and MBF dose dependently with associated decrease in CBF. The pressor and renal cortical vasoconstriction is a result of the vasoconstrictive effect of AII [25] probably mediated through AT1 receptors. Several studies have shown the paradoxical increase in MBF with AII bolus dose [1,19]. AII-induced vasodilatation, as seen in the medulla, could also have been indirect, being dependent on stimulation on the biosynthesis and/or release of vasodilator agents, such as prostaglandins, kinins or NO [26]. In order to determine which of the vasodilatory agents was involved in AII-induced increase in MBF, we examined the effect Copyright © 2012 SciRes. PP  Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels 394 of AII infusion in the presence of indomethacin, a COX inhibitor, L-NNA, a NO synthase inhibitor, ODQ, a sGC inhibitor or glibenclamide, a KATP channels blocker. LNNA or ODQ enhanced AII-induced increase in MABP and decrease in CBF but remarkably inhibited AII- induced increase in MBF. This is in agreement with the studies that showed that inhibition of NO synthesis prevented an increase in perfusion of the medulla after AII [14,27]. NO acts through the stimulation of sGC, with subsequent formation of cyclic GMP. These findings implicate NO involvement via sGC in the increase in MBF induced by AII. These data are in agreement with previous studies [14,27,28]. The rate of synthesis and tissue concentration of prostaglandins is much higher in the medulla compared with the cortex, and AII stimulates prostaglandin synthesis via AT1 receptors [28]. Prosta- glandin E2 (PGE2) is a major renal cyclooxygenase me- tabolite of arachidonate that modulates renal hemody- namics and salt and water excretion [29]. The main- tenance of normal renal blood flow and function during physiological stress is especially dependent on endoge- nous prostaglandin synthesis [30] buffering the vasocon- strictor effects of AII, catecholamines, and vasopressin in the kidney thereby preserving normal renal function. Contrary to previous reports showing a tonic vasodilator influence of prostaglandins on the medullary circulation [31-33] inhibition of prostaglandins by indomethacin in this study enhanced AII-induced increase in MBF. This result suggests that vasodilator prostaglandins may be contributing to the increase in MBF while the vasocon- strictor prostaglandins may be contributing to the in- crease in MABP or decrease in CBF elicited by AII. KATP channel regulation of vasoactivity in vascular beds has been documented and infusion of glibenclamide, a KATP into rats induced mesenteric, skeletal muscle, and renal vasoconstriction [34,35]. In vivo, KATP channel inhibition also increases resistance to blood flow in mesentery [36], renal cortex and medulla [32,37]. Pre- vious studies demonstrated that high concentrations of AII inhibit KATP in the renal medulla [38] and infusions of KATP channel inhibitors have been shown to decrease MBF [15,32]. In the present studies, inhibition of KATP channels with glibenclamide did not significantly change AII-mediated effects on systemic and renal hemodynamics. AII acts at two main receptor subtypes: AT1 and AT2 receptors. AT1 receptors are responsible for mediating most of the known actions of AII, including vasocon- striction [39]. Moreover, a role for the AT2 receptor in opposing the actions of AT1 receptor stimulation has been implicated in growth and cardiovascular function [39-41]. In the kidney, infusions of AII reduce total renal blood flow (RBF) and cortical perfusion measured by laser Doppler flowmetry in rats and rabbits [41]. How- ever, medullary perfusion is relatively insensitive to the vasoconstrictor effects of AII under most experimental conditions [1,23]. The explanation for these observations seems to be that, although AT1-receptor activation causes vasoconstriction within vascular elements controlling MBF, it can also cause vasodilatation by release of nitric oxide and/or prostaglandins [5]. The contributions of AT2 receptors to the control of MBF are less clear. However, recent studies in anaesthetised rabbits suggest that AT2-receptor activation counteracts AT1-mediated vasodilatation in the renal medulla, as the AT2 antagonist PD123319 revealed dose-dependent increases in Medul- lary Laser Doppler Flux (MLDF) during renal arterial in- fusion of AII [19] implying an AT2-mediated medullary vasoconstriction but AT1-mediated vasodilation in the rabbit. This observation is at odds with the current study and contrary to the conventional view that AT2 receptors mediate vasodilatation [42]. In our studies, blockade of AT2 receptors with PD123319 attenuated AII-induced increase in MBF, suggesting that the increase in MBF was AT2 receptor mediated. These data were further con- firmed by infusion of CGP41112, an AT2 receptor agonist in the absence and presence of PD123319 to determine the effect of AT2 receptor on basal MABP, CBF and MBF. PD123319 had no detectable effect on resting systemic or renal hemodynamics. Thus, AT2 receptors did not appear to contribute greatly to the control of resting MBF in anesthetized rats under these experimental conditions. Activation of AT2 receptor by CGP41112 decreased basal MABP while increasing CBF and MBF. These responses were attenuated by PD123319, con- firming that AT2 receptor is not only involved in in- creasing MBF but also appears to be involved in the increase in CBF and decrease in MABP. These data are at odds with studies that showed that the increased me- dullary perfusion was AT1 receptor-mediated [1,6,19] in rats. In conclusion, AII evoked differential effects on intra- renal haemodynamics in the rat evoking cortical vaso- constriction but medullary vasodilation. AT2 re-ceptor appears to mediate AII-induced increase in MBF by me- chanisms involving guanylase cyclase, nitric oxide and dilator prostanoids but not KATP channels. 5. Acknowledgements This study was supported by National Institutes of Health grant HL03674. The facilities of the RCMI program at Texas Southern University were used for this study. REFERENCES [1] B. Badzyńska, M. Grzelec-Mojzesowicz, L. Dobrowolski, and J. Sadowski, “Differential Effect of Angiotensin II on Blood Circulation in the Renal Medulla and Cortex of Anaesthetised Rats,” Journal of Physiology, Vol. 538, No. Copyright © 2012 SciRes. PP  Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels 395 1, 2002, pp. 159-166. doi:10.1113/jphysiol.2001.012921 [2] A. W. Cowley, “Role of the Renal Medulla in Volume and Arterial Blood Pressure Regulation,” American Jour- nal of Physiology, Vol. 273, No. 1, 1997, pp. R1-R15. [3] R. M. Carey, “Update on the Role of the AT2 Receptor,” Current Opinion in Nephrology and Hypertension, Vol. 14, No. 1, 2005, pp. 67-71. doi:10.1097/00041552-200501000-00011 [4] W. A. Cupples, T. Sakai and D. J. Marsh, “Angiotensin II and Prostaglandins in Control of Vasa Recta Blood Flow,” American Journal of Physiology, Vol. 254, No. 3, 1988, pp. F417-F424. [5] R. G. Evans, A. C. Madden and K. M. Denton, “Diversity of Responses of Renal Cortical and Medullary Blood Flow to Vasoconstrictors in Conscious Rabbits,” Acta Phy- siology Scandinavia, Vol. 169, No. 4, 2000, pp. 297-308. doi:10.1046/j.1365-201x.2000.00741.x [6] L. L. Walker, A. A. Rajaratne, J. R. Blair-West and P. J. Harris, “The Effects of Angiotensin II on Blood Perfusion in the Rat Renal Papilla,” Journal of Physiology, Vol. 519, No. 1, 1999, pp. 273-278. doi:10.1111/j.1469-7793.1999.0273o.x [7] D. L. Mattson, H. Raff and R. J. Roman, “Influence of Angiotensin II on Pressure Natriuresis and Renal Hemo- dynamics in Volume Expanded Rats,” American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, Vol. 260, No. 6, 1991, pp. R1200-R1209. [8] C. L. Huang, G. Davis and E. J. Johns, “A Study of the Action of Angiotensin II on Perfusion through the Cortex and Papilla of the Rat Kidney,” Experimental Physiology, Vol. 76, No. 5, 1991, pp. 787-798. [9] T. L. Pallone, C. R. Robertson and R. L. Jamison, “Renal Medullary Microcirculation,” Physiological Reviews, Vol. 70, No. 3, 1990, pp. 885-920. [10] S. Lu, D. L. Mattson, R. J. Roman, C. G. Becker and A. W. Cowley Jr., “Assessment of Changes in Intrarenal Blood Flow in Conscious Rats Using Laser-Doppler Flowmetry,” American Journal of Physiology Renal Fluid Electrolyte Physiology, Vol. 264, No. 2, 1993, pp. F956- F962. [11] M. S. Nobes, P. J. Harris, H. Yamada and F. A. O. Men- delsohn, “Effects of Angiotensin on Renal Cortical and Papillary Blood Flows Measured by Laser-Doppler Flow- metry,” American Journal of Physiology Renal Fluid Elec- trolyte Physiology, Vol. 261, No. 6, 1991, pp. F998- F1006. [12] N. W. Rajapakse, J. J. Oliver and R. G. Evans, “Nitric Oxide in Responses of Regional Kidney Blood Flow to Vasoactive Agents in Anesthetized Rabbits,” Journal of Cardiovascular Pharmacology, Vol. 40, No. 2, 2002, pp. 210-219. doi:10.1097/00005344-200208000-00006 [13] J. J. Oliver, N. W. Rajapakse and R. G. Evans, “Effects of Indomethacin on Responses of Regional Kidney Perfu- sion to Vasoactive Agents in Rabbits,” Clinical Experi- mental Pharmacology and Physiology, Vol. 29, No. 10, 2002, pp. 873-879. doi:10.1046/j.1440-1681.2002.03742.x [14] A. P. Zou, F. Wu and A. W. Cowley Jr., “Protective Ef- fect of Angiotensin II-Induced Increase in Nitric Oxide in the Renal Medullary Circulation,” Hypertension, Vol. 31, No. 2, 1998, pp. 271-276. doi:10.1161/01.HYP.31.1.271 [15] A. O. Oyekan, “Contributions of Nitric Oxide and Prost- anoids and Their Signaling Pathways to the Renal Me- dullary Vasodilator Effect of U46619 (9-11-Dideoxy-11α, 9a-epoxymethano-prostaglandin F2a) in the Rat,” Jour- nal of Pharmacology and Experimental Therapeutics, Vol. 304, No. 2, 2003, pp. 507-512. doi:10.1124/jpet.102.040170 [16] J. L. Williams, D. Cartland, A. Hussain and S. Egginton, “A Differential Role for Nitric Oxide in Two Forms of Physiological Angiogenesis in Mouse,” Journal of Physi- ology, Vol. 570, No. 3, 2006, pp. 445-454. doi:10.1113/jphysiol.2005.095596 [17] S. Crestani, Y. D. Rattmann, T. R. Cipriani, L. M. de Souza, M. Iacomini, C. A. Kassuya, M. C. Marques and J. E. da Silva-Santos, “A Potent and Nitric Oxide-Depend- ent Hypotensive Effect Induced in Rats by Semi-Purified Fractions from Maytenus Ilicifolia,” Vascular Pharma- cology, Vol. 51, No. 1, 2009, pp. 57-63. doi:10.1016/j.vph.2009.02.005 [18] H. C. D. Souza, H. C. Salgado, G. Ballejo and M. C. O. Salgado, “SR141716A-Sensitive Enhancement of ET-1 Hypotensive Effect by Chronic NOS Inhibition,” Hyper- tension, Vol. 42, No. 2, 2003, pp. 802-805. doi:10.1161/01.HYP.0000088362.50484.4C [19] L. M. Duke, G. A. Eppel, R. E. Widdop and R. G. Evans, “Disparate Roles of AT2 Receptors in the Renal Cortical and Medullary Circulations of Anesthetized Rabbits,” Hypertension, Vol. 42, No. 2, 2003, pp. 200-205. doi:10.1161/01.HYP.0000083341.64034.00 [20] S. Y. Chou, J. G. Porush and P. F. Faubert, “Renal Me- dullary Circulation: Hormonal Control,” Kidney Inter- national, Vol. 37, No. 1, 1990, pp. 1-13. doi:10.1038/ki.1990.1 [21] D. S. A. Majid, M. Godfrey and L. G. Navar, “Pressure Natriuresis and Renal Medullary Blood Flow in Dogs,” Hypertension, Vol. 29, No. 4, 1997, pp. 1051-1057. doi:10.1161/01.HYP.29.4.1051 [22] S. A. Omoro, D. S. A. Majid, S. S. El-Dahr and L. G. Navar, “Kinin Influences on Renal Regional Blood Flow Responses to Angiotensin-Converting Enzyme Inhibition in Dogs,” American Journal of Physiology Renal Physi- ology, Vol. 276, No. 2, 1999, pp. F271-F277. [23] N. Parekh, L. Dobrowolski, A. P. Zou and M. Stein- hausen, “Nitric Oxide Modulates Angiotensin II- and Norepinephrine-Dependent Vasoconstriction in Rat Kid- ney,” American Journal of Physiology Regulatory Inter- grative and Comparative Physiology, Vol. 270, 1996, pp. R630-R635. [24] R. G. Evans, G. BergstroÈm and A. J. Lawrence, “Effects of the Vasopressin V1 Agonist [Phe2,Ile3,Orn8]-Vaso- pressin on Regional Kidney Perfusion and Renal Excre- tory Function in Anesthetized Rabbits,” Journal of Car- diovascular Pharmacology Vol. 32, No. 4, 1998, pp. 571- 581. doi:10.1097/00005344-199810000-00009 Copyright © 2012 SciRes. PP  Differential Effects of Angiotensin II on Intra-Renal Hemodynamics in Rats; Contribution of Prostanoids, NO and K+ Channels Copyright © 2012 SciRes. PP 396 [25] L. G. Navar, B. L. Harrison, J. D. Imig, et al., “Role of AT1 Receptor in Target Organ Disease: A Functional Perspective,” American Journal of Hypertension, Vol. 13, No. 1, 2000, pp. 45S-54S. doi:10.1016/S0895-7061(99)00248-4 [26] R. G. Evans, G. A. Head, G. A. Eppel, S. L. Burke and N. W. Rajapakse, “Frontiers in Research Series: Neural, Hormonal and Renal Interactions in Long-term Blood Pressure Control II: Angiotensin II and neurohumoral Control of the Renal Medullary Circulation,” Clinical and Experimental Pharmacology and Physiology, Vol. 37, No. 2, 2010, pp. 58-69. doi:10.1111/j.1440-1681.2009.05233.x [27] P. A. Ortiz, N. J. Hong, D. Wang and J. L. Garvin, “Gene Transfer of eNOS to the Thick Ascending Limb of eNOS-KO Mice Restores the Effects of L-Arginine on NaCl Absorption,” Hypertension, Vol. 42, No. 4, 2003, pp. 674-679. doi:10.1161/01.HYP.0000085561.00001.81 [28] H. M. Siragy and R. M. Carey, “The Subtype-2 (AT2) Angiotensin Receptor Regulates Renal Cyclic Guanosine 3,5-Monophosphate and AT1 Receptor-Mediated Pros- taglandin E2 Production in Conscious Rats,” Journal of Clinical Investigation, Vol. 97, No. 8, 1996, pp. 1978- 1982. doi:10.1172/JCI118630 [29] M. D. Breyer and R. M. Breyer, “Prostanglandin E Re- captors and the Kidney,” American Journal of Physiology, Renal Physiology, Vol. 279, No. 1, 2000, pp. F12- F23. [30] A. Yared, V. Kon and I. Ichikawa, “Mechanism of Pres- ervation of Glomerular Perfusion and Filtration during Acute Extracellular Volume Depletion: Importance of In- trarenal Vasopressin Prostaglandin Interaction for Pro- tecting Kidneys from Constrictor Action of Vasopressin,” Journal of Clinical Investigation, Vol. 75, No. 5, 1985, pp. 1477-1487. doi:10.1172/JCI111851 [31] F. G. Knox and J. P. Granger, “Control of Sodium Excre- tion: An Integrative Approach,” In: E. E. Windhager, Ed., Renal Physiology, Oxford University Press, New York, 1992. [32] J. Sadowski, E. Kompanowska-Jezierska, L. Dobrowolski, A. Walkowska and B. Badzynska, “Simultaneous Re- cording of Tissue Ion Content and Blood Flow in Rat Renal Medulla: Evidence on Interdependence,” American Journal of Physiology Renal Physiology, Vol. 273, No. 4, 1997, pp. F658-F662. [33] E. Kompanowska-Jezierska, A. Walkowska and J. Sadowski, “Exaggerated Volume Expansion Natriuresis in Rats Pre- loaded with Hypertonic Saline: A Paradoxical Enhance- ment by Inhibition of Prostaglandin Synthesis,” Acta Physi- ologica Scandinavica, Vol. 167, No. 3, 1999, pp. 189-194. doi:10.1046/j.1365-201x.1999.00604.x [34] H. G. Klieber and J. Daut, “A Glibenclamide-Sensitive Potassium Conductance in Terminal Arterioles Isolated from Guinea Pig Heart,” Cardiovascular Research, Vol. 28, No. 6, 1994, pp. 823-830. doi:10.1093/cvr/28.6.823 [35] S. M. Gardiner, P. A. Kemp, J. E. March, B. Fallgren and T. Bennett, “Effects of Glibenclamide on the Regional Haemodynamic Actions of α-Trinositol and Its Influence on Responses to Vasodilators in Conscious Rats,” British Journal of Pharmacology, Vol. 117, No. 3, 1996, pp. 507- 515. doi:10.1111/j.1476-5381.1996.tb15219.x [36] A. L. Salzman, A. Vromen, A. Denenberg and C. Szabo, “KATP-Channel Inhibition Improves Hemodynamics and Cellular Energetics in Hemorrhagic Shock,” American Journal of Physiology Heart Circulation Physiology, Vol. 272, No. 2, 1997, pp. H688-H694. [37] T. Mimuro, T. Kawata, T. Onuki, S. Hashimoto, K. Tsu- chiya, H. Nihei and T. Koike, “The Attenuated Effect of ATP-Sensitive K+ Channel Opener Pinacidil on Renal Haemodynamics in Spontaneously Hypertensive Rats,” European Journal of Pharmacology, Vol. 358, No. 2, 1998, pp. 153-160. doi:10.1016/S0014-2999(98)00573-1 [38] C. Cao, W. Lee-Kwon, E. P. Silldorff and T. L. Pallone, “KATP-Channel Conductance of Descending Vasa Recta Pericytes,” American Journal of Physiology Renal Physi- ology, Vol. 289, No. 6, 2005, pp. F1235-F1245. doi:10.1152/ajprenal.00111.2005 [39] M. DeGasparo, K. J. Catt and T. Inagami, “International Union of Pharmacology XXIII. The Angiotensin II Re- ceptors,” Pharmacological Reviews, Vol. 52, No. 3, 2000, pp. 415-472. [40] H. Matsubara, T. Sugaya, S. Murasawa, Y. Nozawa, Y. Mori and H. Masaki, “Tissue-Specific Expression Of Human Angiotensin II AT1 and AT2 Receptors and Cel- lular Localization of Subtype mRNAs in Adult Human Renal Cortex Using in Situ Hybridization,” Nephron, Vol. 80, No. 1, 1998, pp. 25-34. doi:10.1159/000045121 [41] L. M. Duke, R. E. Widdop, M. M. Kett and R. G. Evans “AT2 Receptors Mediate Tonic Renal Medullary Vaso- constriction in Renovascular Hypertension,” British Jour- nal of Pharmacology, Vol. 144, No. 4, 2005, pp. 486-492. doi:10.1038/sj.bjp.0706036 [42] R. E. Widdop, E. S. Jones, R. E. Hannan and T. A. Gas- pari, “Angiotensin AT2 Receptors: Cardiovascular Hope or Hype?” British Journal of Pharmacology, Vol. 140, No. 5, 2003, pp. 809-824. doi:10.1038/sj.bjp.0705448
|