Journal of Modern Physics
Vol.2 No.11(2011), Article ID:8650,9 pages DOI:10.4236/jmp.2011.211175
Three Techniques Used to Produce BaTiO3 Fine Powder
Physics Department, College of Science, Al-Nahrain University, Baghdad, Iraq
E-mail: eks2000@hotmail.com
Received September 8, 2011; revised October 12, 2011; accepted October 28, 2011
Keywords: X-Ray Diffraction, Fine Powder, Williamson-Hall Method
ABSTRACT
Homogeneous BaTiO3 fine powder has been synthesized at (80˚C) by using three different chemical methods using the roots TiCl4, BaCl2 and NaOH or Oxalic acid. The resultant powders were characterized using x-ray diffraction (XRD) to estimate the crystal structure, lattice parameters and the crystallite size to investigate the favor method in producing BaTiO3 fine powder. The criteria that was dependent on considering the favor method that was given better results of XRD and demand a least time in preparation which tend to consume a lowest energy.
1. Introduction
Barium Titanate is one of the most important ferroelectric materials which have many applications [1]. It belongs to a peroviskite structure as a ceramic so it needs to prepare BaTiO3 ultrafine powder in order to reduce the calcination temperature, porosity, sintering temperature and sintering time [1]. All above parameters will lead to reduce the cost through reducing the energy and increase efficiency. The wide range of applications of BaTiO3 includes preparing multi-layer ceramic capacitors (MLCCs), electro optical device, PTC and NTC resistors, piezoelectric actuators, transducers and chemical gas sensors. So that ultrafine powder is very necessary for these applications especially in (MLCCs) [1-7]. In this study, we try to discuss three chemical methods were used to prepare BaTiO3 fine powder to conclude the best one according to the resultant phases, crystallite size, cost effective and time management.
2. Experimental Work
In general, the three chemical techniques have been done depends on the roots those were used represented by, Titanium tetrachloride (TiCl4) was used as a titanium source, Barium chloride (BaCl2∙2H2O) as a barium source and Sodium hydroxide (NaOH) and Oxalic acid (COOH)2∙2H2O were used as catalysts.
The First method (Sodium hydroxide-ethanol method): (10 ml) of TiCl4 was added to (20 ml) of Absolute Ethanol to get yellow gummy solution. There is (10 ml) of distilled water was added gradually until got transparent solution. The resultant transparent solution was mixed with (70 ml) of (0.385 M) of BaCl2, (220 ml) of (12 M) NaOH was added to the mixture. The reaction was carried out in water bath at (80˚C) for (5 hr). Then the resultant precipitate was subjected for washing and filtering (5 times), dried at (90˚C) for (12 hr). Calcination process was carried out at (850˚C) for (5 hr) to get BaTiO3 ultrafine powder. The schematic diagram of the first method is shown in Figure 1.
The Second method (Oxalate method); this method was carried out by mixing (50 ml) of (1 M) of TiCl4 (aqueous solution) with (50 ml) of (1.05 M) of Barium chloride (aqueous solution). The mixture was then added gradually to (50 ml) of (2.2 M) of Oxalic acid [8]. The reaction was carried out in water bath at (80˚C) for (15 min) the resultant powder was BariumTetanyl oxalate tetrahydrate [BaTiO(C2O4)∙4H2O]. It was separated by filtration; washed and dried at (90˚C) for (12 hr). Calcination process was performed on two different parts, the first one was subjected to (600˚C) for (5 hr), the second part subjected to (850˚C) for (5 hr) to get BaTiO3 ultrafine powder. The schematic diagram for the second method is shown in Figure 2.
The Third method (Sodium hydroxide method), the preparation was done by mixing (50 ml) of (1 M) TiCl4 (aqueous solution) with (50 ml) of (1.05) M Barium chloride (aqueous solution). The mixture was added to (50 ml) of (7 M) of NaOH gradually. The reaction was carried out in water bath at (80˚C) for (5 hr) the resultant precipitate was then washed five times and dried at (90˚C)

Figure 1. Schematic diagram for the first method.
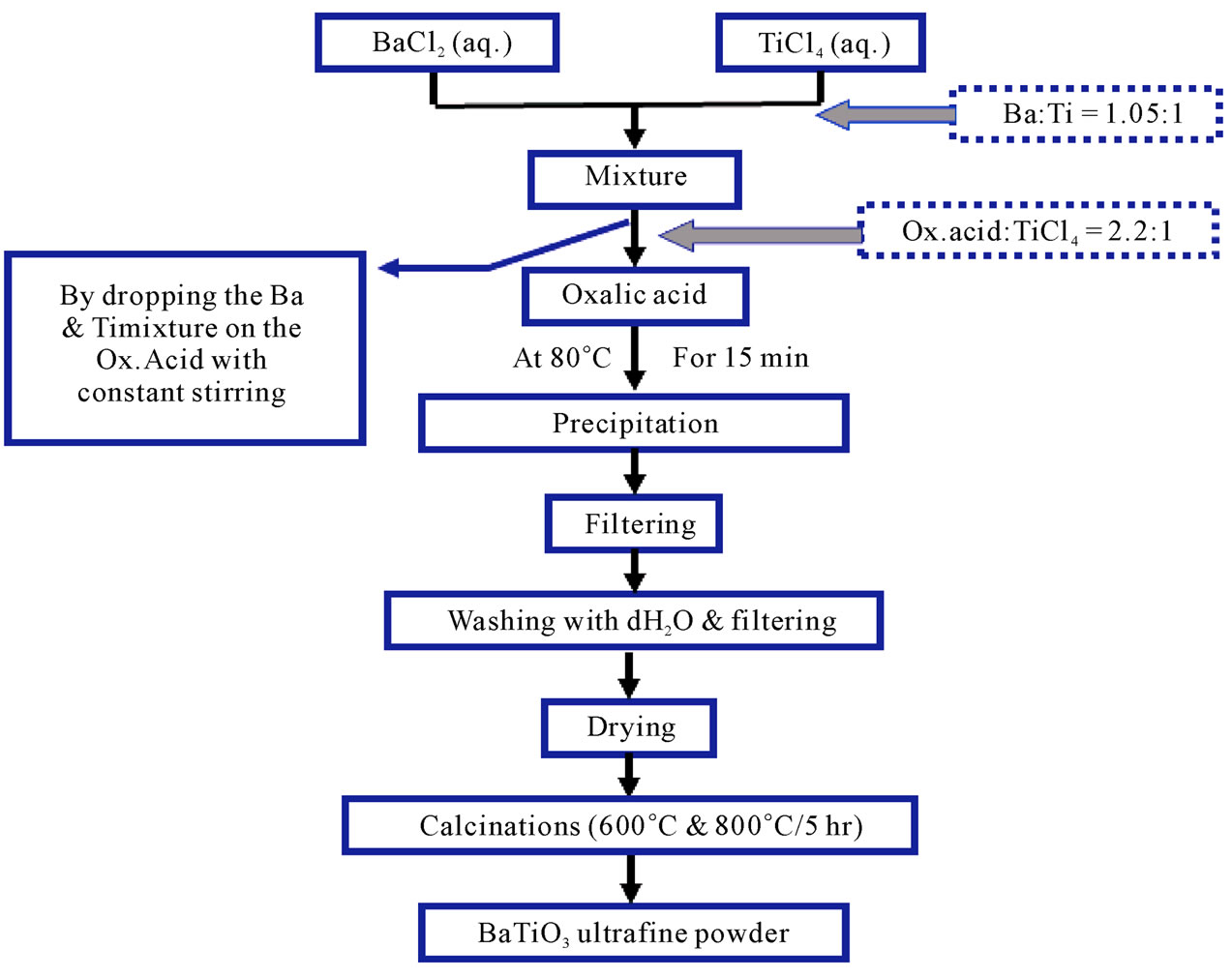
Figure 2. The Schematic diagram for the second method.
for (12 hr). Precipitate calcination was done at (850˚C) for (5 hr) to get the fine powder. The schematic diagram for the third method is shown in Figure 3.
As well known, there are many techniques used to conclude the homogeneous powder represented by XRD, SEM and TEM. In our research it is concentrated on XRD to obtain the better method in preparation for simple reason represented by the availability of this apparatus in Iraq. Our study is concentrated on the searching of better method in preparation during different parameter as mentioned before, so, just only XRD technique is suitable to give us indication about which method is favor to produce BaTiO3 fine powder through the investigation of crystal phase. We used for this purpose, X-ray diffractometer with CuKα1 (λ = 1.5406 Ǻ) with voltage of (40 kV) and current of (10 mA). It was used to investigate the structure of BaTiO3 powders at room temperature. The analysis was performed using XPowder software (http://www.xpowder.com) for calculations of lattice parameters and crystallite size. The second one is FullProfSuit software for Drawing XRD patterns and space group determination (http://www.ccp14.ac.uk). In addition to that there is XRD Database (PDF2# 05-626 for BaTiO3) from International Center for Diffraction Data (ICDD) used to complete the specification of the powder produced.
3. Results and Discussion
Regarding to our expert in preparation of ceramic powder, it was clear that the chemical methods (sol-precipitation) were used to get homogeneous and ultrafine powder whereas the conventional one (solid state reaction) needs high temperature of approximately (1000˚C - 1400˚C) followed by grinding. The resultant microstructure has a large grain size and multiple phases (i.e., heterogeneous) and is inventably porous with high sintering temperature of about (1350˚C - 1450˚C). While the chemical method can produce ultrafine powder, homogeneous, high purity and low grain size and porosity which lead to reduce calcination temperature in the range (600˚C - 900˚C) in comparable with solid state reaction. Solid state reaction might be considered as indirect method while chemical methods (solution methods) are direct method because that the reaction was done in the solution directly between Ba+2, Ti+4 and O–2 directly to get BaTiO3 precursor, that was clear by XRD as shown in Figure 4.
The results, for the first method, showed that the crystalline phase with tetragonal structure (a = b = 3.9841 Ǻ, c = 4.0162 Ǻ) with space group (P4 mm) was predominate. The crystallite size was calculated by using XPowder software according to Williamson Hall equation.
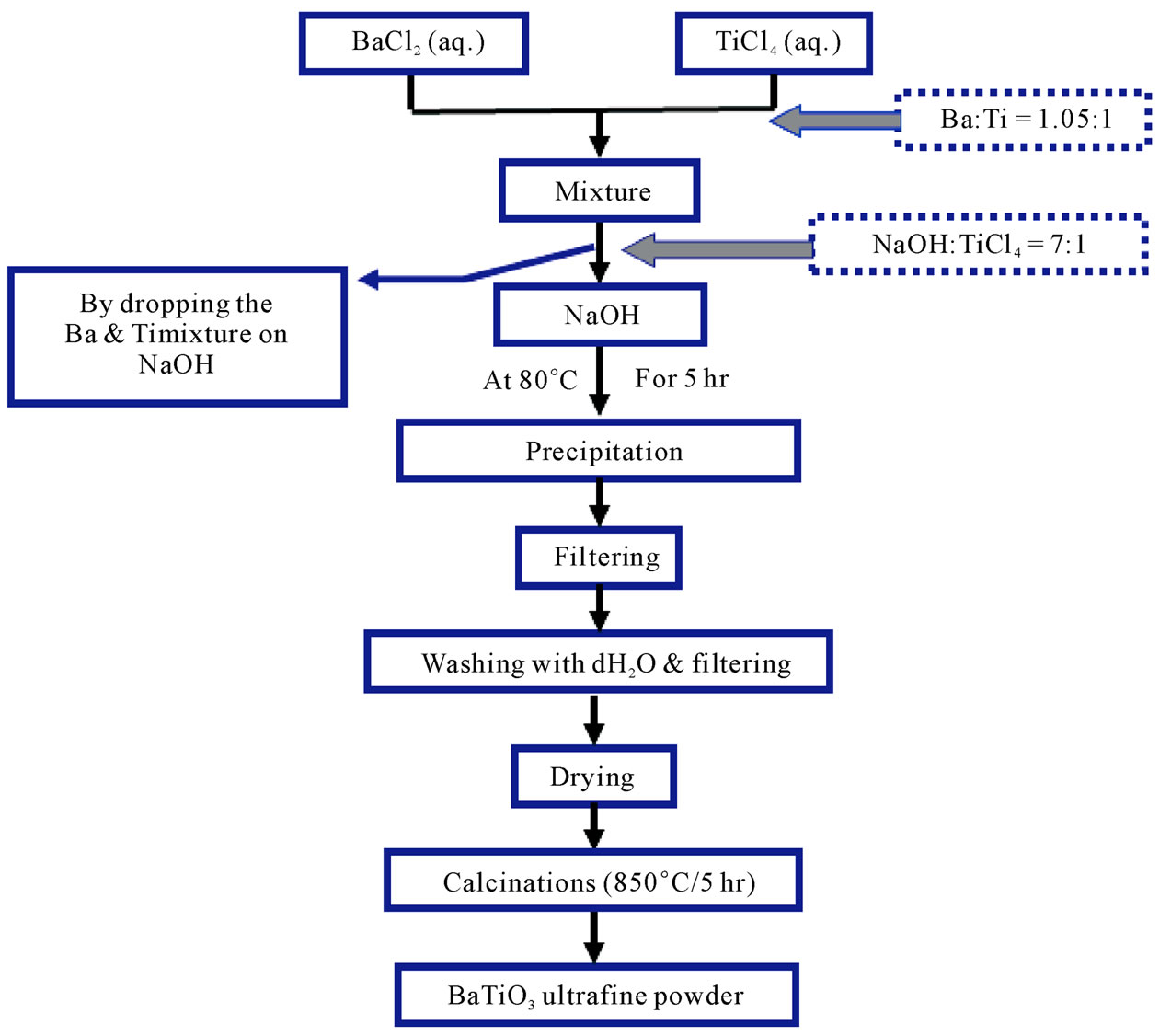
Figure 3. The Schematic diagram for the third method.

Figure 4. XRD pattern for the sample prepared by the first chemical method, (a) before calcinations, (b) after calcinations at 850˚C/5hr.

where B is full width at the half maximum (FWHM), d is the crystallite size, (ε) elastic strain and (θ) is the diffracted angle as shown in Figure 5. The results showed that the crystallite size is in the range (26 - 42 nm) and the non-uniform strain is about (0.18). While Guangneng [2], obtained cubic structure for BaTiO3 before calcination.
The results of XRD for Oxalate method as shown in Figure 6, it was clear that before calcination we got amorphous phase for BaTiO(C2O4)∙4H2O as a result of presence the carbon atoms and the water in the mixture. After calcinations, at (600˚C), the crystalline phase with cubic structure (a = 4.0073 Ǻ) was appeared with space group (Pm - 3 m). The calcination at (850˚C/5hr) tend to produce the crystalline phase with tetragonal phase (a = b = 3.999 Ǻ and c = 4.0053 Ǻ) with space group (P4mm). On the other hand, the crystallite size was in the range (21 - 25 nm) and the non-uniform strain is (0.246) for the sample calcined at (600˚C/5hr). Whereas the crystallite size was in the range (25 - 37 nm) and the non-uniform strain is (0.253) for the sample calcined at (850˚C/5hr). Hwu [1], obtained cubic structure BaTiO3 for (600˚C/6hr) calcination and tetragonal BaTiO3 for (800˚C/6hr) calcination, and the crystalline size were (32.41 nm) and (41.32 nm) for (600˚C) and (800˚C) calcination respectively, So Hwu’s results are similar to our obtainable result .
The results of XRD for the sample prepared by third chemical method as shown in Figure 9, it was clear that the prepared powder before calcination showed the crystalline phase with cubic lattice constant (a = 4.0247 Ǻ) with space group (Pm - 3 m), and after calcination at (850˚C) crystalline phase was still remains cubic lattice with lattice constant (a = 4.0025 Ǻ) and space group (Pm - 3 m) with slightly deviation in some of diffracted peaks as appeared in Figure 9. There are many unknown peaks as mentioned in (Figures 4, 9), return to the phase of TiO2 that was present within the BiTiO3 phase. In order to eliminate the unknown peaks in the 1st and the 3rd methods, it became necessary the increasing of NaOH molarity or increasing the reaction time and that demands more power in production tends to consider a high expensive methods.
The crystallite size was in the range (26 - 36 nm) and the non-uniform strain is (0.152) for the sample before

Figure 5. Williamson Hall plot for the 1st method after calcinations 850˚C/5hr.
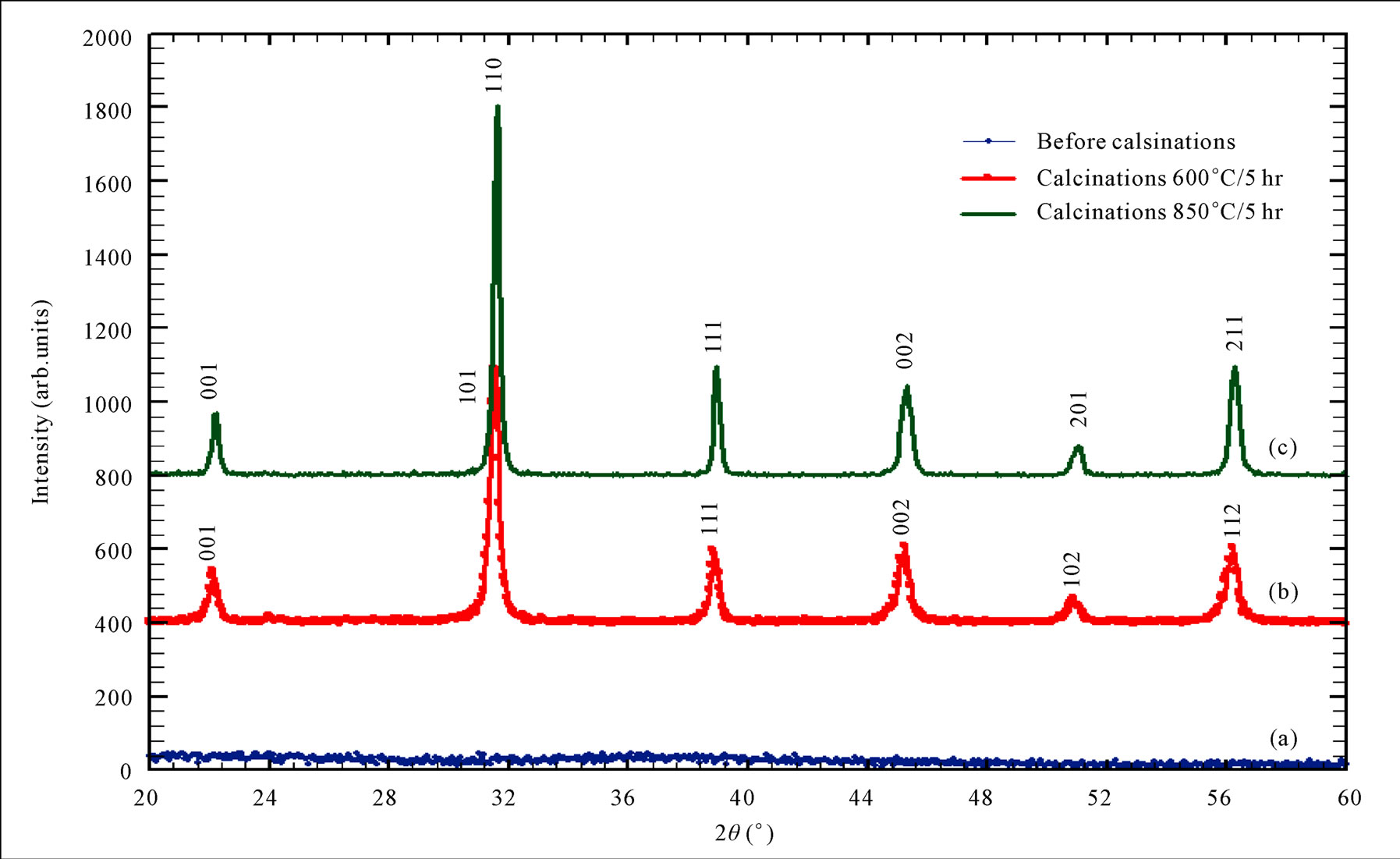
Figure 6. XRD pattern for Oxalate method, (a) before calcination (b) after calcination at 600˚C/5hr, (c) after calcination at 850˚C/5hr.
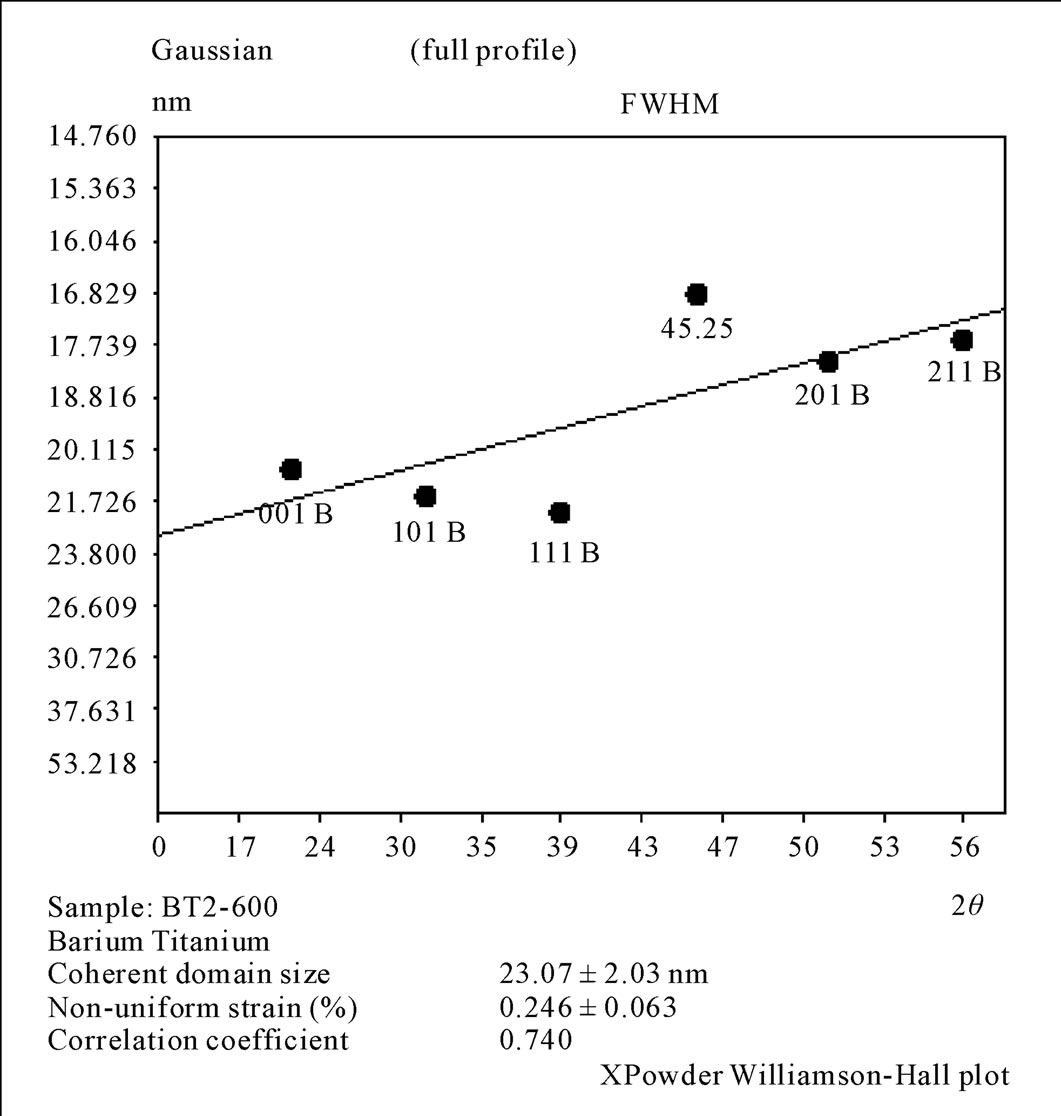
Figure 7. Williamson Hall plot for the 2nd method calcinations at 600˚C/5hr.
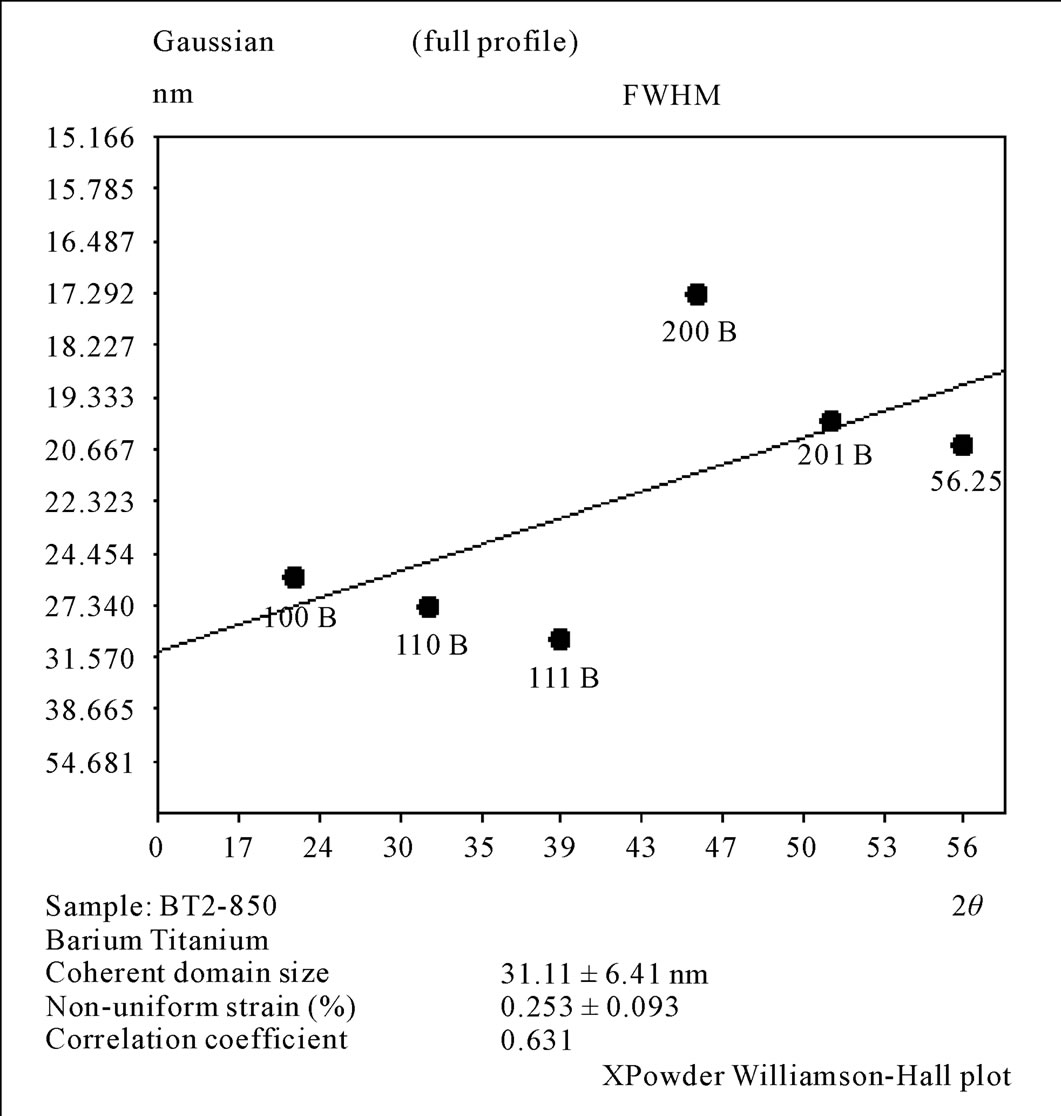
Figure 8. Williamson Hall plot for the 2nd method calcinations at 850˚C/5hr.
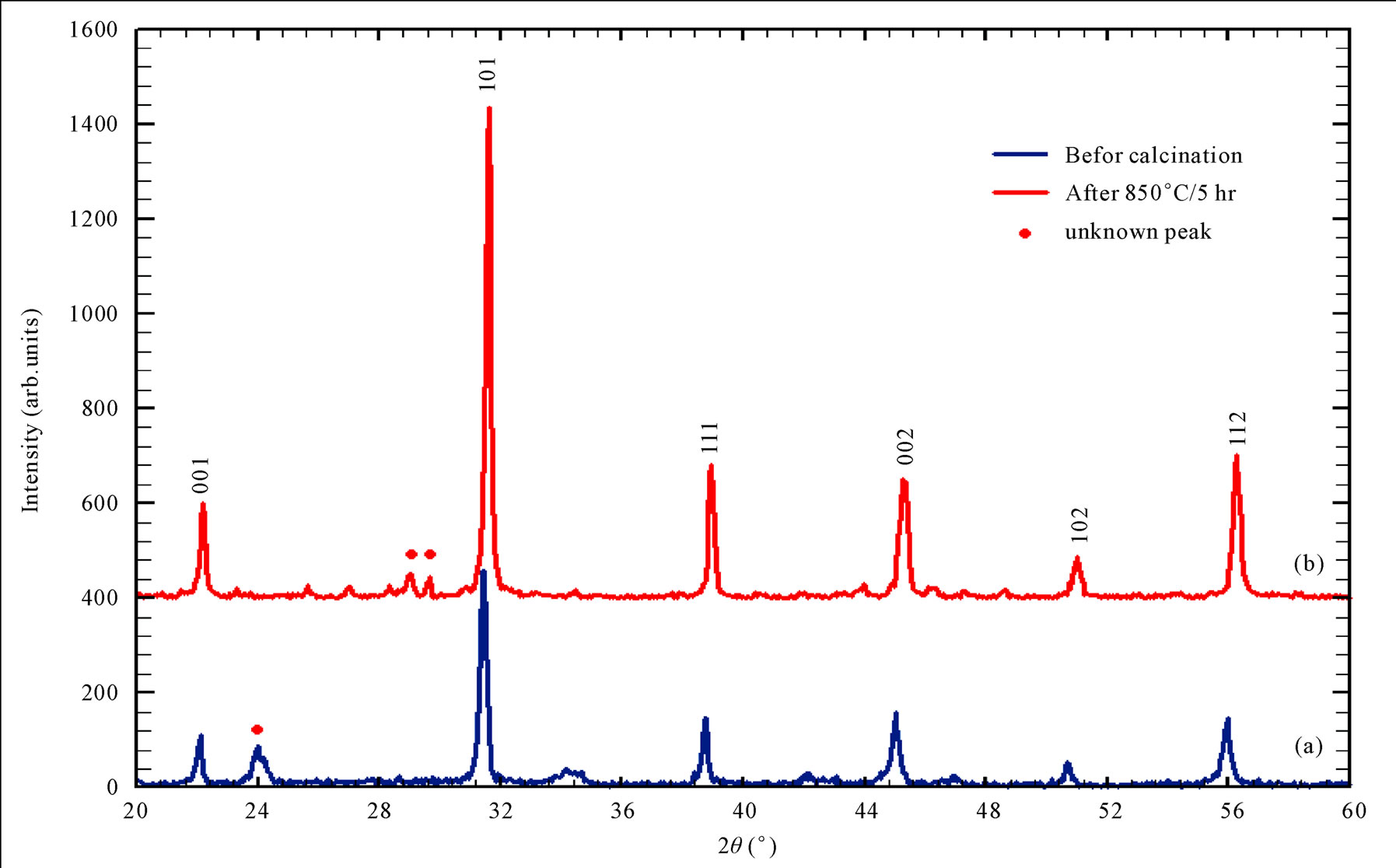
Figure 9. XRD pattern for NaOH method (a) before calcination (b) after calcination at 850˚C/5hr.
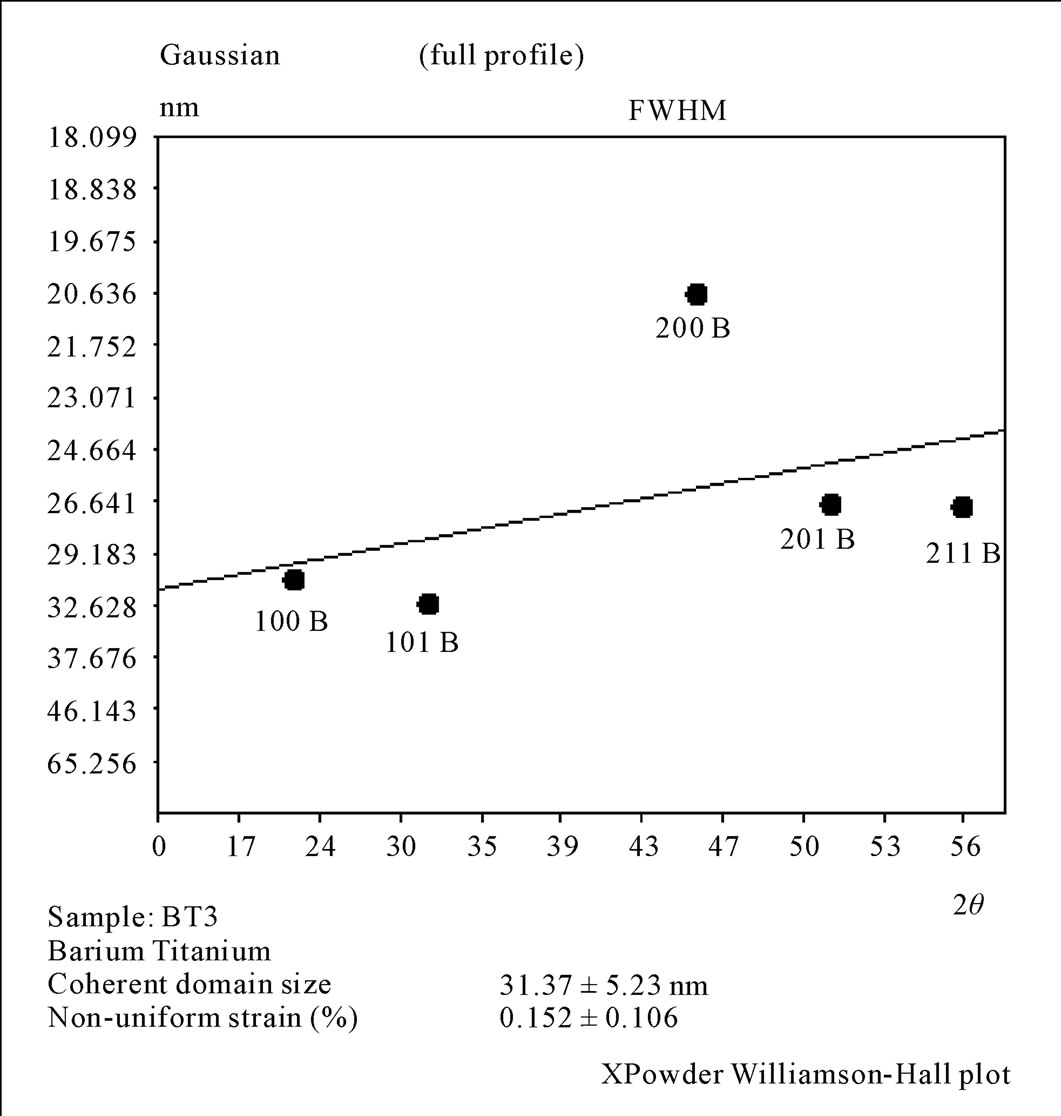
Figure 10. Williamson Hall plot for the 3rd method before calcinations.

Figure 11. Williamson Hall graph for the 3rd method calcinations at 850˚C/5hr.
calcinations. The crystallite size was in the range (27 - 35 nm) and the non-uniform strain is (0.184) for the sample calcined at (850˚C).
4. Conclusions
Regarding to the obtainable results by three chemical methods used to prepare fine powder of BaTiO3, we conclude that the best method for producing BaTiO3 was the 2nd method (Oxalate method) according to XRD. The XRD pattern for the 2nd method shows all the peaks of BaTiO3 and the crystallite size is in the range (21 - 25 nm) and (25 - 37 nm) for the sample calcined at (600˚C) and (850˚C) respectively. While the first and the third methods shows unknown peaks, which belong to TiO2, in XRD pattern and have a larger crystallite sizes. The reason for selected the 2nd method is the reaction time, which was (15 min) and it did not require washing and filtering. While the reaction time for the 1st and 3rd method was (5 hr) and it required washing and filtering for (5 times). The catalyst (Oxalic acid), the molarity was (2.2 M), while catalyst (NaOH), the molarity was (12 M) and (7 M) for the 1st and 3rd methods respectively. All these reasons, leads to conclude that the Oxalate method is the better one. For the third method the suggestion is to increase the NaOH molarity (i.e. NaOH: TiCl4 = 8:1, 9:1, ) to eliminate the unknown peak in XRD.
) to eliminate the unknown peak in XRD.
REFERENCES
- J. M. Hwu, W. H. Yu, W. C. Yang, Y. W. Chen and Y. Y. Chou, “Characterization of Dielectric Barium Titanate Powders Prepared by Homogeneous Precipitations Chemical Reaction for Embedded Capacitor Applications,” Materials Research Bulletin, Vol. 40, No. 10, 2005, pp. 1662-1679. doi:10.1016/j.materresbull.2005.05.019
- F. Guangneng, H. Lixia and H. Xueguang, “Synthesis of Single Crystal BaTiO3 Nanoparticles via One Step SolPriciptation Route,” Journal of Crystal Growth, Vol. 279, 2002, pp. 489-493. doi:10.1016/j.jcrysgro.2005.02.054
- Z. Chen, W. Zhang, J. Chen and Y. Jimmy, “Low Temperature One Step Synthesis of Barium Titanate: Particle Size Mechanism and Large Scale Synthesis,” Chinese Journal of Chemical Engineering, Vol. 14, No. 5, 2006, pp. 642-648. doi:10.1016/S1004-9541(06)60128-6
- X. Hurarui and G. Lain, “New Evidence of a Dissolution Precipitation Mechanism in Hydrothermal Synthesis of BaTiO3 Powder,” Material Letters, Vol. 57, No. 2, 2002, pp. 490-494. doi:10.1016/S0167-577X(02)00817-0
- N. Xu, Y. Lu, Y. Liu, Sh. Shi, T. Qian and D. Lu, “Sono Chemical Synthesis of Monosized Spherical BaTiO3 Particles,” Power Technology, Vol. 161, No. 3, 2006, pp. 185-189. doi:10.1016/j.powtec.2005.10.001
- H. Xu, L. Geo and J. Guo, “Preparation and Characterizations of Tetragonal BaTiO3 by Hydrothermal Method,” Journal of European Ceramic Society, Vol. 22, No. 7, 2002, pp. 1163-1170. doi:10.1016/S0955-2219(01)00425-3
- Y. Khollam, A. Deshpande, H. Potdar, S. Deshpande, S. Date and A. Patil, “A Self Sustaining Acid Base Reaction in Semi-Aqueous Media for Synthesis of Bariumtitanyl Oxalate Leading to BaTO3 Powder,” Material Letters, Vol. 55, No. 3, 2002, pp. 175-181. doi:10.1016/S0167-577X(01)00642-5
- K. Hur and J. Lee, “Method for Preparing BaTiO3 Powder by Oxalate Synthesis,” United State Patent US6692721B2, 2004.

