Paper Menu >>
Journal Menu >>
 World Journal of Nano Science and Engineering, 2012, 2, 126-133 http://dx.doi.org/10.4236/wjnse.2012.23016 Published Online September 2012 (http://www.SciRP.org/journal/wjnse) Electron Emission of Graphene-Diamond Hybrid Films Using Paraffin Wax as Diamond Seeding Source Deepak Varshney1,2*, Chitturi Venkateswara Rao3, Fr a nk Men do za1,2, Kenneth Perez1,2, Maxime J.-F. Guinel1,2,3, Yasuyuki Ishikawa2,3, Brad R. Weiner2,3, Gerardo Morell1,2 1Department of Physics, University of Puerto Rico, San Juan, USA 2Institute of Functional Nanomaterials, University of Puerto Rico, San Juan, USA 3Department of Chemistry, University of Puerto Rico, San Juan, USA Email: *deepvar20@gmail.com Received June 6, 2012; revised July 3, 2012; accepted July 20, 2012 ABSTRACT We present a scalable, reproducible and economic process for the fabrication of diamond and diamond-graphene hybrid films using paraffin wax as a seeding source for diamond. The films were characterized using Raman spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and electron energy loss spectroscopy (EELS). Raman spectra show the characteristic band of diamond at 1332 cm−1 and the D, G, and 2D bands of graphene at 1360, 1582 and 2709 cm−1, respectively. Electron microscopy confirms the microcrystalline nature of the diamond films with crystal size in the range of 0.5 μm to 1.0 μm, and the hybrid film consists of microcrystalline diamond at- tached to thin, semi-transparent graphene flakes. The graphene-diamond hybrid films exhibit a turn-on field of about 3.6 V/μm with a prolonged current stability of at least 135 h. Keywords: Graphene; Chemical Vapor Deposition; Diamond Films; Electron Field Emission 1. Introduction The exceptional properties (e.g. high thermal conduc- tivity, high strength, and lowest compressibility) of dia- mond make it an ideal material for many applications, such as in cutting tools, coatings for magnetic disks, op- tical switches [1], electronic devices [2], spintronics de- vices, and quantum computational components [3] and more. Various surface pre-treatment methods have been used to enhance the nucleation density of diamond [4-8]. All these reported methods lead to surface alteration or damage (usually by the formation of nano-scale pits, scratches and defects concentration) and even contami- nate the substrate. Furthermore, surface pre-treatment cannot be easily applied to substrates with complex ge- ometries, and is often incompatible with industrial appli- cations because of the increased cost. It is therefore im- portant to nucleate diamond on an electrically conducting surface without the need for surface pre-treatment while maintaining high nucleation density and good adhesion. Isolated graphene, a one-atom-thick layer of graphitic carbon, was first reported in 2004 [9], and has been dubbed as the wonder material. It opens new opportuni- ties because of its unique electrical and mechanical properties. Its potential applications are many, including solar cells, field-emission devices and batteries [10,11]. The field emission properties of diamond [12], amor- phous carbon [13], and vertically aligned multi- and sin- gle-walled carbon nanotubes [14,15] for cold cathode applications have been extensively investigated but the field emission properties of graphene films have only been reported very recently [16,17]. These studies on the field emission from various carbon materials focused on the importance of the field enhancement factor according to the morphological and topographical structure of the cathode surface. The excellent electrical and thermal properties of graphene and diamond respectively can be combined into one hybrid material for advanced applica- tions. We have recently reported the direct nucleation of diamond from kitchen wrap-polyethylene [18]. In the present work, we report a new method for diamond nu- cleation using paraffin wax as the seeding material. Gra- phenediamond hybrid films were also fabricated using the same seeding source for diamond. The method here- by described is simple, cost effective and handy. The graphene-diamond hybrid films show excellent field emission characteristics with a low turn-on field and are able to withstand for longer times compared to graphene alone. *Corresponding author. C opyright © 2012 SciRes. WJNSE  D. VARSHNEY ET AL. 127 2. Experimental For the synthesis of diamond films, paraffin wax (ca. 5 g) was melted on a hot plate and a small portion of the melt was transferred onto a copper disk substrate (14 mm di- ameter) with the help of a glass dropper and allowed to cool to room temperature. It was then introduced into the HFCVD chamber. This wax coated copper substrate was exposed to a gas mixture consisting of 0.3% methane in hydrogen with 10% methane and 99.7% of hydrogen for 4 h, at a constant pressure of 20 Torr and a total gas flow of 100 sccm. This gaseous mixture was activated by a rhenium filament (8 cm in length and 0.5 mm in diameter) positioned 9 mm above the substrate. The temperature of the substrate and the filament was kept at approximately 450˚C and 2500˚C, respectively. The graphene-diamond hybrid films were fabricated using a similar procedure. An amount of 0.02 g of pre- synthesized graphene powder [19] was mixed with the paraffin melt to obtain a homogeneous suspension which was then transferred onto a copper disk substrate and subjected to the same reaction conditions as those used in the fabrication of pure diamond films. Method of Characterization The Raman scattering spectra were obtained on a triple monochromator (ISAJ-Y Model T 64000) with appro- ximately 1 cm−1 resolution using the 514.5 nm line of Ar laser. Scanning electron microscopy (SEM) images were recorded using a JEOL JSM-7500F SEM also equipped with a transmission electron detector. The samples were analyzed using a Carl Zeiss LEO 922 energy filtered transmission electron microscope (TEM) operated at 200 kV. The field emission I-V characteristics of the fabricated films were measured using a custom built system [20, 21]. 3. Results and Discussion 3.1. Raman Analysis The First, The paraffin wax crystallites act as nucleation sites for diamond growth in the presence of hydro-carbon radicals and atomic hydrogen in the CVD system. When the melted paraffin wax cools below its equilibrium melting temperature, its crystallization via self-nuclea- tion becomes thermodynamically favorable and it acts as a seeding source for diamond nucleation. Also, in the present case, the use of non-carbide forming substrate is important as it provides a surface with minimal surface interactions, facilitating the formation of crystallites in the lowest energy diamond shape [22,23]. The first order Raman spectrum of the wax-coated substrate is shown in the inset of Figure 1(a). It clearly shows distinctive Raman contributions at 1063 cm−1, 1133 cm−1, 1296 cm−1, and 1441 cm−1. These are attri- buted to C-C (carbon-carbon) stretching and CH2 and CH3 deformation [24], as would be expected given the straight-chain hydrocarbon structure of wax. Figure 1(a) shows the Raman spectrum recorded from the film obtained from the CVD process of wax coated copper substrate. The intense band at 1332.6 cm−1 is charac- teristic of diamond [25]. The disappearance of the typical vibrational modes of paraffin wax and the appearance of band at 1332.6 cm−1 clearly indicates the growth of diamond films from paraffin wax. The fabrication of diamond film using paraffin wax as a seeding source follows the same mechanism as reported in case of kitchen wrap polyethylene [18]. The Raman spectrum of the pre-synthesized graphene is shown in inset of Figure 1(b) showing the presence of D band corresponding to the disorder induced in sp2 car- bon [26] and a band around 1582 cm−1 corresponds to G-band [27]. The position and shape of the prominent 2D peak in the Raman spectrum can be used to clearly dis- tinguish the number of layers in the graphene sample [28]. Broad 2D band observed at 2685 ± 5 cm−1 in dif- ferent regions indicate that the material is few layered (5 - 10 layers) graphene. The Raman spectrum of the gra- phene-diamond hybrid material in the range 1000 - 3000 cm−1 is shown in Figure 1(b). The band at 1582 cm−1 corresponds to the G band arising from the in-plane vi- brations of sp2 carbon atoms of graphene, or the doubly degenerate (TO and LO) phonon mode (E2 g symmetry) at the Brillouin zone center. The band at 1332.6 cm−1 remains present and is that of diamond (sp3 C). A shoul- der at 1360 cm−1 is identified as the D-band which is attributed to the disorder-induced in sp2-bonded carbon [29]. The 2D band at 2709 cm−1 originates from a two phonon double resonance Raman process and is closely related to the band structure of graphene and is used to confirm the presence of graphene [30]. The Raman spec- trum in the range of 2600 - 2900 cm−1 is shown in the inset of Figure 1(b). It clearly depicts two bands at 2709 and 2845 cm−1 corresponding to the 2D and S3 peaks for graphene [31]. The 2D band, which is a characteristic of graphene, is used to determine the number of layers of graphene in the sample [32]. The broad and less intense 2D band seen here indicates that the hybrid material con- sists of few layered graphene. 3.2. Scanning Electron Microscopy The morphology of the diamond and graphene-diamond hybrid films was ascertained by scanning electron mi- croscopy (SEM). Figure 2(a) shows an overview SEM image of the diamond film composed of microcrystalline diamond. The inset of Figure 2(a) shows a diamond rystal with well-defined facets having a size of c Copyright © 2012 SciRes. WJNSE 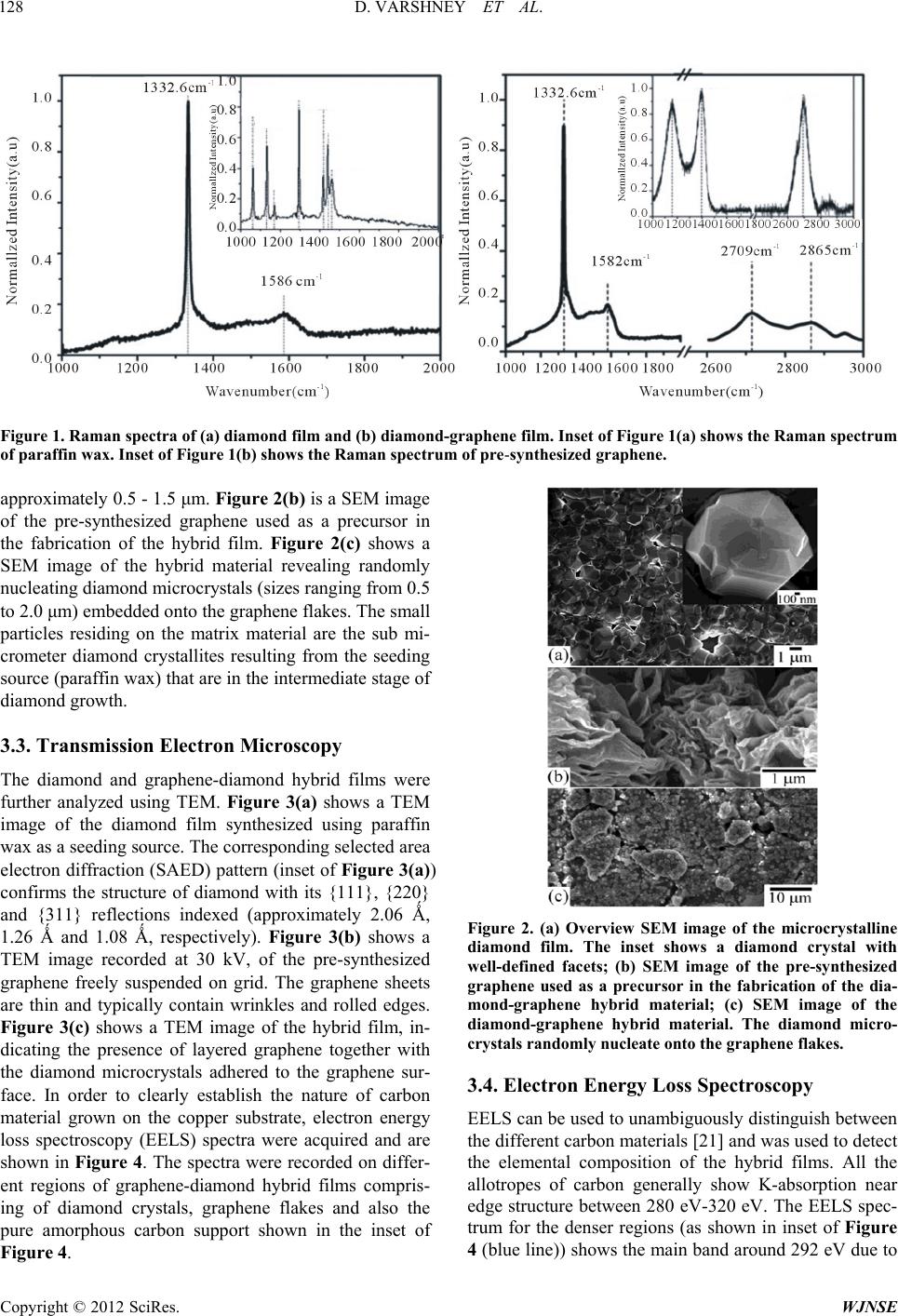 D. VARSHNEY ET AL. Copyright © 2012 SciRes. WJNSE 128 Figure 1. Raman spectra of (a) diamond film and (b) diamond-graphene film. Inset of Figure 1(a) shows the Raman spectrum of paraffin wax. Inset of Figure 1(b) shows the Raman spectrum of pre-synthesi zed graphene. approximately 0.5 - 1.5 μm. Figure 2(b) is a SEM image of the pre-synthesized graphene used as a precursor in the fabrication of the hybrid film. Figure 2(c) shows a SEM image of the hybrid material revealing randomly nucleating diamond microcrystals (sizes ranging from 0.5 to 2.0 μm) embedded onto the graphene flakes. The small particles residing on the matrix material are the sub mi- crometer diamond crystallites resulting from the seeding source (paraffin wax) that are in the intermediate stage of diamond growth. 3.3. Transmission Electron Microscopy The diamond and graphene-diamond hybrid films were further analyzed using TEM. Figure 3(a) shows a TEM image of the diamond film synthesized using paraffin wax as a seeding source. The corresponding selected area electron diffraction (SAED) pattern (inset of Figure 3(a)) confirms the structure of diamond with its {111}, {220} and {311} reflections indexed (approximately 2.06 Ǻ, 1.26 Ǻ and 1.08 Ǻ, respectively). Figure 3(b) shows a TEM image recorded at 30 kV, of the pre-synthesized graphene freely suspended on grid. The graphene sheets are thin and typically contain wrinkles and rolled edges. Figure 2. (a) Overview SEM image of the microcrystalline diamond film. The inset shows a diamond crystal with well-defined facets; (b) SEM image of the pre-synthesized graphene used as a precursor in the fabrication of the dia- mond-graphene hybrid material; (c) SEM image of the diamond-graphene hybrid material. The diamond micro- crystals randomly nucleate onto the graphe ne flakes. Figure 3(c) shows a TEM image of the hybrid film, in- dicating the presence of layered graphene together with the diamond microcrystals adhered to the graphene sur- face. In order to clearly establish the nature of carbon material grown on the copper substrate, electron energy loss spectroscopy (EELS) spectra were acquired and are shown in Figure 4. The spectra were recorded on differ- ent regions of graphene-diamond hybrid films compris- ing of diamond crystals, graphene flakes and also the pure amorphous carbon support shown in the inset of Figure 4. 3.4. Electron Energy Loss Spectroscopy EELS can be used to unambiguously distinguish between the different carbon materials [21] and was used to detect the elemental composition of the hybrid films. All the allotropes of carbon generally show K-absorption near edge structure between 280 eV-320 eV. The EELS spec- trum for the denser regions (as shown in inset of Figure blue line)) shows the main band around 292 eV due to 4 ( 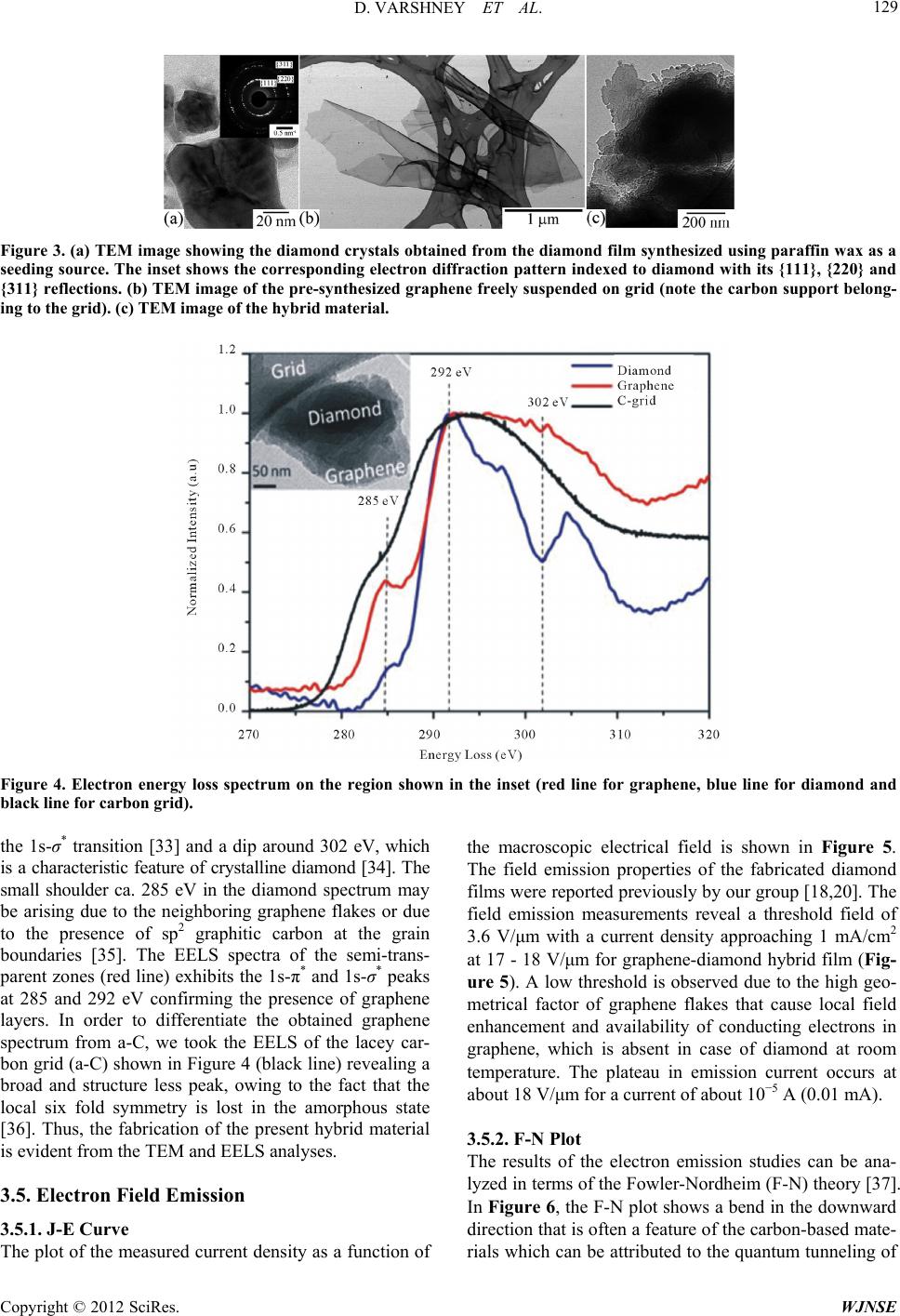 D. VARSHNEY ET AL. 129 Figure 3. (a) TEM image showing the diamond crystals obtained from the diamond film synthesized using paraffin wax as a seeding source. The inset shows the corresponding electron diffraction pattern indexed to diamond with its {111}, {220} and {311} reflections. (b) TEM image of the pre-synthesized graphene freely suspended on grid (note the carbon support belong- ing to the grid). (c) TEM image of the hybrid material. Figure 4. Electron energy loss spectrum on the region shown in the inset (red line for graphene, blue line for diamond and black line for carbon grid). the 1s-σ* transition [33] and a dip around 302 eV, which is a characteristic feature of crystalline diamond [34]. The small shoulder ca. 285 eV in the diamond spectrum may be arising due to the neighboring graphene flakes or due to the presence of sp2 graphitic carbon at the grain boundaries [35]. The EELS spectra of the semi-trans- parent zones (red line) exhibits the 1s-π* and 1s-σ* peaks at 285 and 292 eV confirming the presence of graphene layers. In order to differentiate the obtained graphene spectrum from a-C, we took the EELS of the lacey car- bon grid (a-C) shown in Figure 4 (black line) revealing a broad and structure less peak, owing to the fact that the local six fold symmetry is lost in the amorphous state [36]. Thus, the fabrication of the present hybrid material is evident from the TEM and EELS analyses. 3.5. Electron Field Emission 3.5.1. J-E Curve The plot of the measured current density as a function of the macroscopic electrical field is shown in Figure 5. The field emission properties of the fabricated diamond films were reported previously by our group [18,20]. The field emission measurements reveal a threshold field of 3.6 V/μm with a current density approaching 1 mA/cm2 at 17 - 18 V/μm for graphene-diamond hybrid film (Fig- ure 5). A low threshold is observed due to the high geo- metrical factor of graphene flakes that cause local field enhancement and availability of conducting electrons in graphene, which is absent in case of diamond at room temperature. The plateau in emission current occurs at about 18 V/μm for a current of about 10−5 A (0.01 mA). 3.5.2. F- N Plot The results of the electron emission studies can be ana- lyzed in terms of the Fowler-Nordheim (F-N) theory [37]. In Figure 6, the F-N plot shows a bend in the downward direction that is often a feature of the carbon-based mate- rials which can be attributed to the quantum tunneling of Copyright © 2012 SciRes. WJNSE 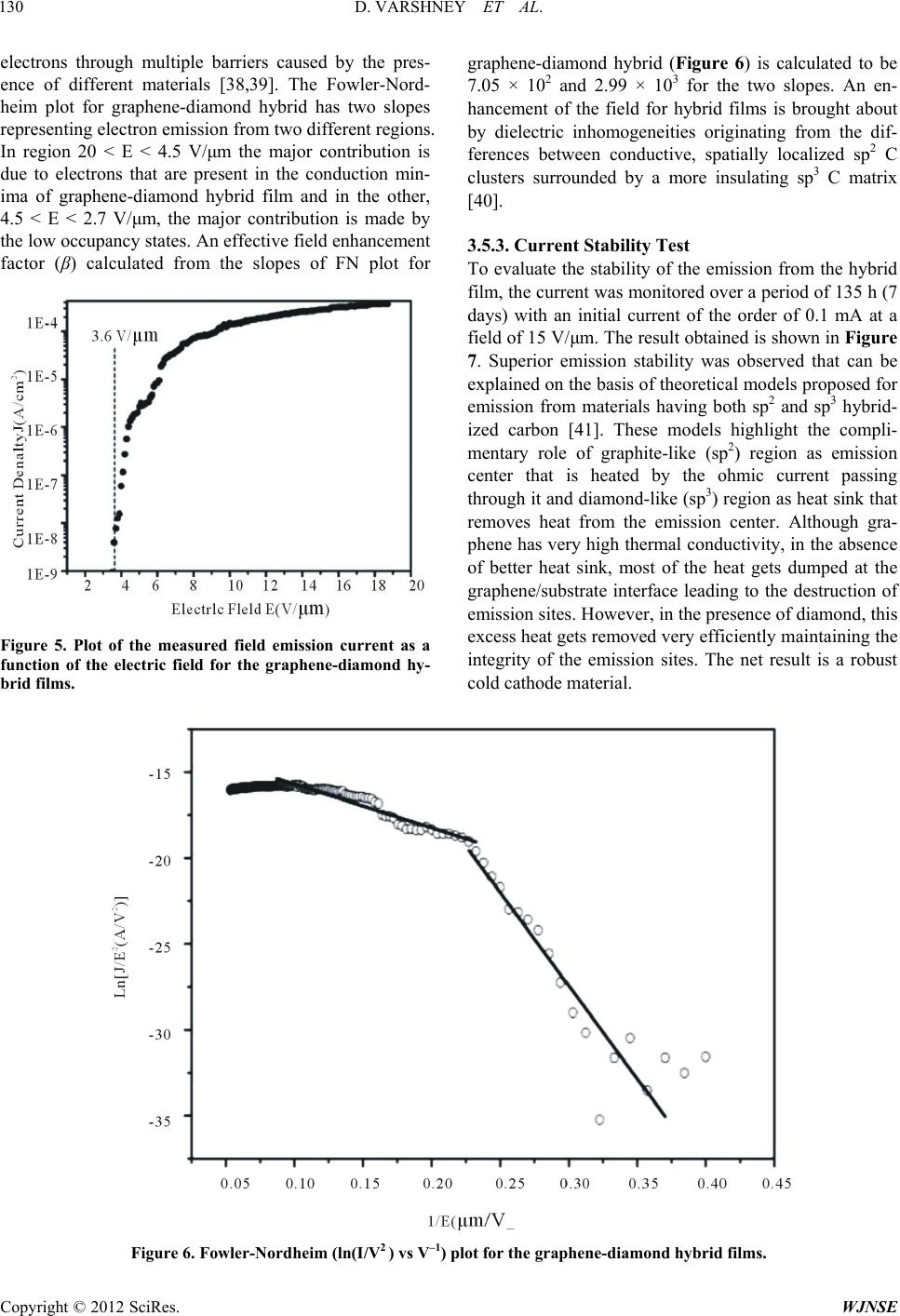 D. VARSHNEY ET AL. 130 electrons through multiple barriers caused by the pres- ence of different materials [38,39]. The Fowler-Nord- heim plot for graphene-diamond hybrid has two slopes representing electron emission from two different regions. In region 20 < E < 4.5 V/μm the major contribution is due to electrons that are present in the conduction min- ima of graphene-diamond hybrid film and in the other, 4.5 < E < 2.7 V/μm, the major contribution is made by the low occupancy states. An effective field enhancement factor (β) calculated from the slopes of FN plot for Figure 5. Plot of the measured field emission current as a function of the electric field for the graphene-diamond hy- brid films. graphene-diamond hybrid (Figure 6) is calculated to be 7.05 × 102 and 2.99 × 103 for the two slopes. An en- hancement of the field for hybrid films is brought about by dielectric inhomogeneities originating from the dif- ferences between conductive, spatially localized sp2 C clusters surrounded by a more insulating sp3 C matrix [40]. 3.5.3. Current Stability Test To evaluate the stability of the emission from the hybrid film, the current was monitored over a period of 135 h (7 days) with an initial current of the order of 0.1 mA at a field of 15 V/μm. The result obtained is shown in Figure 7. Superior emission stability was observed that can be explained on the basis of theoretical models proposed for emission from materials having both sp2 and sp3 hybrid- ized carbon [41]. These models highlight the compli- mentary role of graphite-like (sp2) region as emission center that is heated by the ohmic current passing through it and diamond-like (sp3) region as heat sink that removes heat from the emission center. Although gra- phene has very high thermal conductivity, in the absence of better heat sink, most of the heat gets dumped at the graphene/substrate interface leading to the destruction of emission sites. However, in the presence of diamond, this excess heat gets removed very efficiently maintaining the integrity of the emission sites. The net result is a robust cold cathode material. Figure 6. Fowler-Nordheim (ln(I/V2 ) vs V–1) plot for the graphene-diamond hybrid films. Copyright © 2012 SciRes. WJNSE  D. VARSHNEY ET AL. 131 Figure 7. Plot of the emission current density versus time for a period of 135 h recorded from the graphene-diamond hybrid film. The diamond-graphene hybrid film of this study shows better emission stability as compared to that of pure gra- phene films or graphene composite thin films (Table 1). In order to confirm the reproducibility of field emission characteristics, I-V measurements were performed at several locations. The observed field emission character- istics were almost independent of the locations, due to nearly uniform fabrication over the whole substrate sur- face. 4. Conclusion Diamond and graphene-diamond hybrid films were fab- ricated by the HFCVD technique using paraffin wax as a seeding material for diamond. The characteristic bands of diamond and graphene were evident from Raman spec- troscopy. Microscopic images reveal the presence of mi- cron-sized diamond crystals in the fabricated films. The graphene-diamond hybrid films exhibits a turn-on field of 3.8 V/μm with an emission current density approach- ing 0.3 mA/cm2 at a field of 20 V/μm. The hybrid film exhibits good emission current stability of 135 h. The present study provides an inexpensive fabrication ap- proach towards a graphene-diamond hybrid films that exhibit excellent field emission properties and can be a competitive candidate for efficient field emitter material. 5. Acknowledgements This research was made possible by funds from the In- stitute for Functional Nanomaterials (NSF Grant # 1002410), PR NASA EPSCoR (NASA Cooperative Agreement # NNX07AO30A and NNX08BA48A), and Table 1. Comparison of emission characteristics of present graphene-diamond hybrid film with existing literature. Field ehan cment factor Turn-on Field (V/μm) Current stability (minutes) Reference Graphene N/A 1.35 - 6.20 5500 [21] Graphene paper 3.2 × 103 - 4.1 × 103 1.6 - 2.4 1500 - 1700 [42] Graphene on polymer film 1.0 × 103 1.8 >180 [43] Graphene-diamond Hybrid material 5.9 × 102 - 1.1 × 104 3.8 >8100 Present work Copyright © 2012 SciRes. WJNSE  D. VARSHNEY ET AL. 132 PR DOE EPSCoR (DOE Grant # DEFG02-08ER46526). D.V would like to acknowledge the help of Mr. W. Pérez for the Raman spectroscopy measurements. Microscopes are operated at the Nanoscopy facility at UPR. REFERENCES [1] K. Baba, Y. Aikawa, N. Shohata, H. Yoneda and K. I. Ueda, “Photo Conductive Switch with CVD Diamond Films by Ultraviolet Light Pulse,” NEC Research De- velopment, Vol. 36, No. 3, 1995, pp. 369-375. doi:10.1016/0925-9635(95)00448-3 [2] L. S. Pan and D. R. Kania, “Diamond: Electronic Proper- ties and Applications: Kluwer Academic Publishers,” Kluwer Academic Publishers, Boston, 1995. [3] D. A. David, R. Epstein and R. Hanson, “The Diamond Age of Spintronics,” Scientific American, Vol. 297, No. 4, 2007, pp. 84-91. doi:10.1038/scientificamerican1007-84 [4] B. R. Stoner, G. H. M. Ma, S. D. Wolter and J. T. Glass, “Characterization of Bias-Enhanced Nucleation of Dia- mond on Silicon by Invacuo Surface Analysis and Transmission Electron Microscopy,” Physical Review B, Vol. 45, No. 19, 1992, pp. 11067-11084. doi:10.1103/PhysRevB.45.11067 [5] K. Uppireddi, O. Resto, B. R. Weiner and G. Morell, “Iron Oxide Nanoparticles Employed as Seeds for the Induction of Microcrystalline Diamond Synthesis,” Na- noscale Research Letters, Vol. 3, No. 2, 2008, pp. 65-70. doi:10.1007/s11671-008-9117-5 [6] S. Biwu and W. M. L. Leo, “Apparatus and Method for Nucleation and Deposition of Diamond Using Hot-Fila- ment Dc Plasma,” US Patent No. 6161499, 2000. [7] G. Alfred, L. C. Robert and J. D. Patrick, “Grown Dia- mond Mosaic Separation,” US Patent No. 2007/0017437, 2007. [8] Q. Wang, R. Schliesing, H. Zacharias and V. Buck, “En- hancement of Diamond Nucleation on Silicon Substrates in Pulsed Laser Assisted Hot Filament CVD,” Applied Surface Science, Vol. 138-139, 1999, pp. 429-433. doi:10.1016/S0169-4332(98)00436-X [9] K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, “Electric Field Effect in Atomically Thin Carbon Films,” Science, Vol. 306, No. 5696, 2004, pp. 666-669. doi:10.1126/science.1102896 [10] X. Wang, L. J. Zhi and K. Mullen, “Transparent, Conduc- tive Graphene Electrodes for Dye-Sensitized Solar Cells,” Nano Letters, Vol. 8, No. 1, 2008, pp. 323-327. doi:10.1021/nl072838r [11] G. Eda and M. Chhowalla, “Graphene-Based Composite Thin Films for Electronics,” Nano Letters, Vol. 9, No. 2, 2009, pp. 814-818. doi:10.1021/nl8035367 [12] K. Okano, S. Koizumi, S. R. P. Silva and G. A. J. Amara- tunga, “Low-Threshold Cold Cathodes Made of Nitro- gen-Doped Chemical-Vapour-Deposited Diamond,” Na- ture, Vol. 381, No. 9, 1996, pp. 140-141. doi:10.1038/381140a0 [13] G. A. J. Amaratunga and S. R. P. Silva, “Nitrogen Con- taining Hydrogenated Amorphous Carbon for Thin-Film Field Emission Cathodes,” Applied Physics Letters, Vol. 68, No. 18, 1996, pp. 2529-2531. doi:10.1063/1.116173 [14] W. A. De Heer, A. Chatelain and D. Ugarte, “A Carbon Nanotube Field-Emission Electron Source,” Science, Vol. 270, No. 5239, 1995, pp. 179-180. doi:10.1126/science.270.5239.1179 [15] R. B. Rakhi, A. L. M. Reddy, M. M. Shaijumon, K. Sethupati and S. Ramaprabhu, “Electron Field Emitters Based on Multi-Walled Carbon Nanotubes Decorated with Nanoscale Metal Clusters,” Journal of Nanoparticle Research, Vol. 10, No. 1, 2008, pp. 179-189. doi:10.1007/s11051-007-9240-8 [16] G. Eda, H. E. Unalan, N. Rupesinghe, G. A. J. Amara- tunga and M. Chhowalla, “Field Emission from Graphene Based Composite Thin Films,” Applied Physics Letters, Vol. 93, No. 23, 2008, pp. 233502-233505. doi:10.1063/1.3028339 [17] A. Malesevic, R. Kemps, A. Vanhulsel, M. P. Chowdhury, A. Volodin and C. V. Haesendonck, “Field Emission from Vertically Aligned Few-Layer Graphene,” Journal of Applied Physics, Vol. 104, No. 8, 2008, pp. 084301- 084305. doi:10.1063/1.2999636 [18] D. Varshney, V. I. Makarov, P. Saxena, M. J.-F. Guinel, A. Kumar, J. F. Scott, B. R. Weiner and G. Morell, “Electron Emission from Diamond Films Seeded Using Kitchen-Wrap Polyethylene,” Journal of Physics D: Ap- plied Physics, Vol. 44, No. 8, 2010, pp. 085502-085508. doi:10.1088/0022-3727/44/8/085502 [19] K. Uppireddi, C. V. Rao, Y. Ishikawa, B. R. Weiner and G. Morell, “Temporal Field Emission Current Stability and Fluctuations from Graphene Films,” Applied Physics Letters, Vol. 97, No. 6, 2010, pp. 62106-62108. doi:10.1063/1.3474800 [20] G. Morell, A. Gonzalez-Berrios, B. R. Weiner and S. Gupta, “Synthesis, Structure, and Field Emission Proper- ties of Sulfur-Doped Nanocrystalline Diamond,” Journal of Materials Science: Materials in Electronics, Vol. 17, No. 6, 2006, pp. 443-451. [21] A. González-Berríos, F. Piazza and G. Morell, “Effects of Adsorbates on Field Emission Reproducibility of Sul- fur-Incorporated Nanocomposite Carbon Films,” Journal of Vacuum Science & Technology B, Vol. 25, No. 2, 2005, pp. 318-323. [22] Y. Wang, M. Rafailovich, J. Sokolov, D. Gersappe, T. Araki, Y. Zou, A. D. L. Kilcoyne, H. Ade, G. Marom and A. Lustiger, “Substrate Effect on the Melting Tempera- ture of Thin Polyethylene Films,” Physical Review Let- ters, Vol. 96, No. 2, 2006, pp. 028303-028306. doi:10.1103/PhysRevLett.96.028303 [23] Z. Stephen and D. Cheng, “Materials Science: Polymer Crystals Downsized,” Nature, Vol. 448, No. 157, 2007, pp. 1006-1007. doi:10.1038/4481006a [24] M. Zheng and W. Du, “Phase Behavior, Conformations, Thermodynamic Properties, and Molecular Motion of Multicomponent Paraffin Waxes: A Raman Spectroscopy Copyright © 2012 SciRes. WJNSE  D. VARSHNEY ET AL. 133 Study,” Vibrational Spectroscopy, Vol. 40, No. 2, 2006, pp. 219-224. [25] J. E. Field, “The Properties of Diamond,” Academic Press, London, 1979, pp. 281-324. [26] D. Varshney, C. V. Rao, M. J.-F. Guinel, Y. Ishikawa, B. R. Weiner and G. Morell, “Free Standing Graphene- Diamond Hybrid Films and Their Electron Emission Properties,” Journal of Applied Physics, Vol. 110, No. 4, 2011, pp. 044324-0443249. doi:10.1063/1.3627370 [27] M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. G. Cancado, A. Jorio and R. Saito, “Studying Disorder in Graphite-Based Systems by Raman Spectroscopy,” Phy- sical Chemistry Chemical Physics, Vol. 9, No. 11, 2007, pp. 1276-1290. doi:10.1039/b613962k [28] A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, “Raman Spectrum of Graphene and Graphene Layers,” Physical Review Letters, Vol. 97, No. 18, 2006, pp. 187401-187403. doi:10.1103/PhysRevLett.97.187401 [29] C. Thomsen and S. Reich, “Double Resonant Raman Scattering in Graphite,” Physical Review Letters, Vol. 85, No. 25, 2000, pp. 5214-5217. [30] L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S. Dresselhaus, “Raman Spectroscopy in Grapheme,” Phys- ics Reports, Vol. 473, No. 5-6, 2009, pp. 51-87. doi:10.1016/j.physrep.2009.02.003 [31] J. Wang, M. Zhu, R. A. Outlaw, X. Zhao, D. M. Manos and B. C. Holloway, “Synthesis of Carbon Nanosheets by Inductively Coupled Radio-Frequency Plasma Enhanced Chemical Vapor Deposition,” Carbon, Vol. 42, No. 14, 2004, pp. 2867-2872. doi:10.1016/j.carbon.2004.06.035 [32] A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, “Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition,” Nano Letters, Vol. 9, No. 1, 2009, pp. 30-35. doi:10.1021/nl801827v [33] D. Varshney, B. R. Weiner and G. Morell, “Growth and Field Emission Study of a Monolithic Carbon Nano- tube/Diamond Composite,” Carbon, Vol. 48, No. 18, 2010, pp. 3353-3358. doi:10.1016/j.carbon.2010.05.025 [34] R. Arenal, P. Bruno, D. J. Miller, M. Bleuel, J. Lal and D. M. Gruen, “Diamond Nanowires and the Insulator-Metal Transition in ultrananocrystalline Diamond Films,” Phy- sical Review B, Vol. 75, No. 19, 2007, pp. 195431- 195441. doi:10.1103/PhysRevB.75.195431 [35] J. E. Butler and A. V. Sumant, “The CVD of Nanodia- mond Materials,” Chemical Vapor Deposition, Vol. 14, No. 7-8, 2008, pp. 145-160. doi:10.1002/cvde.200700037 [36] S. Muto, T. Tanabe, A. Hirota, M. Rubel, V. Philipp and T. Maruyama, “TEM and EELS Characterization of Car- bon Dust and Co-Deposited Layers from the TEXTOR Tokamak,” Journal of Nuclear Materials, Vol. 307-311, 2002, pp. 1289-1293. doi:10.1016/S0022-3115(02)01118-2 [37] R. H. Fowler and L. Nordheim, “Electron Emission in Intense Electric Fields,” Proceedings of the Royal Society of London. Series A, Vol. 119, No. 781, 1928, pp. 173- 181. [38] C. Ducati, E. Barborini, P. Piseri, P. Milani and J. Robertson, “Influence of Cluster-Assembly Parameters on the Field Emission Properties of Nanostructured Carbon Films,” Journal of Applied Physics, Vol. 92, No. 9, 2002, pp. 5482-5489. doi:10.1063/1.1512969 [39] M. Liao, Z. Zhang, W. Wang and K. Liao, “Field-Emis- sion Current from Diamond Film Deposited on Molyb- denum,” Journal of Applied Physics, Vol. 84, No. 2, 1998, pp. 1081-1084. doi:10.1063/1.368096 [40] J. D. Carey, R. D. Forrest and S. R. P. Silva, “Origin of Electric Field Enhancement in Field Emission from Amorphous Carbon Thin Films,” Applied Physics Letters, Vol. 78, No. 16, 2001, pp. 2339-2341. doi:10.1063/1.1366369 [41] E. D. Eidelman and A. Y. Vul, “The Strong Thermoelec- tric Effect in Nanocarbon Generated by the Ballistic Pho- non Drag of Electrons,” Journal of Physics: Condensed Matter, Vol. 19, No. 26, 2007, pp. 266210-266221. [42] J. Liu, B. Zeng, Z. Wu, J. Zhu and X. Liu, “Improved Field Emission Property of Graphene Paper by Plasma Treatment,” Applied Physics Letters, Vol. 97, No. 3, 2010, pp. 033109-033111. doi:10.1063/1.3467042 [43] V. P. Verma, S. Das, I. Lahiri and W. Choi, “Large-Area Graphene on Polymer Film for Flexible and Transparent Anode in Field Emission Device,” Applied Physics Let- ters, Vol. 96, No. 20, 2010, pp. 203108-203110. Copyright © 2012 SciRes. WJNSE |

