Peculiarities of Heavy Metals Accumulation by the Plants of Meadow Phytocenosis 277
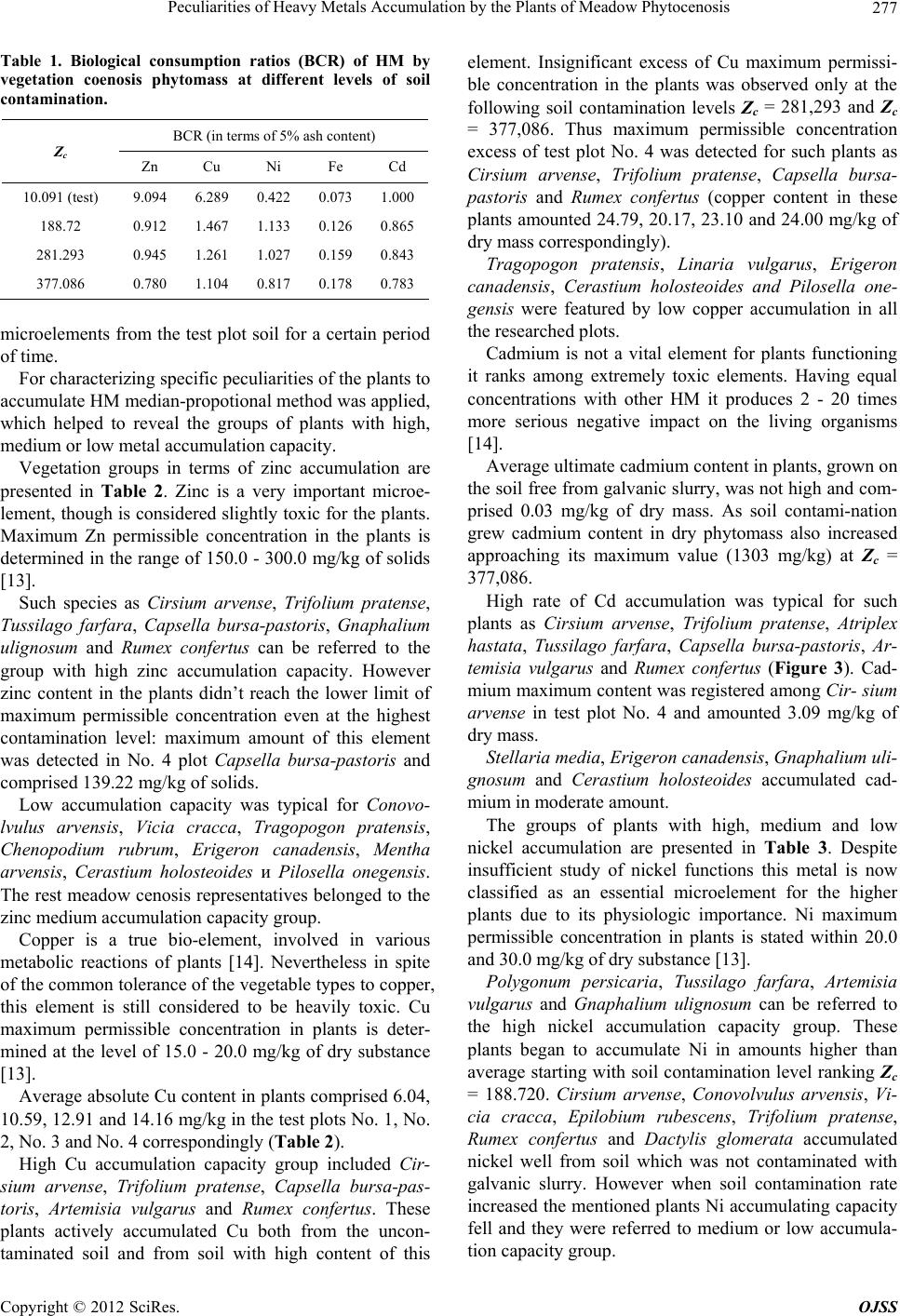
Table 1. Biological consumption ratios (BCR) of HM by
vegetation coenosis phytomass at different levels of soil
contamination.
BCR (in terms of 5% ash content)
Zс
Zn Cu Ni Fe Cd
10.091 (test) 9.094 6.289 0.422 0.073 1.000
188.72 0.912 1.467 1.133 0.126 0.865
281.293 0.945 1.261 1.027 0.159 0.843
377.086 0.780 1.104 0.817 0.178 0.783
microelements from the test plot soil for a certain period
of time.
For characterizing specific peculiarities of the plants to
accumulate HM median-propotional method was applied,
which helped to reveal the groups of plants with high,
medium or low metal accumulation capacity.
Vegetation groups in terms of zinc accumulation are
presented in Table 2. Zinc is a very important microe-
lement, though is considered slightly toxic for the plants.
Maximum Zn permissible concentration in the plants is
determined in the range of 150.0 - 300.0 mg/kg of solids
[13].
Such species as Cirsium arvense, Trifolium pratense,
Tussilago farfara, Capsella bursa-pastoris, Gnaphalium
ulignosum and Rumex confertus can be referred to the
group with high zinc accumulation capacity. However
zinc content in the plants didn’t reach the lower limit of
maximum permissible concentration even at the highest
contamination level: maximum amount of this element
was detected in No. 4 plot Capsella bursa-pastoris and
comprised 139.22 mg/kg of solids.
Low accumulation capacity was typical for Conovo-
lvulus arvensis, Vicia cracca, Tragopogon pratensis,
Chenopodium rubrum, Erigeron canadensis, Mentha
arvensis, Cerastium holosteoides и Pilosella onegensis.
The rest meadow cenosis representatives belonged to the
zinc medium accumulation capacity group.
Copper is a true bio-element, involved in various
metabolic reactions of plants [14]. Nevertheless in spite
of the common tolerance of the vegetable types to copper,
this element is still considered to be heavily toxic. Cu
maximum permissible concentration in plants is deter-
mined at the level of 15.0 - 20.0 mg/kg of dry substance
[13].
Average absolute Cu content in plants comprised 6.04,
10.59, 12.91 and 14.16 mg/kg in the test plots No. 1, No.
2, No. 3 and No. 4 correspondingly (Table 2).
High Cu accumulation capacity group included Cir-
sium arvense, Trifolium pratense, Capsella bursa-pas-
toris, Artemisia vulgarus and Rumex confertus. These
plants actively accumulated Cu both from the uncon-
taminated soil and from soil with high content of this
element. Insignificant excess of Cu maximum permissi-
ble concentration in the plants was observed only at the
following soil contamination levels Zс = 281,293 and Zс
= 377,086. Thus maximum permissible concentration
excess of test plot No. 4 was detected for such plants as
Cirsium arvense, Trifolium pratense, Capsella bursa-
pastoris and Rumex confertus (copper content in these
plants amounted 24.79, 20.17, 23.10 and 24.00 mg/kg of
dry mass correspondingly).
Tragopogon pratensis, Linaria vulgarus, Erigeron
canadensis, Cerastium holosteoides and Pilosella one-
gensis were featured by low copper accumulation in all
the researched plots.
Cadmium is not a vital element for plants functioning
it ranks among extremely toxic elements. Having equal
concentrations with other HM it produces 2 - 20 times
more serious negative impact on the living organisms
[14].
Average ultimate cadmium content in plants, grown on
the soil free from galvanic slurry, was not high and com-
prised 0.03 mg/kg of dry mass. As soil contami-nation
grew cadmium content in dry phytomass also increased
approaching its maximum value (1303 mg/kg) at Zс =
377,086.
High rate of Cd accumulation was typical for such
plants as Cirsium arvense, Trifolium pratense, Atriplex
hastata, Tussilago farfara, Capsella bursa-pastoris, Ar-
temisia vulgarus and Rumex confertus (Figure 3). Cad-
mium maximum content was registered among Cir- sium
arvense in test plot No. 4 and amounted 3.09 mg/kg of
dry mass.
Stellaria media, Erigeron canadensis, Gnaphalium uli-
gnosum and Cerastium holosteoides accumulated cad-
mium in moderate amount.
The groups of plants with high, medium and low
nickel accumulation are presented in Table 3. Despite
insufficient study of nickel functions this metal is now
classified as an essential microelement for the higher
plants due to its physiologic importance. Ni maximum
permissible concentration in plants is stated within 20.0
and 30.0 mg/kg of dry substance [13].
Polygonum persicaria, Tussilago farfara, Artemisia
vulgarus and Gnaphalium ulignosum can be referred to
the high nickel accumulation capacity group. These
plants began to accumulate Ni in amounts higher than
average starting with soil contamination level ranking Zс
= 188.720. Cirsium arvense, Conovolvulus arvensis, Vi-
cia cracca, Epilobium rubescens, Trifolium pratense,
Rumex confertus and Dactylis glomerata accumulated
nickel well from soil which was not contaminated with
galvanic slurry. However when soil contamination rate
increased the mentioned plants Ni accumulating capacity
fell and they were referred to medium or low accumula-
tion capacity group.
Copyright © 2012 SciRes. OJSS