 Vol.2, No.7, 726-731 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.27090 Copyright © 2010 SciRes. OPEN ACCESS Review on dermatomycosis: pathogenesis and treatment Deepika T. Lakshmipathy, Krishnan Kannabiran* Division of Biomolecules and Genetics,School of Biosciences and Technology, VIT University, Vellore, India; *Corresponding Author: kkb@vit.ac.in Received 24 February 2010; revised 25 March 2010; accepted 3 April 2010. ABSTRACT Dermatophytes, a group of keratinophilic fungi thriving on the keratin substrate are the etio- logical agents responsible for causing cutane- ous infections. Dermatophytosis is currently treated with the commercially available topical and oral antifungal agents in spite of the exist- ing side effects. Treatment of these cutaneous infections with secondary metabolites produced by marine microorganisms is considered as a novel approach. For many years these organ- isms have been explored with the view of de- veloping antibacterial, antifungal, antiviral, anti- cancer and antiparasitic drugs. Exploring the unexplored aspect of actinobacteria for devel- oping antidermatophytic drugs is a novel at- tempt which needs further investigation. Keywords: Trichophyton; Microsporum; Epidermophyton; Tinea Infections; Novel Approach; Actinobacteria 1. INTRODUCTION 1.1. Dermatophytes Infections pertaining to mankind particularly those af- fecting the keratinized tissues are of serious concerns worldwide and are increasing on a global scale. Derma- tomycoses are infections of the skin, hair and nail caused as a result of colonization of the keratinized layers of the body. This colonization is brought about by the organ- isms belonging to the three genera namely Trichophyton, Microsporum and Epidermophyton [1,2]. Infection may also be caused rarely by the members of the genus Can- dida and by non-dermatophytic moulds belonging to the genera Fusari um, Scopulariopsis and Aspergillus [3,4]. Interestingly dermatophytic infections are predominant in the tropical and subtropical countries; especially in the developing countries like India where the hot climate and humid weather is favourable to the acquisition and maintenance of the disease [5,6] and currently no race is totally free from dermatophytoses. 2. ECOLOGICAL CLASSIFICATION In the course of evolution these pathogens have devel- oped host specificity. This host specificity is ascribed to the difference in the composition of keratin [7]. Based on their host specificity dermatophytes are classified into three ecological groups namely geophiles (soil), an thro- pophiles (man) and zoophiles (animals) [8]. The geo- philic dermatophytes are generally saprophytic and de- rive nutrients from keratinous substrates. Rarely these pathogens cause infection in animals and man. Examples include Trichophyton ajelloi, Trichophyton terrestre, Microsporum fulvum, Micropsorum gypseum, Micro- sporum cookie and Epidermophyton stockdaleae [9-11]. Zoophiles are pathogens with only one animal host and grow as saprophytes on animal materials. Zoophiles are also reported to infect human beings. Human beings acquire the infection from infected animals. Examples include Trichophyton simii (monkeys), Trichophyton mentagrophytes (rodents), Trichophyton equinum (hor- ses), Microsporum canis (cats) and Micropsorum nan- num (pigs) [12,13]. The primary hosts of anthropophilic species are hu- man beings but they may also cause infection in animals. Transmission of infection is from man to man. Examples include Trichophyton rubrum, Trichophyton kanei, Trichophyton schoenleini, Trichophyton concentricum, Trichophyton tonsurans, Micropsorum gypseum, audou- inii, Microsporum ferrugineum and Epidermophyton floccosum [14,15]. 2.1. Trichophyton The genus Trichophyton includes 24 species. The colo- nies on agar media are powdery, velvety or waxy. The predominant spore type is micro conidia with sparse 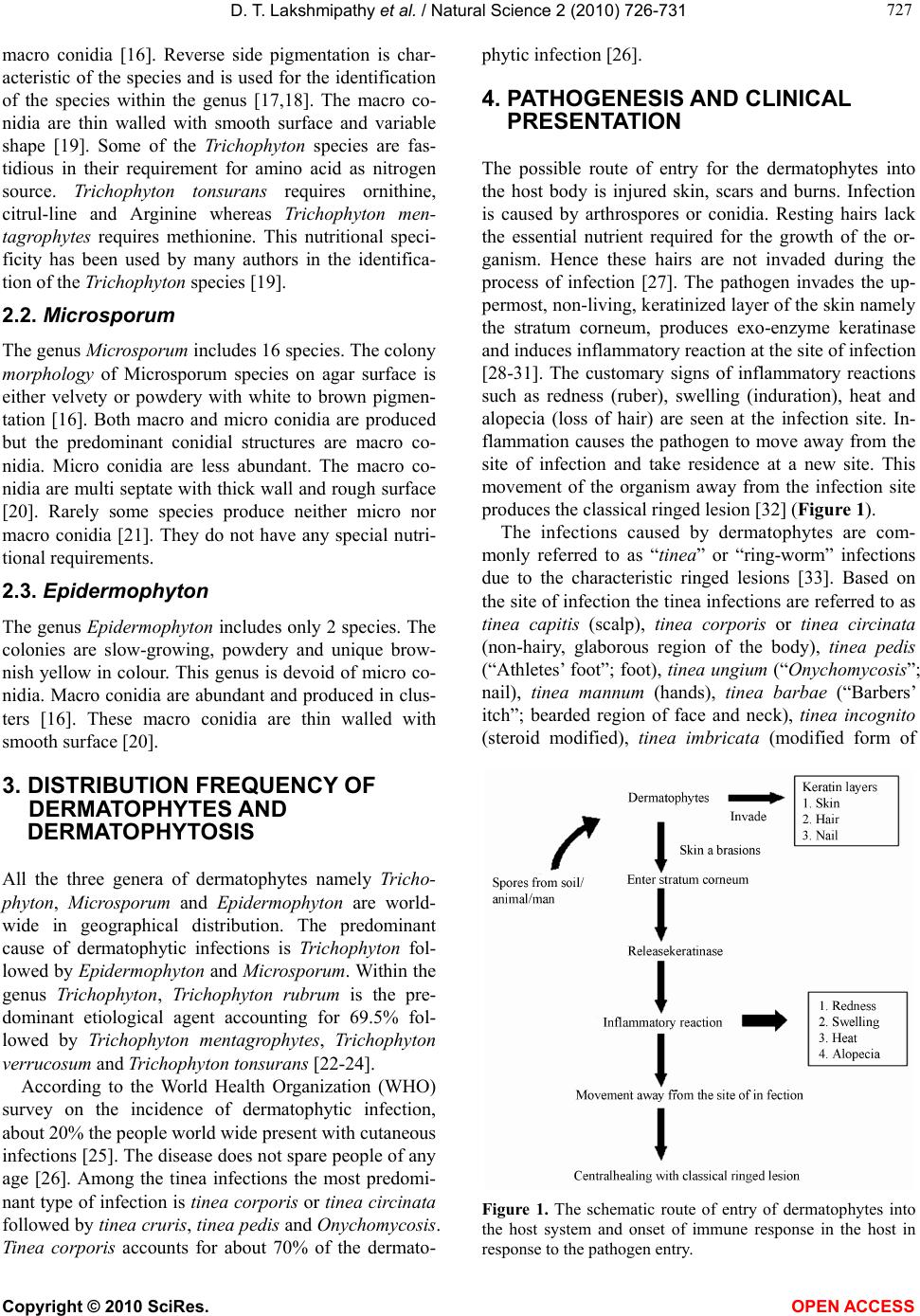 D. T. Lakshmipathy et al. / Natural Science 2 (2010) 726-731 Copyright © 2010 SciRes. OPEN ACCESS 727 727 macro conidia [16]. Reverse side pigmentation is char- acteristic of the species and is used for the identification of the species within the genus [17,18]. The macro co- nidia are thin walled with smooth surface and variable shape [19]. Some of the Trichophyton species are fas- tidious in their requirement for amino acid as nitrogen source. Trichophyton tonsurans requires ornithine, citrul-line and Arginine whereas Trichophyton men- tagrophytes requires methionine. This nutritional speci- ficity has been used by many authors in the identifica- tion of the Trichoph yton species [19]. 2.2. Microsporum The genus Microsporum includes 16 species. The colony morphology of Microsporum species on agar surface is either velvety or powdery with white to brown pigmen- tation [16]. Both macro and micro conidia are produced but the predominant conidial structures are macro co- nidia. Micro conidia are less abundant. The macro co- nidia are multi septate with thick wall and rough surface [20]. Rarely some species produce neither micro nor macro conidia [21]. They do not have any special nutri- tional requirements. 2.3. Epidermophyton The genus Epidermophyton includes only 2 species. The colonies are slow-growing, powdery and unique brow- nish yellow in colour. This genus is devoid of micro co- nidia. Macro conidia are abundant and produced in clus- ters [16]. These macro conidia are thin walled with smooth surface [20]. 3. DISTRIBUTION FREQUENCY OF DERMATOPHYTES AND DERMATOPHYTOSIS All the three genera of dermatophytes namely Tricho- phyton, Microsporum and Epidermophyton are world- wide in geographical distribution. The predominant cause of dermatophytic infections is Trichophyton fol- lowed by Epidermophyton and Microsporum. Within the genus Trichophyton, Trichophyton rubrum is the pre- dominant etiological agent accounting for 69.5% fol- lowed by Trichophyton mentagrophytes, Trichophyton verrucosum and Trichophyton tonsurans [22-24]. According to the World Health Organization (WHO) survey on the incidence of dermatophytic infection, about 20% the people world wide present with cutaneous infections [25]. The disease does not spare people of any age [26]. Among the tinea infections the most predomi- nant type of infection is tinea corporis or tinea circinata followed by tinea cruris, tinea pedis and Onychomycosis. Tinea corporis accounts for about 70% of the dermato- phytic infection [26]. 4. PATHOGENESIS AND CLINICAL PRESENTATION The possible route of entry for the dermatophytes into the host body is injured skin, scars and burns. Infection is caused by arthrospores or conidia. Resting hairs lack the essential nutrient required for the growth of the or- ganism. Hence these hairs are not invaded during the process of infection [27]. The pathogen invades the up- permost, non-living, keratinized layer of the skin namely the stratum corneum, produces exo-enzyme keratinase and induces inflammatory reaction at the site of infection [28-31]. The customary signs of inflammatory reactions such as redness (ruber), swelling (induration), heat and alopecia (loss of hair) are seen at the infection site. In- flammation causes the pathogen to move away from the site of infection and take residence at a new site. This movement of the organism away from the infection site produces the classical ringed lesion [32] (Figure 1). The infections caused by dermatophytes are com- monly referred to as “tinea” or “ring-worm” infections due to the characteristic ringed lesions [33]. Based on the site of infection the tinea infections are referred to as tinea capitis (scalp), tinea corporis or tinea circinata (non-hairy, glaborous region of the body), tinea pedis (“Athletes’ foot”; foot), tinea ungium (“Onychomy cosi s”; nail), tinea mannum (hands), tinea barbae (“Barbers’ itch”; bearded region of face and neck), tinea incognito (steroid modified), tinea imbricata (modified form of Figure 1. The schematic route of entry of dermatophytes into the host system and onset of immune response in the host in response to the pathogen entry.  D. T. Lakshmipathy et al. / Natural Science 2 (2010) 726-731 Copyright © 2010 SciRes. OPEN ACCESS 728 tinea corporis), tinea gladiatorium (common among wrestlers’) and tinea cruris (“Jocks’ itch”; groin) [34]. 5. IMMUNITY BEHIND DERMATOPHYTIC INFECTION Host immune response to the invading pathogen is re- sponsible for the clinical manifestations. The fungal pathogens induce both immediate hypersensitivity as well as cell mediated or delayed type hypersensitivity. Acquired resistance to the infection may also result from dermatophytic infection. The fungal growth is restricted by the inflammatory reactions produced as a result of infection with dermatophytes [35]. 6. TREATMENT Despite the advancements of science and technology, surprisingly the development of novel and efficient an- tifungal drugs is still lagging behind due to the very fact that fungi are also eukaryotic and have mechanisms similar to human beings [36]. Hence it becomes very difficult to develop an antifungal agent that is more spe- cific in targeting the fungi alone without any damage to human beings. For successful treatment of the disease, proper diagnosis of the disease is always essential. The treatment is chosen based on the infection site, etiological agent and penetration ability of the drug. The penetration ability and retention in the site of infection of the agent determines its efficacy and frequency of utility. Since the dermatophytes reside in the stratum corneum especially within the keratinocytes, the anti- fungal agents should have a good penetrating ability. The duration of treatment mainly depends on the type of in- fection and symptom. Generally a two-three week treat- ment is required for skin lesions whereas four-six week for feet inflammation [37]. Earlier, dermatomycosis was treated with the tradi- tional topical antifungal agent Whitfield’s ointment, a combination of 3% salicylic acid and 6% benzoic acid in a Vaseline base [38]. Next came into existence, Castel- lani’s paint, a deep red coloured liquid, specifically ef- fective against tinea ungium. Another topical preparation of importance was a combination of silver nitrate and tincture iodine. This preparation was effective against multiple lesions [39]. In general the dosage depends on the severity of infection, location and the efficacy of the drug. These topical preparations were applied twice a day for 2-3 weeks to prevent relapse condition. In addi- tion to the above mentioned topical agents, tolnaftate, undecylenic acid, haloprogin, triacetin were in use for the treatment of dermatophytosis [39]. The year 1970 saw the release of Miconazole, the first in the line of azoles group. Since then many more were subsequently synthesized and added to this list during the same period. These antimycotic drugs belonged to the Azoles class of antifungal drugs. The major target of the azoles unlike the other antifungal agents is the cytochrome P450 en- zyme [40] (Figure 2). Based on the number of nitrogen atoms the azoles derivatives are classified into 2 groups as imidazoles and triazoles [16]. Imidazoles include miconazole (1970), clotrimazole, ketaconazole (1978), econazole, bifonazole, tioconazole and oxiconazole [41]. The chronological order of the imidazoles to get FDA approval in United States is as follows miconazole (1974), econazole (1982), keta- conazole (1985), oxiconazole (1988) and clotrimazole (1993) [42]. The most recent drug to clear the FDA trials (2003) is Sertaconazole, a novel imidazole with broad spectrum antifungal activity [43]. In general the imida- zoles exhibit side effects such as anorexia, constipation, headache, hepatitis, pruritis, exanthema and inhibition of synthesis of steroid hormone [44]. Triazoles include flu- conazole, voriconazole, itraconazole (1980), posacona- zole, teraconazole and ravuconazole. In comparison to the imidazoles, the triazoles exhibit lesser degree of side effects which includes nausea, dizziness and gastrointes- tinal upset [45]. Allylamines and benzyl amines were synthesized in the 1980s’. Allylamines include naftifine and terbinafine. Naftifine, terbinafine and benzylamine obtained FDA approval in United States in the year 1988, 1992 and 2001, respectively. The mode of action of these drugs is inhibition of the key enzyme squalene epoxidase, an essential enzyme involved in the synthesis of squa- lene epoxide from squalene [46] (Figure 2). Figure 2. Schematic representation of the site of action of azoles, allylamines and benzyl amines.  D. T. Lakshmipathy et al. / Natural Science 2 (2010) 726-731 Copyright © 2010 SciRes. OPEN ACCESS 729 729 Amorolfine, a morpholine drug targets the ergosterol synthesis similar to the azoles but at a site different from that of the azoles [47]. A new class of antifungal drug called hydroxypyridones became available since the year 2000. Ciclopiroxolamine, the representative drug of this class targets the cell membrane and affects the cell per- meability. Apart from the above mentioned synthetic drugs many drugs such as Pyrrolo [1,2-a] [1,4] benzodi- azepine with less side effects are being synthesized and experimented for treating dermatophytosis [48]. Griseo- fulvin, from Penicillium chrysogenum was isolated in 1930. Its antibacterial and antifungal potential was not fully understood until late 1950s’. It is the first antimy- cotic drug with a microbial origin [49]. Griseofulvin is a narrow spectrum antimycotic drug with fungistatic ac- tivity. It is very effective against all the dermatomycoses. The side effects include headache, nausea, bad taste, skin rash, systemic lupus erythematosus (SLE), porphyria and arthralgia. With all its side effects, griseofulvin still re- mains to be the gold-standard for treating dermatophytic infections [50]. Treatment of cutaneous infection using natural sources is the ongoing research work of many research groups across the globe. Compounds from the plants Psorolea corylifolia [51], Azadirachta indica [52], Melaleuca alternifolia, Melaleuca dissitiflora, Me- laleuca linariifolia [53], Nandina domestica [54], Didis- cus oxeata [55] have been reported to exhibit potential anti-dermatophytic activity. Further confirmation on the activity of these compounds is under investigation. 7. A NOVEL APPROACH TO SOLVE THE PROBLEM More recently the scientific community has turned its attention to secondary metabolites from actinobacteria and its exploitation for various purposes which include therapeutic, environmental and industrial applications. With developing microbial resistance and need for safe and cost-effective antidermatophytic drugs, screening of actinobacteria for potential bioactive secondary metabo- lites becomes indispensible [56]. About 75-80% of the antibiotics that are available in the market are derived from Streptomyces [57]. To the best of our knowledge antidermatophytic secondary metabolite from Strepto- myces rochei AK39 is the first report on antidermato- phytic activity of actinobacteria [58]. Our investigation on the antidermatophytic activity of Streptomyces spp isolated from the saltpan region yielded three potential strains. The morphological, physiological and bioche- mical properties of these three potential isolates namely VITDDK1, VITDDK2 and VITDDK3 have been studied and reported [56,57]. The 16 S rRNA sequence of three strains Streptomyces spp. VITDDK1, Streptomyces spp. VITDDK2 and Streptomyces spp. VITDDK3 was sub- mitted to the GenBank, NCBI under the accession num- bers, GU223091, GU223092 and GU223093 respec- tively. The antidermatophytic activity of these three strains is anticipated to be due to high salt concentration of the environment. Under stress conditions microorgan- isms inhabiting the particular environment is said to produce complex chemicals that can be exploited med- icinally. 8. CONCLUSIONS The management of dermatophytic infections needs personal hygiene, awareness of infection, proper diagno- sis and medication. At present there are a large number of antidermatophytic drugs available commercially. With increasing incidence of fungal infection, microbial resis- tance to the existing drugs, cost and side effects, there is a need for an antifungal drug that can overcome all these limitations. Streptomyces remains to be an unexhausted source of bioactive compounds and a boon to the medi- cal field. Screening of Streptomyces from stressed envi- ronment can be a novel approach for obtaining potential lead molecules for clinical trials and later treatment of dermatomycosis. 9. ACKNOWLEDGEMENTS The authors wish to thank the management of VIT University for pro- viding the facilities to carry out this study and the International Foun- dation for Science (IFS, F 4185-1), Sweden and the Organization for the Prohibition of Chemical Weapons (OPCW), The Hague to one of the author (KK) for providing financial support. REFERENCES [1] Emmons, C.W., Bindford, C.H., Utz, J.P. and Kwon- Chung, K.L. (1977) Dermatophytoses. Medical Mycol- ogy, 3rd Edition, Lea and Febiger, Philadelphia, 117-167. [2] Luilma, A.G., Sidrimb, J.J.C., Domingos, T.M., Cechinel, V.F. and Vietla, S.R. (2005) In vitro antifungal activity of dragon’s blood from Croton urucurana against dermato- phytes. Journal of Ethnopharmacology, 97(2), 409-412. [3] Pinto, E., Pina-Vaz, C., Salgueiro, L., Goncalves, M.J., Costa-de-Oliveira, S., Carlos, C., Palmeira, A., Rodrigues, A. and Martinez-de-Oliveira, J. (2006) Antifungal activ- ity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. Journal of Medi- cal Microbiology, 55(10), 1367-1373. [4] Naveed, A.M., Naeem, R. and Nasiruddin (2009) Non- dermatophyte moulds and yeasts as causative agents in Onychomycosis. Journal of Pakistan Association of Der- matologists, 19(2), 74-78. [5] Rippon, J.W. (1988) The Pathogenic Fungi and Patho- genic Actionmycetes, 3rd Edition. WB Saunders, Phila-  D. T. Lakshmipathy et al. / Natural Science 2 (2010) 726-731 Copyright © 2010 SciRes. OPEN ACCESS 730 delphia, 1988. [6] Rao, A. (1959) Mycotic diseases in India - a critical re- view. Bulletin of School of Tropical Medicine Calcutta, 13-22. [7] Georg, L.K. (1959) Animals ringworm in public health. Diagnosis and Nature, Bulletin, 57 . [8] Rippon, J.W. (1982) Host specificity in dermatophytoses. Proceedings of the Eight Congress of the International Society for Human and Animal Mycology, 28-33. [9] De Vroey, C. (1984) Ecological and epidemiological aspects in dermatophytoses. Zbl Bakt Hyg., 257(2), 234- 239. [10] Baxter, M. and Pearson, R.D. (1969) The occurence of Microsporum nanumas a human pathogen and animal pathogen in New Zealand. New Zealand Journal of Medical Laboratory Technology, 23, 87-90. [11] Connole, M.D. (1990) Review of animal mycoses in Australia. Mycopathologia, 111(3), 133-164. [12] English, M.P. (1972) The epidemiology of animal ring- worm in man. British Journal of Dermatology, 86(3), 78-87. [13] Marples, M.J. (1956) The ecology of Microsporum canis bodin in New Zealand. Journal of Hygiene, 54(3), 378-387. [14] Georg, L.K. (1960) Epidemiology of dermatophytes sources of infection, modes of transmission and epi- demicity. Annals of the New York Academy of Sciences, 89(2-3), 69-77. [15] Kaplan, W. and Gump, R.H. (1958) Ringworm in the dog caused by Trichophyton rubrum. Veterinary Medicine, 53(1), 139-142. [16] Jagdish, C. (1995) Dermatophytoses. Medical Mycology, 1st Etdition, 106-107. [17] Wagner, D.K. and Sohnle, P.G. (1995) Cutaneous de- fenses against dermatophytes and yeasts. Clinical Micro- biology Reviews, 8(3), 317-335. [18] Larone, D.H. (1995) Medically important fungi: A guide to identification, 3rd Edition, American Society for Mi- crobiology. [19] Philpot, C.M. (1977) Use of nutritional tests for the dif- ferentiation of dermatophytes. Sabouraudia, 15(2), 141- 150. [20] Emmons, C.W. (1934) Dermatophytes-natural grouping based on the form of the spores and accessory organs. Arch Dermatol Syphil, 30(3), 337-362. [21] St-Getmain, G. and Summerbell, R. (1996) Identifying filamentous fungi: A clinical laboratory handbook. Star Publishing, Belmont. [22] Chen, B.K. and Friedlander, S.F. (2001) Tinea capitis update: A continuing conflict with an old adversary. Cur- rent Opinion in Pediatrics, 13(4), 331-335. [23] Ciavaglia, M.C., de Carvalho, T.U. and de Souza, W. (1993) Interaction of trypanosoma cruzi with cells with altered glycosylation patterns. Biochemical and Bio- physical Research Communication, 193(2), 718-721. [24] Coloe, S.V. and Baird, R.W. (1999) Dermatophyte infec- tions in Melbourne: Trends from 1961/64 to 1995/96. Pathology, 31(4), 395-397. [25] Marques, S.A., Robles, A.M., Tortorano, A.M., Tuculet, M.A., Negroni, R. and Mendes, R.P. (2000) Mycoses as- sociated with AIDS in the Third World. Medical Mycol- ogy, 38(Suppl. 1), 269-279. [26] Vander Straten, M.R., Hossain, M.A. and Ghannoum, M.A. (2003) Cutaneous Infections dermatophytosis, onychomycosis, and tinea versicolor. Infectious Disease Clinics of North America, 17(1), 87-112. [27] Weirzman, I. and Summerbell, R.C. (1995) The der- matophytes. Clinical Microbiology Reviews, 8(2), 240- 259. [28] Wawrzkiewicz, K., Wolski, T. and Lobarzewski, J. (1991) Screening the keratinolytic activity of dermatophytes in vitro. Mycopathologia, 114(3), 1-8. [29] Lopez-Martinez, R., Manzano-Gayosso, P., Mier, T., Mendez-Tovar, L.J. and Hernandez-Hernandez, F. (1994) Exoenzymes of dermatophytes isolated from acute and chronic tinea. Revista Latinoamericana de Microbiología, 36(1), 17-20. [30] Siesenop, U. and Bohm, H. (1995) Comperative studies on keratinase production of Trichophyton mentagro- phytes strains of animal origin. Mycoses, 38(5-6), 205- 209. [31] Muhsin, T.M., Aubaid, A.H. and Al-Duboon, A.H. (1997) Extracellular enzyme activities of dermatophytes and yeast isolates on solid media. Mycoses, 40(11-12), 465-469. [32] Dahl, M.V. (1994) Dermatophytosis and immune re- sponse. Journal of the American Academy of Dermatol- ogy, 31(3), S34-S41. [33] Theodore, C.W., Brian, G.O., Yvonne, G. and Matthew, R.H. (2008) Generating and testing molecular hypotheses in the dermatophytes. Eukaryotic Cell, 7(8), 1238-1245. [34] Judith, A.W. (2005) Allergy and dermatophytes. Clinical Microbiology Reviews, 30-43. [35] Grappel, S.F., Bishop, C.T. and Blank, F. (1974) Immu- nology of dermatophytes and dermatophytosis. Bacteri- ology Reviews, 38(3) , 222-250. [36] Berdy, J. (1989) The discovery of new bioactive micro- bial metabolites: Screening and identification. In: Bioac- tive Metabolites from Microorganisms, Bushell, M.E., Grafe, U., Eds., Elsevier, Amsterdam, 3-25. [37] Elewski, B.E. and Hazen, P.G. (1989) The superficial mycoses and the dermatophytes. Journal of the American Academy of Dermatology, 21(4), 655-673. [38] Pavithran, K. (1985) Fungal infections of the skin. Der- mato-Venero-Leprology, 77. [39] Pasricha, J.S. (1981) Infections and infestations. Trea t- ment of Skin Diseases, 82(1), 73-74. [40] Elewski, B.E. (1993) Mechanisms of action of systemic antifungal agents. Journal of the American Academy of Dermatology, 28(3), 28-34. [41] Michael, A.P. and Deanna, A.S. (2006) Review of in vitro activity of sertaconazole nitrate in the treatment of super- ficial fungal infections. Diagnostic Microbiology Infec- tious Disease, 56(4), 147-152. [42] Van Cutsem, J.M. and Thienpont, D. (1972) Miconazole, a broad-spectrum antimycotic agent with antibacterial activity. Chemotherapy, 17(1), 392-404. [43] Agut, J., Palacı´n, C., Salgado, J., Casas, E., Sacrista´n, A. and Ortiz, J.A. (1992) Direct membrane-damaging effect of sertaconazole on Candida albicans as a mechanism of its fungicidal activity. Arzneimittelforschung, 42(5A), 721-724. [44] Katsambas, A., Antoniou, C.H., Frangouli, E., Avgerinou, G., Michailidis, D. and Stratigos, J. (1989) A double-  D. T. Lakshmipathy et al. / Natural Science 2 (2010) 726-731 Copyright © 2010 SciRes. OPEN ACCESS 731 731 blind trial of treatment of seborrhoic dermatitis with 2% ketaconazole cream compared with 1% hydrocortisone cream. British Journal of Dermatology, 12(Suppl 14), 353-357. [45] Grant, S.M. and Clissold, S.P. (1989) Itraconazole: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in superficial and systemic mycosis. Drugs, 37(3), 310-344. [46] Wolverton, S.E. (2001) Systemic antifungal drugs. Com- prehensive Dermatologic Drug Therapy, 2nd Edition, Saunders. [47] Andriole, V.T. (2000) Current and future antifungal ther- apy: New targets for antifungal therapy. International Journal of Antimicrobial Agents, 16(3), 317-322. [48] Lieven, M., Jef, V.G., Frans, V.G., Filip, W., Patrick, M., Vic, S., Gilkerson, T., Roger, N., David, C. and Richardsc, R.D. (2005) Pyrrolo[1,2-a][1,4]benzodiazepine: A novel class of non-azole anti-dermatophyte anti-fungal agents. Bioorganic & Medicinal Chemistry Letters, 15(14), 3453-3458. [49] Oxford, A.E., Raistruck, H. and Simonarat, P. (1939) Studies in the biochemistry of microorganisms, Griseo- fulvin CHOCI, a metabolic product of Penicillium gri- seofulvin dierck. Biochemistry, 33(2), 248-252. [50] Gentles, J.C. (1958) Experimental ringworm of guinea pigs: Oral treatment with griseofulvin. Nature, 182(4633), 476-477. [51] Rajendra, P.N., Anandi, C., Balasubramanian, S. and Pugalendi, K.V. (2004) Antidermatophytic activity of ex- tracts from Psoralea corylifolia (Fabaceae) correlated with the presence of a flavonoid compound. Journal of Ethnopharmacology, 91(1), 21-24. [52] Natarajan, V., Venugopal, P.V. and Menon, T. (2003) Effect of Azadirachta indica (Neem) on the growth pat- tern of dermatophytes. Indian Journal of Medical Micro- biology, 21(2), 98-101. [53] Nenoff, P., Haustein, U.F. and Brandt, W. (1996) Anti- fungal activity of essential oil of Melaleuca alternifolia against pathogenic fungi in vitro. Skin Pharmacology, 9(6), 388-394. [54] Vivek, K.B., Jung, I.Y. and Sun, C.K. (2009) Antifungal potential of essential oil and various Organic extracts of Nandina domestica Thunb against skin infectious fungal pathogens. Applied Microbiology and Biotechnology, 83(6), 1127-1133. [55] Helena, G., Sonia, S.F., Ana, I.R. and Rob, V.S. (2004) Antifungal activity of (+) -curcuphenol, a metabolite from the marine sponge didiscus oxeata. Marine Drugs, 2(1), 8-13. [56] Deepika, T.L. and Kannabiran, K. (2009) A morpho- logical, biochemical and biological studies of halophilic Streptomyces sp isolated from saltpan environment. American Journal of Infectious Diseases, 5(3), 207- 213. [57] Deepika, T.L. and Kannabiran, K. (2009) A report on antidermatophytic activity of actinomycetes isolated from Ennore coast of Chennai, Tamil Nadu, India. Inter- national Journal of Integrative Biology, 6(3), 132-136. [58] Vijayakumar, R., Muthukumar, C., Thajuddin, N., Pan- neerselvam, A. and Saravanamuthu, R. (2007) Studies on the diversity of actinomycetes in the Palk Strait re- gion of Bay of Bengal, India. Actinomycetologica, 21(2), 59-65.
|