 Vol.2, No.7, 718-725 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.27089 Copyright © 2010 SciRes. OPEN ACCESS Residues of 862, 921 of VP3 are associated with virulence in infectious bursal disease virus strain Harbin-1 Renmao Li1,2, Haiying Wang1,3, Guangming Huang1, Manfu Zhang1* 1Lab for Animal Molecular Virology, College of Biological Sciences, China Agricultural University, Beijing, China; *Corresponding Author: Manfuzhang@yahoo.com 2Life Sciences and Technology School, Zhanjiang Normal University, Zhanjiang, China 3Department of Biochemistry and Molecular Biology, Peking University Health Center, Beijing, China Received 25 February 2010; revised 28 April 2010; accepted 6 May 2010. ABSTRACT Reverse genetics was used to study the effect of particular amino acids of infectious bursal dis- ease virus (IBDV) on virulence. Using site-di- rected mutagenesis, altering of two amino acids in VP2 (Q253H, A284T) and VP3 (H783Q, V862M, I921V) in the segment A of a Chinese very virulent IBDV field strain Harbin-1, 4 virus mutants in- cluding H253/284, H783/862, H862/921, H921/783 were res- cued. To evaluate the characteristics of the re- covered viruses in vivo, we inoculated 4-week-old chickens with virus mutants and rescued Harbin- 1 (rHarbin-1), analyzed their bursae for patho- logical lesions 4 days postinfection. rHarbin-1 and H783/862, H253/284 caused severe bursal lesion, milder lesion for H862/921, mildest for H921/783. How- ever, H253/284 caused the lowest mortality. The re- sults showed that residue at position Q253, A284 of VP2 and V862, I921 of VP3 gene are involved with virulence, but there is difference between VP2 and VP3’s role in virulence. The ability of 862 and 921 to control virulence in VP3 is stronger than 253 and 284. Keywords: IBDV; VP3; Mutagenesis; Reverse Genetics; Virulence 1. INTRODUCTION Infectious bursal disease (IBD) is a highly contagious disease among young chickens and characterized by the destruction of the bursa of Fabricius. IBD was first de- scribed by Cosgrove [1], but in China the first case was reported in 1979 [2]. Nowadays IBD has spread world- wide and continues to threat the poultry industry. Infec- tious bursal disease virus (IBDV) is the causative agent of the disease, belonging to Avibirnavirus genus of the Birnaviridae family [3]. Europe had experienced the emergence of very virulent infectious bursal disease vi- rus (vvIBDV) which can cause up to 70% flock mortal- ity [4,5]. Meanwhile, vvIBDV infections also have been observed in Asia and in South America [6]. The genome of IBDV consists of two segments of double-stranded RNA (dsRNA), approximately 3.4 kb (segment A) and 2.7 kb (segment B) in length [7]. Seg- ment A contains two partially overlapping open reading frames (ORFs). The larger ORF encodes a polyprotein (1,012 amino acids, 110 kDa) that is autocatalytically cleaved to yield the viral proteins pVP2 (VPX) (48 kDa), VP4 (29 kDa) and VP3 (33 kDa). During virus matura- tion, pVP2 is processed into matured VP2 (41 to 38 kDa), probably resulting from site-specific cleavage of pVP2 by a host cell-encoded protease [8]. The smaller ORF encodes the nonstructural protein VP5 (145 to 149 amino acids, 17 kDa). Segment B encodes VP1 (970 kDa) having putative RNA-dependent RNA polymerase activity [9,10]. This protein is covalently linked to the 5' ends of the genomic RNA segments or present at a free form [11,12]. VP2 and VP3 are the major structural pro- tein of the virion. The VP2 is the major host-protective antigen of IBDV and contains the determinants respon- sible for causing antigenic variation [13-15]. Position 279 and 284 amino acids in the VP2 variable region possibly contribute to virulence of IBDV [16]. Residues 253 and 284 of the VP2 protein of the variant virus are necessary for tissue culture infectivity [17]. The viru- lence and pathogenic-phenotype markers of IBDV reside in VP2 and residues at position 253 (Gln), 279 (Asp) and 284 (Ala) of VP2 are involved in the virulence and *Part of the contents in this article was presented in Shanghai Univer- sity in June of 2009.  R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 719 719 pathogenic phenotype of virulent IBDV [18-20]. How- ever, recent study demonstrated VP2 is not the sole de- terminant of the very virulent phenotype [21]. C-termi- nal part of VP3 may play a decisive role in controlling the virulence [22]. VP3 could play an important role in receptor-mediated virus-cell attachment, which implied that VP3 has relation with virulence [23]. In order to verify if VP3 have molecular determi- nant of virulence for Chinese vvIBDV strain Harbin-1, amino acids in VP3 among Harbin-1, D78 (vaccine strain), TY89 (IBDV serotype II) were aligned, the different amino acids among them were listed in Ta- ble 1. TY89 could not infect B lymphocytes, having no virulence to B lymphocytes, and D78 has mild virulence to B lymphocytes. Based on the result of alignment the amino acids in VP3 that maybe in- volved in virulence could be found. Position 783 and 862 in Harbin-1 have different amino acids from D78, however, position 921 is different from TY89. To prove their role in virulence, position 783, 862 and 921 in VP3 were mutated subsequently to obtain the combination of two points mutation. As a control, position 253 and 284 in VP2 hypervarible region was mutated at the same time. By use of cRNA-based re- verse-genetics system for IBDV [20], four virus mu- tants were recovered. Furthermore, the characteristics of recovered virus in vitro and in vivo were described and the amino acids responsible for virulence. In this paper we report the discovery that residues of 783, 862, 921 of VP3 are associated with virulence of IBDV. 2. MATERIALS AND METHODS 2.1. Virus and Cells The very virulent strain Harbin-1 was kindly given by Harbin Veterinary Research Institute of the Chinese Aca- demy of Agricultural Sciences. Harbin-1 causes 100% morbidity and mortality of specific-pathogen-free (SPF) chickens, the mean infection lethal dose (ILD50) for SPF embryo is 10-4/0.2 ml. Primary bursal cells were derived from 18-day-old embryonated SPF eggs (Merial, Beijing, China) and were grown in Dulbecco’s minimal essential medium (DMEM, Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal calf serum (FCS) and maintained with DMEM with 5% FBS [22]. Transfec- tion experiments were performed on primary bursal cells. All virus mutants including H253/284, H783/862, H862/921, H921/783 and rHarbin-1, Harbin-1 were used as the viruses for challenge at a dose of 1200 pfu per animal via eye and nose drop. 2.2. Construction of Full-Length CDNA Clones Several clones for segment A and segment B of Harbin- 1 were constructed, pGEM-T-HA (coding sequence of segment A clone), pGEM-T-H5′-A (5′ non-coding se- quence of segment A clone), and pGEM-T-H3′A (3′ non-coding sequence of segment A clone), pGEM-T-HB (coding sequence of segment B clone), pGEM-T-H5′B (5′ non-coding sequence of segment B clone), pG EM-T-H3′B (3′ non-coding sequence of segment B clone). All recombinant plasmids were based on pGEM- T (Promega, Madison, WI, USA). There is partly over- lapped area between CR (coding region) clone and NCR (non-coding region) clone for segment A and B. But the overlapped area lack appropriate restriction site, thus fusion PCR was used to ligate the NCR and CR to obtain the full length cDNA clone for segment A and B. Oli- gonucleotides HACR1, HACR2, HANCR1, HAN CR2, HBCR1, HBCR2, HBNCR1, HBNCR2 (Table 2) were adopted for segment A and B. For transcription in vitro, EcoRI site and T7 promoter was introduced into 5′ end of oligonucleotides; XbaI site at 3′ end in segment A and XhoI site at 3′ end in segment B. The fusion PCR prod- uct of segment A and B was ligated into the T-vector (Takara Bio, Dalian, China) to obtain full-length cDNA clone named as pRHA and pRHB respectively. The se- quence of final products was determined by Takara Bio Ltd. 2.3. Site-Directed Mutagenesis Mutations were introduced into the cDNA of segment A of Harbin-1 according to the manufacture’s instruction of QuickChange site-directed mutagenesis kit (Strata- gene, La Jolla, CA, USA) with minor modification. Amino acid residues 253, 284, 783, 862, 921 were lo- cated in large open reading frame of segment A and their Table 1. Different AA in VP3 for Harbin-1 and vaccine strain, serotype II strain. AA site 767 773 783 787 815 862 899 905 921 947 981 990 992 1005 AA site strain strain Harbin-1 S E H S R V D L I K P V T A Harbin-1 D78 S E Q S R M D L I K L A T A D78 TY89 D D R Q K M E P V R P A S T TY89 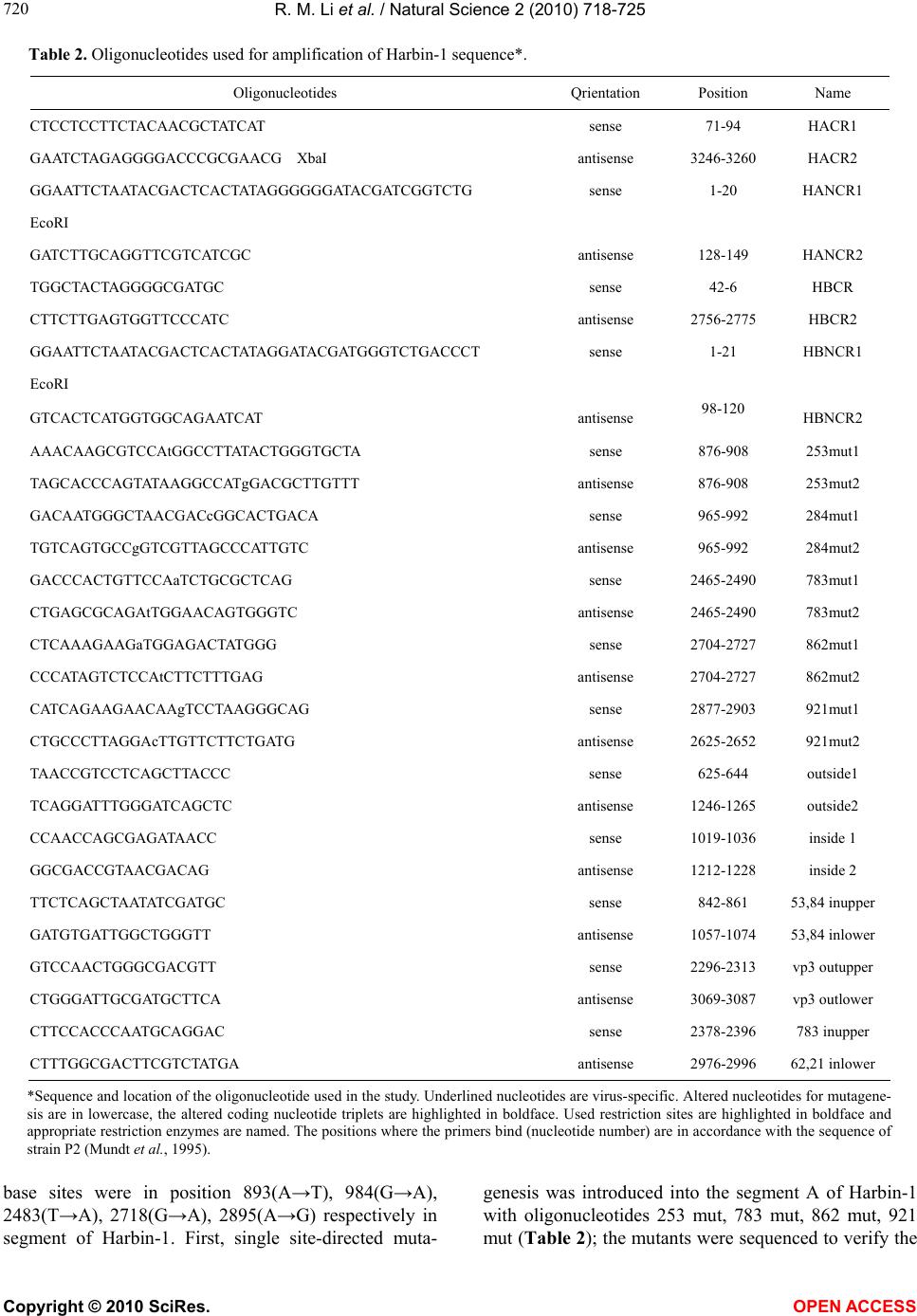 R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 720 Table 2. Oligonucleotides used for amplification of Harbin-1 sequence*. Oligonucleotides Qrientation Position Name CTCCTCCTTCTACAACGCTATCAT sense 71-94 HACR1 GAATCTAGAGGGGACCCGCGAACG XbaI antisense 3246-3260 HACR2 GGAATTCTAATACGACTCACTATAGGGGGGATACGATCGGTCTG sense 1-20 HANCR1 EcoRI GATCTTGCAGGTTCGTCATCGC antisense 128-149 HANCR2 TGGCTACTAGGGGCGATGC sense 42-6 HBCR CTTCTTGAGTGGTTCCCATC antisense 2756-2775 HBCR2 GGAATTCTAATACGACTCACTATAGGATACGATGGGTCTGACCCT sense 1-21 HBNCR1 EcoRI GTCACTCATGGTGGCAGAATCAT antisense 98-120 HBNCR2 AAACAAGCGTCCAtGGCCTTATACTGGGTGCTA sense 876-908 253mut1 TAGCACCCAGTATAAGGCCATgGACGCTTGTTT antisense 876-908 253mut2 GACAATGGGCTAACGACcGGCACTGACA sense 965-992 284mut1 TGTCAGTGCCgGTCGTTAGCCCATTGTC antisense 965-992 284mut2 GACCCACTGTTCCAaTCTGCGCTCAG sense 2465-2490 783mut1 CTGAGCGCAGAtTGGAACAGTGGGTC antisense 2465-2490 783mut2 CTCAAAGAAGaTGGAGACTATGGG sense 2704-2727 862mut1 CCCATAGTCTCCAtCTTCTTTGAG antisense 2704-2727 862mut2 CATCAGAAGAACAAgTCCTAAGGGCAG sense 2877-2903 921mut1 CTGCCCTTAGGAcTTGTTCTTCTGATG antisense 2625-2652 921mut2 TAACCGTCCTCAGCTTACCC sense 625-644 outside1 TCAGGATTTGGGATCAGCTC antisense 1246-1265 outside2 CCAACCAGCGAGATAACC sense 1019-1036 inside 1 GGCGACCGTAACGACAG antisense 1212-1228 inside 2 TTCTCAGCTAATATCGATGC sense 842-861 53,84 inupper GATGTGATTGGCTGGGTT antisense 1057-1074 53,84 inlower GTCCAACTGGGCGACGTT sense 2296-2313 vp3 outupper CTGGGATTGCGATGCTTCA antisense 3069-3087 vp3 outlower CTTCCACCCAATGCAGGAC sense 2378-2396 783 inupper CTTTGGCGACTTCGTCTATGA antisense 2976-2996 62,21 inlower *Sequence and location of the oligonucleotide used in the study. Underlined nucleotides are virus-specific. Altered nucleotides for mutagene- sis are in lowercase, the altered coding nucleotide triplets are highlighted in boldface. Used restriction sites are highlighted in boldface and appropriate restriction enzymes are named. The positions where the primers bind (nucleotide number) are in accordance with the sequence of strain P2 (Mundt et al., 1995). base sites were in position 893(A→T), 984(G→A), 2483(T→A), 2718(G→A), 2895(A→G) respectively in segment of Harbin-1. First, single site-directed muta- genesis was introduced into the segment A of Harbin-1 with oligonucleotides 253 mut, 783 mut, 862 mut, 921 mut (Table 2); the mutants were sequenced to verify the  R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 721 721 resultant mutation; after then the second site-directed mutagenesis was introduced into the first mutation product with oligonucleotides 284 mut, 862 mut, 921 mut, 783 mut (Table 2) to obtain two point mutagenesis clone named p253/284 m, p783/862 m, p862/921 m, p921/783 m respectively. The second mutation products were sequenced by the company (Takara). The obtained muta-genized plasmids with the alteration of two amino acids, Q253H-A284T, H783Q-V862M, V862M-I921V, H783 Q-I921V were used for subsequent transcription in vitro and transfection experiments. 2.4. Transcription and Transfection of Synthetic RNAs The experiment was performed by the protocol de- scribed by Mundt with minor alterations [24]. For tran- scription in vitro, non-mutation and mutated plasmids of segment A and intact segment B were linearized by cleavage with XbaI and XholI respectively. After re- strictive digestion, the products were adjusted to 0.5% SDS and incubated with proteinase K (0.5 mg/ml) for 1 hr at 37. The linearized DNA templates were reco℃v- ered by ethanol precipitation, and 1 μg linearized DNA was used for transcription. Segment A and segment B was transcribed respectively. Transcription reaction mix- ture (30 µl) containing 40 mM Tris-HCl (pH 7.9), 10 mM NaCl, 6 mM MgCl2, 2 mM spermidine, 0.5 mM ATP, 0.5 mM CTP, 0.5 mM UTP, 0.1 mM GTP, 0.25 mM cap analog [m7G(5′)ppp(5′)G] (Promega), 20 units RNasin, 130 units T7 RNA polymerase (Promega), and incubated at 37 for 1 hr. As controls, the transcri℃ption products were treated with either DNase or RNase (Promega). After primary bursal cells were grown to 80% con- fluency in 35-mm dishes, the cells were washed with DMEM (free serum) and incubated at 37 for 10 mi℃n- utes in a CO2 incubator. The process was repeated again. Simultaneously, 60 μl DMEM (free serum) was incu- bated with 6 μl of Lipofectin reagent (Invitrogen, Carls- bad, CA, USA) for 60 min in a polystyrene tube at room temperature to form Lipofectin-DMEM mixture. Syn- thetic RNA transcripts of both segments resuspended in 30 μl of DEPC treated water were mixed and added to the DMEM-Lipofectin mixture, mixed gently and incu- bated on ice for 5 min. After removing the DMEM from the monolayers in the 35-mm dishes and replacing it with fresh 800 μl of DMEM, the nucleic acid-containing mixture was added drop-wise to the cells and swirled gently. After 2 hours of incubation at 37, the mixture ℃ was replaced with DMEM containing 5% FCS (without rinsing the cells), and further incubated at 37 for d℃e- sired time intervals. 2.5. Virus Recovery from cRNA and Detect the Presence of Virus by AC-ELISA, RT-PCR and Plaque Assay Two days after transfection, cells were frozen -thawed and centrifuged at 700 g to remove cellular debris. The supernatant was passaged for 4 times in the primary bur- sal cells, harvesting the cells for ELISA. In order to screen the recombinant virus from many samples AC- ELISA was performed. Each well of 96-wells polysty- rene ELISA plates (Costar, Cambridge, MA, USA) were coated with 100 μl of chicken polyclonal IBDV antise- rum, diluted in PBS at a ratio of 1:4000. After incubation at 37 for 1 hour, the plate was washed three times with ℃ washing buffer (1% Tween 80 in PBS) and each well was blocked by 100 μl of blocking buffer (0.5% gelatin in PBS) at 37 for 0.5 h. After three washes of the plate ℃ with washing buffer, 100 μl sample including positive and negative control was added in duplicate. The plate was then incubated at room temperature for 1 h and washed with washing buffer before 50 μl of MAbs M6 or B29 [25,26]., diluted 1:2500 and 1:1000 in antibody diluent (5% NaCl and 4% BSA in washing buffer) re- spectively, were added to the wells in duplicate. After incubation for 1 h at room temperature, the plate was washed three times with washing buffer. Subsequently, 50 μl of goat anti-mouse IgG-horseradish peroxidase (Sigma) diluted 1:1000 with antibody diluent was added. One hour later at room temperature, the plate was washed three times with washing buffer. After addition 100 μl TMB peroxidase substrate (Kirkegaard and Perry Laboratories Inc., Gaithersburg, MD, USA) and incu- bated at 37 for 15 min, the reaction was stopped by ℃ adding 100 μl 1 M H3PO4. The result was read by an ELISA reader at the optical density at 450 nm (OD450). If OD value of sample is greater than mean OD value plus 3 times standard deviation of negative control sam- ple, then the sample is considered as positive and was stocked at −86 for future use.℃ The titre of virus mutants was determined using plaque assay [27] and prepared for future animal ex- periment. The titre is represented as PFU/ml. IBDV mutants were reversely transcribed using out- side 1 and nested PCR was amplified using outside 1, outside 2, inside 1 and inside 2 primer (Table 2). 2.6. Genetic Stability Analysis If changes in the amino acid sequence occurred during passaging viral RNA of IBDV before challenge, the identity of virus have to be confirmed. The virus mutants were subjected to RT-PCR using oligonucleotides out- side 1 and outside 2 for IBDV with VP2 mutation, VP3 outupper and VP3 outlower for IBDV with VP3 muata- tion before challenge (Table 2). Nested PCR was ampli-  R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 722 fied with 783 inupper and 62, 21 inlower to identify vi- rus with VP3 alteration (Table 2). Cloned PCR frag- ments of IBDV mutants were sequenced and obtained sequences were analyzed with DNAStar. 2.7. Virulence of IBDV Mutants in Young SPF Chickens Forty eight 4-week-old SPF White Leghorn chickens were divided randomly into six groups including posi- tive control group. Chickens were infected via eye and nose drop with total 1200 PFU. Non-inoculated hatch- mates were used as negative controls. During the course of the experiment animals were observed daily for clini- cal signs and mortality. At 4 days p.i., all alive chickens from each group were bled and euthanized. The bursa of each chicken (include alive and dead) was removed, weighed and subdivided into two parts. One part was used for detecting the presence of IBD viral antigen by means of an AC-ELISA and RT-PCR. The second part was fixed in 10% neutral-buffered formalin for histology. Formalin-fixed bursal samples were embedded in paraf- fin, sectioned and stained with haematoxylin and eosin (H&E). Microscopic bursal lesion score (BLS) was de- termined by histopathological analysis of the bursa. BLS was evaluated on a scale of 0 to 5 as follows: 0, no ab- normalities; 1, 1-20%; 2, 21-40%; 3, 41-60%; 4, 61-80%; and 5, 81-100% lymphocyte depletion [28]. 2.8. Detection of Viral Antigen in Bursae after Challenge Bursae were homogenized with homogenizer. The presence of virus in the bursal homogenate was de- tected with AC-ELISA which incorporated Mab 6 recognizing VP2-located epitopes [25]. 3. RESULTS 3.1. Determination of Nucleotide Sequence of Harbin-1 Mutant To establish a reverse genetics system the complete ge- nomic sequence of Harbin-1 mutants was determined. The mutagenized plasmids were obtained with the al- teration of two amino acids, Q253H-A284T, H783Q- V862M, V862M-I921V, H783Q-I921V. 3.2. Rescue of Recombinant Virus from cDNA Primary bursal cells were transfected with synthesized cRNA of mutated segment A and intact segment B by means of lipofectin (Invitrogen). After every transfection, the resultant supernatant was used for RT-PCR and AC- ELISA to detect the presence of viruses. The samples were performed to RT-PCR after IBDV antigen was de- tectable using AC-ELISA. Electrophoresis result showed that there is one 209 bp band, whose sequence located in VP2 hypervarible region, on 1.2% agarose gel. The re- sult of RT-PCR and AC-ELISA demonstrated that virus mutants were successfully recovered. From 10 transfec- tion samples we obtained four mutant viruses designated as H253/284, H783/862, H862/921, H921/783 and rescued Harbin-1 named rHarbin-1. 3.3. Genetic Stability Analysis Sequence analysis of the RT-PCR products confirmed the identity of the IBDV used. No amino acid substitu- tions compared to the sequence of the used plasmids (p253/284 m, p783/862 m, p861/921 m, p921/783 m) were found within the region flanked by primers used for RT-PCR, proving the genetic stability of the virus during virus pass aging. 3.4. Virulence Determinants for VP2 and VP3 in Chinese vvIBDV Strain To evaluate the virulence of all virus mutants animal experiments were performed. Animals infected with vvIBDV (rHarbin-1) and H783/862 showed severe clinical signs of IBD. The mortality rates were 7/8 for rHarbin-1, 5/8 for H783/862, 1/8 for H253/284 and H862/921. In contrast, none of the animals infected with H921/783 died or showed clinical signs of IBD. Bursae of chickens infected with the different virus mutants showed depletion of bursal cells in lymph nodule with remarkable differences (Fig- ure 1). rHarbin-1 and H253/284, H783/862 induced severe bursal lesion (BLS of 5, 1.6, 3.1 respectively); H862/921 induced mild lesion (BLS 1.5); H921/783 hardly induce lesion (BLS 0). As to the ration of bursal weight and body weight, rHarbin-1 and H783/862 showed severe bur- sal atrophy (3.39, 3.94 respectively). There was no re- markable difference among H253/284, H862/921, H921/783 and negative control (4.0, 4.8, 4.94, 4.71 respectively) (Ta- ble 3). The above-mentioned results demonstrated that V862 and I921 in VP3 are probably the major virulence determinants, furthermore, 862 and 921 in VP3 has the stronger ability to manage virulence than 253 and 284 in VP2. 4. DISCUSSION In recent years, many investigators have shown that mu- tations in the viral genome often lead to changes in the virulence, pathogenesis of animal viruses. A single amino acid substitution in the West Nile Virus Nonstru- ctural protein NS2A disables its ability to inhibit Al- pha/Beta interferon induction and attenuates virus in mice [18]; point mutations in an infectious bovine viral diarrhoea virus type2 cDNA transcript yields an attenu-  R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 723 723 Table 3. Results of chicken challenged by four recombinant viruses. Virus Number of Chickens* Mortality Avg bursa/body wt, Ratio (SD), 103 Avg BLS** (SD) Pathological Lesions H253/284 8 1/8 4.0 (2.0) ab 1.6 (1.1)b individual lymphatic nodule necrosis and atrophy in dead chicken H783/862 8 5/8 3.9 (1.5) ab 3.1 (2.6)c lymphatic nodule severe necrosis and atrophy in dead chickens H862/921 8 1/8 4.8 (1.4) b 1.5 (1.4)b lymphatic nodule partly necrosis and slightly atrophy in dead chicken H921/783 8 0/8 4.9 (0.9)b 0 (0.0)a lymphatic nodule slightly atrophy and widen interstice close to normal rHarbin-1 8 7/8 3.4 (0.9) a 5 (0.0)d lymphatic nodule appear necrosis, congest and hemorrhage Native Harbin-1 8 7/8 3.1 (0.4)a 5 (0.0)d lymphatic nodule appear necrosis, congest and severe hemorrhage, atrophy Negative control 8 0/8 4.7 (0.3)b 0 (0.0)a normal *The indicated number of 4-week-old SPF chickens were infected via the eye and nose drop; **BLS of BF of each chicken investigated. Values within the same row with the same superscript letters are not significant (P < 0.05). Figure 1. Microscopic pathological effect in bursae challenged by virus mutants (10 × 20) (a) H253/284 single lymph nodule necrosis, atrophy, BLS 4; (b) H783/862 lymph nodule severe ne- crosis, atrophy, BLS 4.5; (c) H862/921 lymph nodule partly ne- crotize, BLS 2.3; (d) H921/783 close to normal, BLS 0; (e) rHar- bin-1 lymph nodule necrosis, congest and hemorrhage BLS 5; (f) CK (negative); (g) Harbin-1 (positive control) lymph nod- ule necrosis, congest and severe hemorrhage, atrophy BLS 5. ated and protective viral progeny. Virulence of swine vesicular disease virus is determined at two amino acids in capsid protein VP1 and 2A protease [14]. Above men- tioned phenomena elicit researchers on IBDV and they dedicated to study the virulence mechanism. A number of researchers such as Brandt, Yamaguchi, Lim, Mundt and so forth assumed position 253, 279, 284 amino acids in VP2 hypervarible region control phenotype, and could bind with B lymphocyte [3,17,21,29]. Lots of evidence showed that hypervarible region in VP2 involved in conformation dependent epitope and stimulate the chi- cken to produce protective neutralizing antibody [10, 29]. The result of chickens challenged with viruses showed that H253/284 could induced slighter lesion than parental virus vvIBDV (Harbin-1), but in H783/862 group, there are two kinds of appearance ,the bursa in alive chickens had not showed pathological sign, which could be due to the individual difference, but the bursae of dead chickens showed severe necrosis and atrophy, B lymphocyte de- pletion was up to the same 80-90% as Harbin-1; in H862/921 group, a bursa of dead chicken had the same pathological lesion as Harbin-1, lymphocyte depletion up to above 90%, in other bursae of alive chickens de- pletion is only 10-20%, and appear partly necrosis and atrophy; in H921/783 group bursae had very slightly pathological lesion except minor widening interstice, suggesting bursa was slight swollen. Therefore, H921/783 virus appeared the slightest pathological lesion among all virus mutants. Compared with mDT-VP3C and mDCT-VP3C rescued by Boot who substituted the C- terminal part of VP3 of serotype 1 vvIBDV (isolate D6948) for the corresponding part of serotype 2 IBDV [22], H921/783 induced slighter pathological lesion than  R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 724 mDT-VP3C and mDCT-VP3C. mDT-VP3C and mDCT- VP3C could induced same bursa lesion as wild type D6948 and rD6948, suggesting mDT-VP3C and mDCT- VP3C had stronger residential virulence, but H921/783 virus hardly has no residential virulence. Our experiment demonstrated that VP3 and VP2 con- tain the determinant for virulence too besides VP2 in one strain. However, up to now most researches assume VP2 play an important role in virulence. The reason for this paradox about virulence controlling mechanism is un- known. Molecular determinant of virulence may depend the strains used. In addition we used two alterations of amino acid in this paper. Single alterations of aa 783, 862 and 921 were not tested, further study may be nec- essary to identify if single amino acid function or both of them function in virulence at the same time. 5. CONCLUSIONS V862, I921 in VP3 is obvious virulence marker how- ever I921 has more potential ability to control viru- lence than V862 and H783. Through animal challenge test we make clear the site in VP2 and VP3 involved in virulence, furthermore, the ability of 862 and 921 to control virulence in VP3 is more powerful than 253 and 284 in VP2. 6. ACKNOWLEDGEMENTS We thank Professor Zhizhong Cui in Shandong Agricultural University for his assistance in animal experiment. Professor Zhao Deming in National Animal TSE Lab in China Agricultural University is grate- fully acknowledged for his assistance in Quantitative realtime PCR experiment. This study was supported by Chinese NSFC grant No. 9893290 and INCO-China grant ERBIC18CT98-0330. REFERENCES [1] Cosgrove, A.S. (1962) An apparently new disease of chickens: Avian nephrosis. Avian Diseases, 6(3), 385- 389. [2] Wei, Y.W., Yu, X.P., Zheng, J.T., Chu, W.Y., Xu, H., Yu, X.M. and Yu, L. (2008) Reassortant infectious bursal disease virus isolated in China. Virus Research, 131(2), 279-282. [3] Delmas, B. (2005) Birnaviridae. In: Virus Taxonomy, 8th Report of the International Committee on Tax- onomy of Viruses, Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U. and Ball, L.A., Eds, Elsevier Aca- demic, London, 561-569. [4] Müller, H. (2003) Research on infectious bursal disease- the past, the present and the future. Veterinary Micro- biology, 97(1-2), 153-165. [5] Yamaguchi, T., Ogawa, M., Miyoshi, M., Inoshima, Y., Fukushi, H. and Hirai, K. (1997) Sequence and phy- logenetic analyses of highly virulent infectious bursal disease virus. Archives of Virology, 142(7), 1441-1458. [6] Ikuta, N., El-Attrache, J., Villegas, P., Garcia, E.M., Lunge, V.R., Fonseca, A.S., Oliveira, C. and Marques, E.K. (2001) Molecular characterization of Brazilian infectious bursal disease viruses. Avian Diseases, 45(2), 297-306. [7] Dobos, P., Hill, B.J., Hallett, R., Kells, D.T.C., Becht, H. and Teninges, D. (1979) Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. Journal of Virology, 32(2), 593-605. [8] Kibenge, F.S., Qian, B., Cleghorn, J.R. and Martin, C.K. (1997) Infectious bursal disease virus polyprotein pro- cessing does not involve cellular proteases. Archives of Virology, 142(12), 2401-2419. [9] Spies, U., Müller, H. and Becht, H. (1987) Properties of RNA polymerase activity associated with infectious bursal disease virus and characterization of its reaction products. Virus Research, 8(2), 127-140. [10] Von Einem, U.I., Gorbalenya, A.E., Schirrmeier, H., Behrens, S.E., Letzel, T. and Mundt, E. (2004) VP1 of infectious bursal disease virus is an RNA-dependent RNA polymerase. Journal of General Virology, 85(8), 2221-2229. [11] Dobos, P. (1993) In vitro guanylylation of infectious pancreatic necrosis virus polypeptide VP1. Virology, 193(1), 403-413. [12] Spies, U. and Müller, H. (1990) Demonstration of enzy- me activities requiredfor cap structure formation in infec- tious bursal disease virus, a member of the birnavirus group. Journal of General Virology, 71(Pt4), 977-981. [13] Brown, M.D., Green, P. and Skinner, M.A. (1994) VP2 sequences of recent European ‘very virulent’ isolates of infectious bursal disease virus are closely related to each other but are distinct from those of ‘classical’ strains. Journal of General Virology, 75(Pt3), 675-680. [14] Fahey, K.J., Erny, K. and Crooks, J. (1989) A con- formational immunogen on VP2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. Journal of General Viro- logy, 70(Pt6), 1473-1481. [15] Letzel, T., Coulibaly, F., Rey, F.A., Delmas, B., Jagt, E.W., van Loon, A.A.M. and Mundt, E. (2007) Mole- cular and structural bases for the antigenicity of VP2 of infectious bursal disease virus. Journal of Virology, 81(23), 12827-12835. [16] Yamaguchi, T., Ogawa, M., Inoshima, Y., Miyoshi, M., Fukushi, H. and Hirai, K. (1996) Identification of sequence changes responsible for the attenuation of highly virulent infectious bursal disease virus. Virology, 223(1), 219-223. [17] Mundt, E. (1999) Tissue culture infectivity of different strains of infectious bursal disease virus is determined by distinct amino acids in VP2. Journal of General Virology,  R. M. Li et al. / Natural Science 2 (2010) 718-725 Copyright © 2010 SciRes. OPEN ACCESS 725 725 80(8), 2067-2076. [18] Jackwood, D.J., Sreedevi, B., Lefever, L.J. and Sommer- Wagner, S.E. (2008) Studies on naturally occurring infectious bursal disease viruses suggest that a single amino acid substitution at position 253 in VP2 increases pathogenicity. Virology, 377(1), 110-116. [19] Brandt, M., Yao, K., Liu, M., Heckert, R.A. and Vak- haria, V.N. (2001) Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. Journal of Virology, 75(24), 11974- 11982. [20] Van Loon, A.A.W.M., de Haas, N., Zeyda, I. and Mundt, E. (2002) Alteration of amino acids in VP2 of very virulent infectious bursal disease virus results in tissue culture adaptation and attenuation in chickens. Journal of General Virology, 83(1), 121-129. [21] Boot, H.J., ter Huurne, A.A., Hoekman, A.J., Peeters, B.P. and Gielkens, A.L. (2000) Rescue of very virulent and mosaic infectious bursal disease virus from cloned cDNA: VP2 is not the sole determinant of the very virulent phenotype. Journal of Virology, 74(15), 6701-6711. [22] Boot, H.J., ter Huurne, A.A., Hoekman, A.J., Pol, J.M, Gielkens, A.L. and Peeters, B.P. (2002) Exchange of the C-terminal part of VP3 from very virulent infectious bursal disease virus results in an attenuated virus with a unique antigenic structure. Journal of Virology, 76(20), 10346-10355. [23] Yamaguchi, T., Kondo, T., Inoshima, Y., Ogawa, M., Miyoshi, M., Yanai, T., Masegi, T., Fukushi, H. and Hirai, K. (1996) In vitro attenuation of highly virulent infectious bursal disease virus: Some characteristics of attenuated strains. Avian Diseases, 40(3), 501-509. [24] Mundt, E. and Vakharia, V.N. (1996) Synthetic trans- cripts of double-stranded Birnavirus genome are infec- tious. Proceedings of National Academy of Sciences of USA, 93(11-12), 11131-11136. [25] Nunoya, T., Tajima, M. and Itakura, C. (1991) Primary culture of chicken bursal plical epithelium. Research in Veterinary Science, 50(3), 352-354. [26] Eterradossi, N., Toquin, D., Rivallan, G. and Guittet, M. (1997) Modified activity of a VP2-located neutralizing epitope on various vaccine, pathogenic and hypervirulent strains of infectious bursal disease virus. Archives of Virology, 142(2), 255-270. [27] Synder, D.B., Lana, D.P., Cho, B.R. and Marquardt, W.W. (1988) Group and strain-specific neutralization sites of infectious bursal disease virus defined with mo- noclonal antibodies. Avian Diseases, 32(5), 527-534. [28] Hierholzer, J.C. and Killington, R.A. (1996) Suspension assay method. Virology Methods Manual, Academic, San Diego, 39-40. [29] Schröder, A., van Loon, A.A.W.M., Goovaerts, D. and Mundt, E. (2000) Chimeras in noncoding regions between serotypes I and II of segment A of infectious bursal disease virus are viable and show pathogenic phenotype in chickens. Journal of General Virology, 81(23), 533-540.
|