Paper Menu >>
Journal Menu >>
 Pharmacology & Pharmacy, 2010, 1, 9-17 10.4236/pp.2010.11002 Published Online July 2010 (http://www.SciRP.org/journal/pp) Copyright © 2010 SciRes. PP 9 Development and Evaluation of a New Interpenetrating Network Bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Rajat Ray, Siddhartha Maity, Sanchita Mandal, Tapan K. Chatterjee, Biswanath Sa The Division of Pharmaceutics, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India. Email: biswanathsa2003@yahoo.com Received June 6th, 2010; accepted July 8th, 2010. ABSTRACT Interpenetrating network (IPN) beads of sodium carboxymethyl xanthan (SCMX) and sodium alginate (SAL) were pre- pared by ionotropic gelation process using AlCl3 as a cross-linking agent. The effect of different formula tion variables like total polymer concentration, gelation time, concentration of cross-linking agent, and drug load on the extent of release of ibuprofen (IBP), a non stero idal anti-inflammatory drug, was examined. The formation of IPN structure was examined using Fourier Transform Infra-red (FTIR) analysis and the compatibility of the drug in the bead was evalu- ated through FTIR, X-ray diffraction (XRD) and Differential Scanning Calorimetry (DSC) analyses. While increase in the concentration o f total polymer, gelation time, and drug load decreased the drug release in both acidic (pH-1.2) and phosphate buffer (PB) solution (pH-6.8), increase in the concentration of cross-linking agent tended to increase the drug release. However, from all the formulations, the drug release in acidic medium was considerably slow and a maximum 14% of the loaded drug was released in 2 h. Complete drug release was achieved in PB solution within 210 to 330 min depending upon the formulation variables. The release of the drug followed non-Fickian transport process in acidic medium and ca se-II transport mechanism in PB solu tion and these release behaviour correlated well with the kinetics of dynamic swelling of IPN beads. The study indicated that the IPN beads of SCMX and SAL could be a suit- able dosage form to minimize the drug release in ac idic solution and to con trol the drug release in PB solution depend- ing upon the need. Keywords: IPN Bead, Ibuprofen, Drug Release, Kinetics, Swelling 1. Introduction Among the most abundant natural polymers, polysaccha- rides are widely used in pharmaceutical dosage forms as excipients like suspending agents, emulsifying agents, tablet binders, gelling agents. With the advent of mac- romolecular chemistry, the use of polysaccharides has been extended towards new applications in pharmaceuti- cal, biomedical, and agricultural fields. Although natu- rally available polysaccharides exhibit certain limitations in terms of their reactivity and processibility, these can be overcome by modification of the polysaccharides through either physical or chemical cross-linking, graft- ing with other materials and developing hydrogels or interpenetrating network(IPN) structures. Since the homopolymers alone can not meet divergent demand in terms of properties and performances, devel- opment of IPN appears to be a better approach [1] and one of the easiest ways for modification of the properties of polysaccharides. IPN consists of two polymers, each in network form, which can be cross-linked in the pres- ence of each other to give a three dimensional network structure [2] and hence, combine the properties of two cross-linked polymers in a network form [3]. IPNs are thus emerging as a rapidly developing branch of polymer blended technology and are finding applications in artifi- cial implants, dialysis, membranes, drug delivery systems [4], and in agricultural field [5]. Sodium alginate (SAL), a hydrophilic biopolymer ob- tained from brown sea weeds, is a polysaccharide com- posed of varying proportions of D-mannuronic acid (M) and L-guluronic acid (G) residues which are arranged in  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 10 MM or GG blocks interspersed with MG blocks [6]. Its unique property of forming water insoluble calcium al- ginate gel through ionotropic gelation with Ca2+ ions in a simple and mild condition has made possible to encapsu- late both macromolecular agents [7] and low molecular weight therapeutic agents [8,9] in calcium alginate beads. However in physiological environment, calcium alginate beads tend to have poor mechanical stability [10]. To overcome this limitation, IPN beads of SAL with gelatin or egg albumin [2], polyvinyl alcohol-graftedpolyacry- lamide [11], N,O-Carboxymethyl chitosan [12] for con- trolled drug delivery, and with gelatin [5] for controlled release of pesticides have been developed. Although xanthan gum, a polysaccharide obtained from Xanthomonas campestris, can not form gel beads, its Na-salt of carboxymethyl derivative is able to form gel beads through ionotropic gelation with Al3+ ions [13]. Sodium carboxymethyl xanthan (SCMX) beads have been found capable of encapsulating albumin [14] and diltiazem hydrochloride [15]. However hitherto there are no reports on IPN beads of SCMX with SAL for drug release study. The objective of the present work was to develop a new IPN bead composed of SCMX and SAL and to eva- luate the beads for encapsulation and release behavior of ibuprofen (IBP). 2. Experimental 2.1 Materials Ibuprofen (Indian Pharmacopoeia) and xanthan gum were obtained as gift samples from respectively M/S Albert David Limited and M/S Deys Medical Stores (Mfg). Pvt. Limited, Kolkata, India. Sodium alginate (Mol. wt. 240kDa), AlCl3·2H2O (SD Fine Chem Pvt. Ltd, Mumbai, India), Monochloro acetic acid (Loba Chemie Pvt. Ltd, Mumbai, India) and all other analytical grade reagents were obtained commercially and used as re- ceived. 2.2 Preparation of Sodium Carboxymethyl Xanthan (SCMX) Xanthan gum was derivatised to SCMX having O-carboxymethyl substitution of 0.8 following the me- thod reported previously [13]. In brief, required amount of xanthan gum was dispersed in ice cold solution of 45% w/v sodium hydroxide. The dispersion was kept at 5-8°C with continuous stirring for 1h. Monochloroacetic acid solution (75% w/v) was added with stirring in the reaction mixture and the temperature was raised slowly to 15-18°C. After 30 min, the temperature was raised to 75°C and maintained for additional 30 min. The reaction mixture was, then cooled to room temperature, cut into small pieces and dried at 50°C. The dried product was milled, washed with 80% v/v methanol and again dried. 2.3 Preparation of Interpenetrating Network (IPN) Bead Required amount of ibuprofen (IBP) was homogenously dispersed in an aqueous solution of SCMX and SAL. The resulting dispersion was extruded through 21 G flat-tip hypodermic needle into AlCl3 solution. Gelation of the beads was carried out for different periods of time. The beads were, then collected by filtration, washed with deionized water, dried at 45°C in a hot air oven to con- stant weight and kept in a dessicator until used. The beads were prepared using the following variables: 1) Keeping the drug load constant at 50% w/w of total polymer and the concentration of AlCl3 constant at 2% w/v, the total polymer concentration was varied from 2-4% w/v (SCMX to SAL weight ratio 1:1) and the gela- tion time was varied from 0.5 to 2 hour. 2) Keeping the drug load constant at 50% w/w of total polymer, the gelation time at 0.5 hour and total polymer concentration 3% w/v, concentration of AlCl3 was varied from 2-8% w/v. 3) Keeping the total polymer concentration fixed at 3% w/v, gelation time at 0.5 hour, and AlCl3 concentra- tion at 2% w/v, drug load was varied from 20-60% w/w of total polymer. The composition of beads is shown in Table 1. Each formulation was prepared in duplicate. Table 1. Composition and drug entrapment efficiency (DEE) of sodium carboxymethyl xanthan (SCMX) and sodium alginate (SAL) IPN beads SCMX%: SAL% Drug load (% w/w of total polymer) Gelation time (hr) Concentration of AlCl3 (% w/v ) DEE (Mean ± SD, n = 4) 1:1 50 0.5 2 93.46 ± 2.18 1.5:1.5 50 0.5 2 97.22 ± 2.45 2:2 50 0.5 2 99.50 ± 2.86 1:1 50 2 2 91.15 ± 1.92 1.5:1.5 50 2 2 94.25 ± 3.37 2:2 50 2 2 95.31 ± 2.72 1.5:1.5 50 0.5 4 97.25 ± 1.38 1.5:1.5 50 0.5 8 97.65 ± 3.84 1.5:1.5 20 0.5 2 98.86 ± 1.26 1.5:1.5 40 0.5 2 99.16 ± 1.74 1.5:1.5 60 0.5 2 96.96 ± 2.52  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 11 2.4 Fourier Transform Infrared (FTIR) Analysis FTIR spectra of SCMX, SAL and drug free IPN bead were recorded in a FTIR spectrophotometer (Perkin- El- mer, model Spectrum RX-1, UK). Each sample was mixed with KBr and converted into disc at 100 kg pres- sure using a hydraulic press. The spectra were recorded within 4000-400 cm-1 wave numbers. Similarly, the FTIR spectra of IBP and drug loaded IPN beads were recorded. 2.5 Powder X-Ray Diffraction (XRD) Analysis Qualitative XRD studies were performed using an X-ray diffractometer (Bruker D8 advanced powder diffracto- meter, USA). Pure IBP and powdered beads were scanned from 5° to 55° diffraction angle (2θ) range under the following conditions: Source, Ni-filtered Cu-Kα (λ = 1.54) radiation; voltage, 40 kV; Current, 40 mA; scan speed, 16°/min 2.6 Differential Scanning Calorimetry (DSC) Study DSC thermograms of IBP and powdered beads were ob- tained in the following way: A weighed amount (about 6 mg) of sample was kept in a hermetically sealed aluminium pan and heated at a scan speed of 10°C /min over a temperature range of 35°C- 310°C in a Differential Scanning Calorimeter (Perkin- Elmer, model Pyris Diamond TG/DTA, UK ) which was calibrated against indium. A nitrogen purge (20 ml/min) was used throughout the runs. 2.7 Photomicrograph Photomicrograph of IPN beads were taken at 4X magni- fication with an optical microscope (Leica DM 2500P) fitted with a camera (Cannon Power Shot S-80, Japan). 2.8 Drug Entrapment Efficiency IPN beads (20 mg) were accurately weighed in an elec- tronic balance (Precisa XB 600 MC, Precisa Instrument Ltd; Switzerland), immersed in 250 ml USP phosphate buffer (PB) solution (pH 6.8), and shaken for 2h on a mechanical shaker. The beads were crushed and further shaken for 1h. The solution was filtered and an aliquot following suitable dilution was analyzed at 222 nm in a UV-Visible spectrophotometer (model Cary-50 Bio-sp- ectrophotometer, VARIAN, Australia)) and the content of the beads was determined using a calibration curve constructed using PB solution of pH 6.8. The reliability of the above analytical method was judged by conducting recovery analysis at three levels of spiked drug solution in the presence or absence of the polymers for three con- secutive days. The recovery averaged 98.45 ± 2.68%. DEE was determined using the following relation: DEE (%) = (Determined drug content/Theoretical drug content) × 100 2.9 In-Vitro Drug Release Study In-vitro drug release study was carried out in acidic solu- tion 0.1 (N) HCl (pH 1.2) and in USP PB solution (pH 6.8) using USP-II dissolution rate test apparatus (model TDP-06P Electro Lab, Mumbai, India). 20 mg beads were placed in 500 ml acidic solution or 500 ml PB solu- tion (37 ± 1°C) and rotated with paddle at 75 rpm. Ali- quot was withdrawn at different times and replenished immediately with the same volume of fresh solution. Undiluted or suitably diluted withdrawn samples were analyzed spectrophotometrically at 220 nm for acidic solution and 222 nm for PB solution. The amount of drug released in acidic solution and PB solution were calcu- lated from the calibration curves drawn respectively, in 0.1 (N) HCl and PB solution (pH 6.8). Each release study was conducted four times. 2.10 Swelling Study Dried drug-free IPN beads (50 mg) were immersed in 25ml acidic solution (pH 1.2) at 37ºC. The beads were removed at different times by filtration and blotted care- fully to remove excess surface water. The swollen beads were weighed. The swelling ratio of the beads were de- termined using the following formula: Swelling ratio = (weight of swollen beads-weight of dry beads)/weight of dry beads Swelling ratio of the beads in PB solution (pH 6.8) was determined in a similar way. 2.11 Statistical Analysis Each formulation was prepared in duplicate, and each analysis was duplicated. Effect of formulation variables on drug release was tested for significance level by using analysis of variance (ANOVA: single factor and two factor) with the aid of Microsoft® Excel 2003. Differ- ence was considered significant when p < 0.05. 3. Results & Discussion 3.1 Formation of IPN IPN beads composed of SCMX and SAL were prepared by inotropic gelation process using AlCl3 as a common cross-linking agent for both the polymers. Formulation of IPN structure was verified by FTIR analysis (Figure 1). FTIR spectrum of SCMX showed the presence of bands corresponding to asymmetric and symmetric carboxylate anions at respectively 1605 cm-1 and 1419 cm-1, a broad band at 3419 cm-1 corresponding to stretching vibration of hydroxyl group, a peak at 1327 cm-1 corresponding to C = O stretching of carboxymethyl group. These results are similar to the findings reported earlier [14]. The spectrum of SAL showed the bands characteristics of  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 12 Figure 1. FTIR spectra of (a) SCMX, (b) SAL, (c) drug free IPN bead asymmetric and symmetric carboxylate anions at respec- tively 1612 cm-1 and 1420 cm-1, a broad peak corre- sponding to the stretching of hydroxyl group at 3468 cm-1. Similar spectrum of SAL has been reported else- where [16]. The FTIR spectrum of drug-free IPN beads showed peaks at 1639 cm-1 and 1425 cm-1 respectively for asymmetric and symmetric carboxylate anions and a peak at 3405 cm-1 for hydroxyl group. Moreover, the peak at 1327 cm-1 assigned for carboxymethyl group was retained. Comparison of the spectra, however, demon- strated shift of the peaks of carboxylate anions to higher wave numbers. The shift of carboxylate bands confirms the formation of complex between the two polymers and Al3+ ions through physical cross-linking. These results suggest the formation of IPN structure wherein both the polymers are present in cross-linked condition. 3.2 Morphology of IPN Bead The composition of IBP-loaded IPN beads has been shown in Table1. The beads were prepared with a SCMX to SAL weight ratio of 1:1 but in different total polymer concentration (1%:1%, 1.5%:1.5%, 2%:2%) and gelling in AlCl3 solution (2-8% w/v) for different periods of time (0.5 to 2 h). Although the shapes of the wet beads were spherical, the shapes distorted after drying. The surface of dried beads was rough and folded (Figure 2) and was due to shrinkage of the beads during the drying process. Similar shape distortion has been reported for chitosan/ Figure 2. Photo micrographs of ibuprofen-loaded IPN beads, prepared under different conditions. (a) SCMX: SAL 1%:1%, 2% w/v AlCl3, 0.5 h, (b) SCMX: SAL 1.5%: 1.5%, 4% w/v AlCl3, 2 h, (c) SCMX: SAL 2%:2%, 8% w/v AlCl3, 0.5 h carageenan beads [17]. Moreover, neither the concentra- tion of cross-linking agent (AlCl3) nor the gelation time had any appreciable effect on morphology of IPN beads. The shape of the beads did not change even when the drug load was varied from 20 to 60% w/w of total poly- mer. 3.3 Compatibility of Drug in IPN Bead Compatibility of IBP in IPN beads was studied using FTIR, XRD and DSC analyses. The characteristics bands corresponding to C=O stretching and –OH stretching of IBP appeared in FTIR spectrum respectively at 1720 cm-1 and 2956 cm-1. The above two bands were also detected at the same positions in the spectrum of drug-loaded IPN beads (Figure 3). XRD analysis showed reflection to the interplanner distances of 14.41, 7.24, 5.32, 5.01, 4.72, 4.65, 4.39, 3.98 and 3.63 Å respectively at 6.13, 12.21, 16.64, 17.68, 18.78, 19.06, 20.20, 22.30 and 24.52˚ 2θ. Drug–loaded IPN beads also exhibited the same reflec- tions at the same 2θ degrees (Figure 4). The result indi- cates that the crystallinity of the drug in IPN beads was retained and no amorphization of the drug took place. Comparison of DSC thermograms revealed that the Figure 3. FTIR spectra of (a) Ibuprofen (b) Ibupro- fen-loaded IPN bead Figure 4. X-ray diffractograms of (a) Ibuprofen (b) Ibupro- fen-loaded IPN bead  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 13 melting endothermic peak of IBP at 79°C also appeared in the DSC curve of drug loaded IPN bead (Figure 5). These studies indicated that apparently no interactions between the drug and the polymers took place during the formation of IPN beads. 3.4 DEE of IPN bead DEE of IPN beads tended to increase as the total polymer concentrations was increased from 2-4% w/w keeping SCMX to SAL weight ratio constant at 1:1 (Table 1). Two way analysis of variance (ANOVA-2) revealed sig- nificant difference (F2,3 > Ftabular at 0.05 level) in DEE in IPN beads prepared with increasing polymer concentra- tions although no significant difference was noted within the batches of each formulation. Same observations were noted with IPN beads which were prepared by gelling in 0.5% AlCl3 solution for two different gelation times. In- crease in DEE with increase in total polymer concentra- tions is related to the higher rigidity of the matrices of IPN beads. Higher encapsulation efficiency of cefadroxyl has been reported for IPN beads prepared using SAL and gelatin or egg albumin [2]. Concentrations of cross-linking agent did not produce any appreciable change in DEE of IPN beads prepared with a total polymer concentra- tion of 3% w/v keeping SCMX to SAL weight ratio constant at 1:1. The results of one way analysis of vari- ance (ANOVA-1) revealed no significant difference in DEE (F2,3 < Ftabular at 0.95 level) of IPN beads prepared with various concentrations of AlCl3 . Similar independ- ence of DEE on the extent of cross-linking has been re- ported for ketorolac loaded IPN beads composed of so- dium carboxymethyl cellulose and gelatin [18]. The time of gelation, however, had an impact on DEE which tended to decrease as gelation time was increased (Table 1). The results are in agreement with the reports of other workers [2]. Although the solubility of IBP in aqueous medium is very less, prolonged exposure in the gelation medium may cause greater leaching of the drug from IPN beads resulting in decreased DEE. IPN beads having 20 to 60% w/w of IBP were prepared using 3% w/w total polymer concentration and gelling for 0.5 h in 2% w/v AlCl3 solution. DEE was found to vary within 96.96 to 99.16% (Table 1). No significant effect of drug loading on DEE was observed. Similar non-dependence of DEE on % of drug loading has been reported for IPN beads composed of sodium carboxymethyl cellulose and gela- tin. 3.5 In-Vitro Drug Release 3.5.1 Effect of Polymer Concentration Release of IBP from IPN beads, prepared using increased polymer concentrations (SCMX: SAL weight ratio 1:1) and gelling for 0.5 h in 2% AlCl3 solution, have been represented in Figure 6. Drug release in acidic medium Figure 5. DSC thermograms of (a) Ibuprofen (b) Ibupro- fen-loaded IPN bead Figure 6. Release profiles of Ibuprofen in acidic solution (open symbols) and phosphate buffer solution (closed sym- bols) from IPN beads prepared using different concentra- tion of SCMX and SAL and gelling in 2% w/v AlCl3 solu- tion for 0.5 h. Key: SCMX: SAL = () 1%:1%, (∆) 1.5%:1.5%, () 2%:2%. Maximum SEM = 1.24(n = 4) was slow and 8.82 to 14.09% of the loaded drug was released in 2 h. In PB solution (pH 6.8), complete drug release was achieved in 210 min to 300 min depending upon the total polymer concentration in the beads. In- crease in total polymer concentration from 2 to 4% w/w decrease the drug release in both the dissolution media. The derived properties obtained from drug release pro- files indicated that the area under the curves (AUCs), determined using trapezoidal rule, in acidic dissolution medium decreased as the polymer concentration in IPN beads increased. Similarly, the time required for 50% (t50%) and 80% (t80%) drug release in PB solution in- creased and AUCs decreased with increase in polymer concentration in the beads. Similar trend in drug release was observed from IPN beads which were prepared by gelling for 2 h (Table 2). Drug release from hydrophilic polymeric beads depends upon the type of matrix used as well as its rigidity [11]. Increase in total polymer con- centration results in a more entangled or more compact gel system with a greater cross-linking density in the 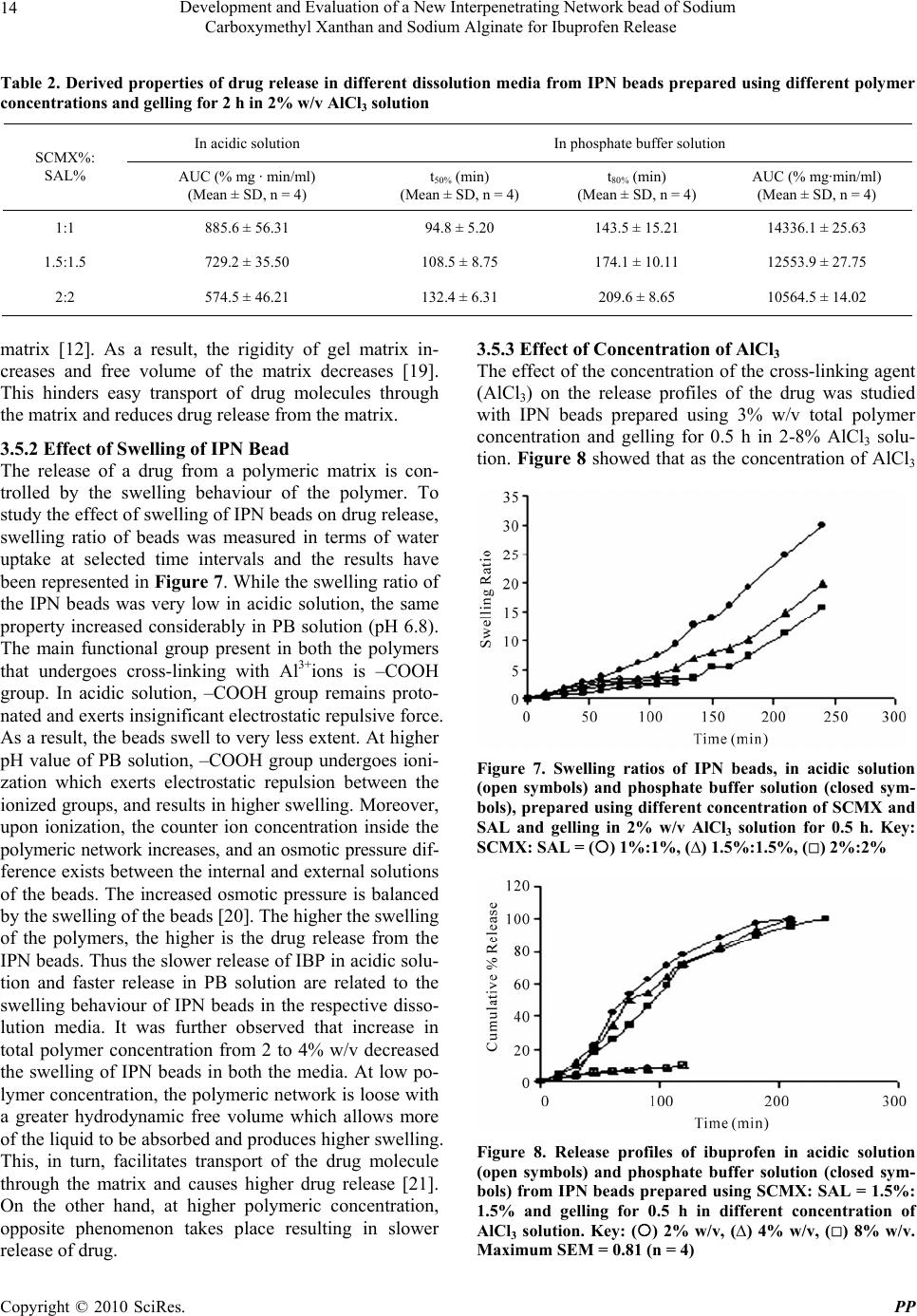 Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 14 Table 2. Derived properties of drug release in different dissolution media from IPN beads prepared using different polymer concentrations and gelling for 2 h in 2% w/v AlCl3 solution In acidic solution In phosphate buffer solution SCMX%: SAL% AUC (% mg · min/ml) (Mean ± SD, n = 4) t50% (min) (Mean ± SD, n = 4) t80% (min) (Mean ± SD, n = 4) AUC (% mg·min/ml) (Mean ± SD, n = 4) 1:1 885.6 ± 56.31 94.8 ± 5.20 143.5 ± 15.21 14336.1 ± 25.63 1.5:1.5 729.2 ± 35.50 108.5 ± 8.75 174.1 ± 10.11 12553.9 ± 27.75 2:2 574.5 ± 46.21 132.4 ± 6.31 209.6 ± 8.65 10564.5 ± 14.02 matrix [12]. As a result, the rigidity of gel matrix in- creases and free volume of the matrix decreases [19]. This hinders easy transport of drug molecules through the matrix and reduces drug release from the matrix. 3.5.2 Effect of Swelling of IPN Bead The release of a drug from a polymeric matrix is con- trolled by the swelling behaviour of the polymer. To study the effect of swelling of IPN beads on drug release, swelling ratio of beads was measured in terms of water uptake at selected time intervals and the results have been represented in Figure 7. While the swelling ratio of the IPN beads was very low in acidic solution, the same property increased considerably in PB solution (pH 6.8). The main functional group present in both the polymers that undergoes cross-linking with Al3+ions is –COOH group. In acidic solution, –COOH group remains proto- nated and exerts insignificant electrostatic repulsive force. As a result, the beads swell to very less extent. At higher pH value of PB solution, –COOH group undergoes ioni- zation which exerts electrostatic repulsion between the ionized groups, and results in higher swelling. Moreover, upon ionization, the counter ion concentration inside the polymeric network increases, and an osmotic pressure dif- ference exists between the internal and external solutions of the beads. The increased osmotic pressure is balanced by the swelling of the beads [20]. The higher the swelling of the polymers, the higher is the drug release from the IPN beads. Thus the slower release of IBP in acidic solu- tion and faster release in PB solution are related to the swelling behaviour of IPN beads in the respective disso- lution media. It was further observed that increase in total polymer concentration from 2 to 4% w/v decreased the swelling of IPN beads in both the media. At low po- lymer concentration, the polymeric network is loose with a greater hydrodynamic free volume which allows more of the liquid to be absorbed and produces higher swelling. This, in turn, facilitates transport of the drug molecule through the matrix and causes higher drug release [21]. On the other hand, at higher polymeric concentration, opposite phenomenon takes place resulting in slower release of drug. 3.5.3 Effect of Concentration of AlCl3 The effect of the concentration of the cross-linking agent (AlCl3) on the release profiles of the drug was studied with IPN beads prepared using 3% w/v total polymer concentration and gelling for 0.5 h in 2-8% AlCl3 solu- tion. Figure 8 showed that as the concentration of AlCl3 Figure 7. Swelling ratios of IPN beads, in acidic solution (open symbols) and phosphate buffer solution (closed sym- bols), prepared using different concentration of SCMX and SAL and gelling in 2% w/v AlCl3 solution for 0.5 h. Key: SCMX: SAL = () 1%:1%, (∆) 1.5%:1.5%, () 2%:2% Figure 8. Release profiles of ibuprofen in acidic solution (open symbols) and phosphate buffer solution (closed sym- bols) from IPN beads prepared using SCMX: SAL = 1.5%: 1.5% and gelling for 0.5 h in different concentration of AlCl3 solution. Key: () 2% w/v, (∆) 4% w/v, () 8% w/v. Maximum SEM = 0.81 (n = 4)  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 15 was increased during the preparation of beads, the release of drug increased in both the dissolution media. Statisti- cal analysis in terms of ANOVA-1 also confirmed this phenomenon as the AUCs in acidic medium increased and t50%, t80% decreased and AUCs increased in PB solu- tion significantly. This unusual release behaviour could be explained in the following way. When the IPN beads were prepared with higher concentration of AlCl3, a thick outer gel layer might have been formed along the periph- ery of the beads. The thicker outer gel layer provided higher diffusional resistance to further influx of Al3+ ions resulting in the formation of inhomogeneous gel beads and less densely cross-linked matrix in the core of the beads. During dissolution study, once the outer thick gel layer swelled, quick drug release occurred from the beads. At lower concentration of AlCl3, Al3+ ions diffuse more uniformly into the beads and form homogenous gel beads resulting in slow drug release. 3.5.4 Effect of Gelation Time The derived properties obtained from drug release pro- files (Table 3) indicated that increase in gelation time decreased the drug release appreciably. The higher the gelation time, the greater is the cross-linking density and rigidity of the matrix which resulted in a fall in drug re- lease. 3.5.5 Effect of Drug Load The effect of drug load on the release dynamics of IBP was studied using IPN beads prepared using 3% w/v total polymer concentration (SCMX:SAL in a weight ratio 1:1) and gelling for 0.5 h in 2% w/v AlCl3 solution, and the results are shown in Figure 9. Increase in drug load from 20 to 60% w/w of total polymer decreased the drug re- lease in both the dissolution media. Generally, higher drug load provides higher concentration gradient be- tween the drug in the dosage form and the external dis- solution medium and results in faster drug release. The release of a drug is governed not only by drug diffusion through the polymeric network but also by the relaxa- tional process of the polymer on solvent penetration. Low drug load in IPN beads forms larger pore fraction resulting in higher swelling and consequently faster drug release. On the other hand, at higher drug load, larger crystalline domain of drug is formed in the beads. This causes reduction as well as shrinkage of pores of the ma- trix and results in fall in drug release. Decrease in drug release with increase in drug load from various IPN beads have been reported [5,18,22-23]. 3.5.6 Release Kinetics Drug release from a swellable matrix primarily depends on the degree of gelation, hydration, chain relaxation, and erosion of polymer. To understand the mode of drug transport through the IPN beads, the release data were fitted to the classical power law expression [24] Mt/Mα = Ktn where Mt and Mα are, respectively, the amount of drug released at time t and at infinite time, K represents a con- stant incorporating structural and geometrical character- istics of the dosage forms, n denotes the diffusion expo- nent indicative of the mechanism of drug release. Values of n ranging from 0.45 to 0.5 indicate Fickian or diffu- sion controlled release, values of n ranging from 0.5 to 0.89 indicate non-Fickian or anomalous release, and val- ues of n ranging from 0.89 to 1.0 indicate Case-II trans- port mechanism. By applying least squares method to release data, the values of n were estimated and have been shown in Table 4 along with the correlation co-efficient (r2). The results indicate that drug release in acidic medium followed non-Fickian mechanism and in PB solution drug release occured following case-II tran- sport mechanism. When the swelling data of drug-free IPN beads were fitted to the above power law expression, it was found that swelling in acidic medium took place following the non-Fickian mechanism and that in PB solu- tion followed Case-II transport mechanism (Table 4). Table 3. Effect of gelation time on derived properties of drug release in different dissolution media from IPN beads prepared using different polymer concentration and gelling in 2% w/v AlCl3 solution for different periods of time In acidic solution In phosphate buffer solution Gelation time 0.5 h Gelation time 2 h Gelation time 0.5 h Gelation time 2 h SCMX%: SAL% AUC (% mg · min/ml) (Mean ± SD, n = 4) AUC (% mg · min/ml) (Mean ± SD, n = 4) t50% (min) (Mean ± SD, n = 4) t80% (min) (Mean ± SD, n = 4) AUC (% mg · min/ml) (Mean ± SD, n = 4) t50% (min) (Mean ± SD, n = 4) t80% (min) (Mean ± SD, n = 4) AUC (% mg · min/ml) (Mean ± SD, n = 4) 1%:1% 970.1 ± 22.63 885.4 ± 56.31 77.5 ± 7.86111.1 ± 11.5313151.5 ± 31.4694.8 ± 5.20 143.5 ± 15.21 14336.1 ± 25.63 1.5%:1.5% 702.2 ± 27.15 729.2 ± 35.50 94.3 ± 10.45148.1 ± 9.4511117.7 ± 20.46108.5 ± 8.75174.1 ± 10.11 12553.9 ± 27.75 2%:2% 565.5 ± 13.36 574.5 ± 46.21 119.7 ± 12.61176.4 ± 14.639230.4 ± 26.81132.4 ± 6.31209.6 ± 8.65 10564.5 ± 14.02  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 16 Table 4. Kinetic data (n) and correlation coefficient (r2) of (A) drug release and (B) swelling of IPN beads prepared by gelling in 2% w/v AlCl3 solution for 0.5 h In acidic solution In phosphate buffer solution SCMX:SAL n r2 n r2 1%:1% 0.73 0.997 1.26 0.989 1.5%: 1.5% 0.69 0.996 1.28 0.994 A 2%:2% 0.87 0.993 1.30 0.989 1%:1% 0.69 0.877 1.40 0.996 1.5%:1.5% 0.64 0.935 1.33 0.982 B 2%:2% 0.80 0.907 1.23 0.923 Figure 9. Effect of drug load on release of ibuprofen in acidic solution (open symbols) and phosphate buffer solu- tion (closed symbols) from IPN beads prepared using SCMX:SAL = 1.5%:1.5% and gelling for 0.5 h in 2% AlCl3 solution. Key: drug load, () 20% w/v, (∆) 40% w/v, () 60%w/v of total polymer. Maximum SEM = 1.27 (n = 4) 4. Conclusions SCMX-SAL interpenetrating network beads were pre- pared by inotropic gelation method using Al3+ ions as cross-linking agent for both the polymers. Formation of IPN structure was verified by FTIR analysis and the ab- sence of drug-polymer interaction in IPN beads was con- firmed by FTIR, XRD, and DSC analysises. DEE of IPN beads were found to be reasonably high (91.15 to 99.50%) and was not affected by formulation variables except the gelation time, the increase of which tended to decrease DEE. While the release of IBP decreased in both acidic (pH 1.2) and PB solution (pH 6.8) with increase in total polymer concentration, gelation time, and drug load, the drug release increased in both the media with increase in the concentration of AlCl3. However, all the formulations showed considerably low release in acidic medium and the release followed non-Fickian transport mechanism due to poor swelling of the beads. Complete drug release was achieved in PB solution (pH 6.8) at different periods of time depending on the formulation variables and the release followed case II transport process due to swelling and erosion of the beads. The results of the study indicate that high drug-loaded IPN beads can be prepared using SCMX and SAL by ionotropic gelation process and could be used to minimize the release of IBP in acidic medium and to modulate the drug release in PB solution (pH 6.8). REFERENCES [1] M. Changez, K. Burugapalli, V. Koul and V. Chowdary, “The Effect of Composition of Poly (Acrylic Ac- id)-Gelatin Hydrogel on Gentamycin Sulphate Release in Vitro,” Biomaterials, Vol. 24, No. 4, 2003, pp. 527-536. [2] A. R. Kulkarni, K. M. Soppimath, T. M. Aminabhavi and W. E. Rudzinski, “In-Vitro Release Kinetics of Ce- fadroxyl Loaded Sodium Alginate Interpenetrating Net- work Beads,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 51, No. 2, 2001, pp. 127-133. [3] T. T. Hsieh, K. H. Hsieh, G. P. Simon and C. Tiu, “Inter- penetrating Polymer Networks of 2-Hydroxylethyl Meth- acrylate Terminated Polyurethanes and Urethanes,” Po- lymer, Vol. 40, No. 11, 1999, pp. 3153-3163. [4] A. K. Bajpai, J. Bajpai and S. Shukla, “Water Sorption through Semi-Interpenetrating Polymer Network with Hydrophilic and Hydrophobic Chains,” Reactive and Functional Polymer s, Vol. 50, No. 1, 2002, pp. 9-21. [5] A. Roy, A. K. Bajpai and J. Bajpai, “Designing Swellable Beads of Alginate and Gelatin for Controlled Release of Pesticide (Cypermethrin),” Journal of Macromolecular Science, Part A, Vol. 46, No. 9, 2009, pp. 847-859. [6] P. Aslani and R. A. Kennedy, “Studies on Diffusion in Alginate Gels 1. Effect of Cross-Linking with Calcium or Zinc Ions on Diffusion of Acetaminophen,” Journal of Controlled Release, Vol. 42, No. 1, 1996, pp. 75-82. [7] T. L. Bowersock, H. HogenEsch, M. Suckow, P. Gui- mond, S.Martin, D. Borie, S.Torregrosa, H. Park and K.Park, “Oral Vaccination of Animals with Antigens En- capsulated in Alginate Microspheres,” Vaccine, Vol. 17, No. 13-14, 1999, pp. 1804-1811. [8] M. L. Gonzalez-Rodriguez, M. A. Holgado, C. San- chez-Lafuente, A. M. Rabasco and A. Finni, “Alginate/ Chitosan Particulate Systems for Sodium Diclofenac Re- lease,” International Journal of Pharmaceutics, Vol. 232, No. 1-2, 2002, pp. 225-234. [9] A. Halder, S. Maiti and B. Sa, “Entrapment Efficiency and Release Characteristics of Polyethyleneimine-Treated or Untreated Calcium Alginate Beads Loaded with Pro- pranolol,” International Journal of Pharmaceutics, Vol. 302, No. 1-2, 2005, pp. 84-94. [10] N. P. Desai, A. Sojomihardjo, Z. Yao, N. Ron and P. Soon-Shiong, “Interpenetrating Polymer Networks of Al- ginate and Polyethylene Glycol for Encapsulation of Is-  Development and Evaluation of a New Interpenetrating Network bead of Sodium Carboxymethyl Xanthan and Sodium Alginate for Ibuprofen Release Copyright © 2010 SciRes. PP 17 lets of Langerhans,” Journal of Microencapsulation, Vol. 17, No. 6, 2000, pp. 677-690. [11] S. G. Kumber and T. M. Aminabhavi, “Preparation and Characterisation of Interpenetrating Network Beads of Poly (Vinyl Alcohol)-Grafted-Poly (Acrylamide) with Sodium Alginate and their Controlled Release Character- istics of Cypermethrin Pesticides,” Journal of Applied Polymer Science, Vol. 84, No. 3, 2002, pp. 552-560. [12] Y. H. Liu, H.-F. Lian, C.-K. Chung, M.-C. Chen and H. -W. Sung, “Physically Cross-Linked Alginate/N,O-Carbo- xymethyl Chitosan Hydrogels with Calcium for Oral De- livery of Protein Drugs,” Biomaterials, Vol. 26, No. 14, 2005, pp. 2105-2213. [13] B. Sa and M. Setty, “Novel Gel Microbeads Based on Natural Polysaccharides,” Indian Patent, No. 224992, 31 October 2008. [14] S. Maiti, S. Roy, B. Mondal, S. Sarkar and B. Sa, “Car- boxymethyl Xanthan Microparticles as a Carrier for Pro- tein Delivery,” Journal of Microencapsulation, Vol. 24, No. 8, 2007, pp. 743-756. [15] S. Ray, S. Maiti and B. Sa, “Preliminary Investigation on the Development of Diltiazem Resin Complex Loaded Carboxymethyl Xanthan Beads,” AAPS PharmSciTech, Vol. 9, No. 1, 2008, pp. 295-301. [16] C. M. Setty, S. S. Sahoo and B. Sa, “Alginate-Coated Alginate-Polyethyleneimine Beads for Prolonged Release of Furosemide in Simulated Intestinal Fluid,” Drug De- velopment and Industrial Pharmacy, Vol. 31, No. 4-5, 2005, pp. 435-446. [17] P. Piyakulawat, N. Praphairaksit, N. Chantarasiri and N. Muangsin, “Preparation and Evaluation of Chitosan/Car- Rageenan Beads for Controlled Release of Sodium Di- clofenac,” AAPS PharmSciTech, Vol. 8, No. 4, 2007, pp. 1-10. [18] A. P. Rokhade, S. A. Agnihotri, S. A. Patil, N. N. Mal- likarjuna, P. V. Kulkarni and T. M. Aminabhavi, “Semi- Interpenetrating Polymer Network Microspheres of Gela- tin and Sodium Carboxymethyl Cellulose for Controlled Release of Ketorolac Tromethamine,” Carbohydrate Po- lymers, Vol. 65, No. 3, 2006, pp. 243-252. [19] S. A. Agnihotri and T. M. Aminabhavi, “Development of Novel Interpenetrating Network Gellan Gum-Poly (Vinyl Alcohol) Hydrogel Microspheres for the Controlled Re- lease of Carvedilol,” Drug Development and Industrial Pharmacy, Vol. 31, No. 6, 2005, pp. 491-503. [20] K. S. Soppimath, A. R. Kulkarni and T. M. Aminabhavi, “Chemically Modified Polyacrylamide-G-Guar Gum Based Crosslinked Anionic Microgels as PH Sensitive Drug Delivery Systems: Preparation and Characteriza- tion,” Journal of Controlled Release, Vol. 75, No. 3, 2001, pp. 331-345. [21] R. V. Kulkarni and B. Sa, “Novel PH-Sensitive Inter- penetrating Network Hydrogel Beads of Carboxymethyl Cellulose-(Polyacryl Amide-Grafted-Alginate) for Con- trolled Release of Ibuprofen: Preparation and Characteri- zation,” Current Drug Delivery, Vol. 5, No. 4, 2008, pp. 256-264. [22] S. Benita, A. Barkai and Y. U. Pathak, “Effect of Drug Loading Extent on the in Vitro Release Kinetic Behaviour of Nifedipine from Polyacrylate Microspheres,” Journal of Controlled Release, Vol. 12, No. 3, 1990, pp. 213-222. [23] K. S. Soppimath, A. R. Kulkarni and T. M. Aminabhavi, “Controlled Release of Antihypertensive Drug from the Interpenetrating Network Poly (Vinyl Alcohol)-Guar Gum Hydrogel Microspheres,” Journal of Biomaterials Science Polymer Edition, Vol. 11, No. 1, 2000, pp. 27-43. [24] P. L. Ritger and N. A. Peppas, “A Simple Equation for Description of Solute Release. II Fickian and Anomalous Release from Swellable Devices,” Journal of Controlled Release, Vol. 5, No. 1, 1987, pp. 37-42. |

