Paper Menu >>
Journal Menu >>
 Vol.2, No.7, 722-730 (2010) doi:10.4236/health.2010.27110 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Influence of poloxamer 407 on fractional and subfractional composition of serum lipoproteins of mice Tatyana A. Korolenko1*, Fedor V. Tuzikov2,3, Thomas P. Johnston4, Natalia A. Tuzikova2,3, Elena E. Filjushina1, Viktoriya M. Loginova1, Natalia G. Savchenko1 1Institute of Physiology, Siberian Branch of Russian Academy of Medical Sciences, Novosibirsk, Russia; *Corresponding Author: t.a.korolenko@physiol.ru 2Boreskov Institute of Catalysis, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia 3Novosibirsk State University, Novosibirsk, Russia 4University of Missouri-Kansas City, Kansas City, USA Received 4 March 2010; revised 22 March 2010; accepted 25 March 2010. ABSTRACT Using a novel small-angle X-ray scattering (SAXS) method for determination of fractional and sub- fractional composition of lipoproteins (LPs), a significant elevation of total cholesterol-lipop- roteins (C-LP) and, especially, total triglyceride- lipoproteins (TG-LP), was shown in this work. Among the LP fractions, poloxamer 407 was shown to significantly increase proatherogenic total C-LDL, TG-LDL and, especially, their pre- cursors C-VLDL and TG-VLDL, while only ex- hibiting a moderate increase in the antiathero- genic C-HDL and TG-HDL fractions. With regard to the VLDL subfractions, significant elevations were observed in both subfractions studied; namely, C-V LDL 1-2 and C-V LDL 3-5. Similar chang- es were no ted in the TG-V LDL1-2 and TG- VLDL 3-5 subfractions. The C-IDL and TG-IDL subfrac- tions were increased significantly (20- to 30- fold), while the C-LDL1-3 subfraction was mod- erately (3- to 5-fold) increased at 48 hrs and at day 4. In the moderately elevated (2- to 4-fold) anti-atherogenic HDL fraction, the C-HDL2 sub- fraction was increased more significantly (4- fold) compared to the C-HDL3 subfraction; how- ever, both C-HDL subfractions returned to base- line by day 4. The elevation in the TG-HDL2 subfraction was observed only at 24 hrs. Mouse models of hyperlipidemia and atherosclerosis are useful to evaluate the role of “individual” LPs, as well as their fractions and subfractions, in hyperlipidemia and the genesis of athero- sclerosis. Keywords: Poloxamer P-407; Dyslipidemia; Serum Lipoprotein Fractions and Subf r actions 1. INTRODUCTION Changes of different classes of circulating lipoproteins are the important indicies of lipid metabolism in physi- ology and pathology; lipoproteins have been shown to also play a regulatory role in vivo [1,2]. The main lipo- protein classes consist of pro-atherogenic low-density li- poproteins (LDL), very-low-density lipoproteins (VLDL), and anti-atherogenic high density lipoproteins (HDL), and are widely used as common lipid biomarkers in atherosclerosis [1]. However, the biological role of sub- classes of lipoproteins is still under investigation. The regulatory role of lipoproteins has been shown to relate to many intracellular processes, primarily to plasma membrane permeability and fluidity as the result of changes in the concentration of plasma membrane cho- lesterol, with subsequent modifications of receptors and transmembrane proteins (transmitters). In this process, the role of total HDL and HDL subfractions have espe- cially high importance in connection with their unique capacity to accept cholesterol from the plasma mem- brane and transport them to other classes of lipoproteins. Hyperlipidemia is one of the main risk factors in the development of cardiovascular and cerebrovascular dis- eases common in contemporary society. In addition to the elevation of plasma LDL-cholesterol and VLDL- cholesterol, hypertriglyceridemia is suggested to be an additional independent risk factor for atherosclerosis and coronary heart disease [3]. According to recent data, atherosclerosis is not only a metabolic lipid disease, but has also been considered to result from inflammation (chronic inflammatory disease). Statins have been re- ported to not only lower LDL by inhibiting the activity  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 723 723 of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, but also by reducing inflammation to endo- thelial cells. For the prevention of atherosclerosis, it is important to study the mechanisms of the early effects of hyperlipi- demia on different cell types involved in the pathogene- sis of atherosclerosis. Therefore, it is necessary to know the types of lipoproteins involved in hyperlipidemia at the early stages of atherosclerosis. It is well known that during the process of atherosclerosis, foam cells initially trap the oxidized LDL molecules via scavenger receptors. The oxidized LDL molecule is digested and transformed, and is then presented to T lymphocytes, initiating the classic immunological reaction, and subsequently stimu- lates the inflammatory response. Formation of lipid-laden cells (mainly smooth muscle cells and macrophages) is followed by increased secretion of pro-inflammatory cytokines (like IL-6) and other factors (several types of matrix metalloproteases), which promote inflammation. Statin treatment has a pleiotropic protective effect, not only by inhibiting HMG-CoA reductase, but also by ex- erting its anti-inflammatory action. The changes of serum lipoprotein levels responsible for pro- and anti-atherogenic action have been studied earlier [2]. However, recently, with help of new methods for characterizing different lipoprotein fractions and subfractions, some new data have been obtained on their role in the pathogenesis of atherosclerosis [4]. For this reason, the investigation of experimental models of hy- perlipidemia is useful. Poloxamer 407 is a block copolymer comprised of polyoxyethylene and polyoxypropylene units, which is known for its biocompatibility and potential to deliver different medications for a variety of disease states [5,6]. Following acute administration, poloxamer 407 was shown to induce significant hyperlipidemia, a model which has been used for testing several hypolipidemic drugs (statins, fibrates, and nicotinic acid) [7-11]. With chronic administration of poloxamer 407 (4 months) to mice, fibrofatty aortic lesions are developed, which are similar in size and number to those observed in classic diet-induced mouse models of atherogenesis [12,13]. Hyperlipidemia in acute poloxamer 407-induced ad- ministration to rodents was characterized by a dramatic elevation of both plasma cholesterol and triglycerides, which is usually not observed in patients with athero- sclerosis. In the poloxamer 407 mouse model of athero- sclerosis, the mechanism of cholesterol and TG elevation is associated with inhibition of cholesterol 7-hydro- xylase and lipoprotein lipase, respectively [13], and not dependent on PPARα [14]. It was also shown that a sin- gle injection of poloxamer 407 administration to mice caused hypercholesterolemia by inducing transient cho- lesterolgenesis and down-regulating low-density lipo- protein receptor expression [6]. The mechanism of ele- vation of different classes and, especially subfractions of lipoproteins with pro- and anti-atherogenic effects, is still not known. Small-angle X-ray scattering (SAXS) is a small-angle scattering technique where the elastic scattering of X-rays (wavelength 0.1 to 0.2 nm) by a sample, which has inhomogeneities in the nanometer range, is recorded at very low angles. This angular range contains informa- tion about the shape and size of macromolecules, char- acteristic distances of partially ordered materials, pore sizes, and other data. SAXS is capable of delivering structural information of macromolecules between 5 and 25 nm, of repeat distances in partially ordered systems of up to 150 nm. SAXS is used in the characterization of various materials. In the case of biologic macromole- cules, such as proteins, the advantage of SAXS, over crystallography, is that a crystalline sample is not needed. The materials can be solid or liquid and they can contain solid, liquid, or gaseous domains (so-called particles) of the same or another material in any combination. SAXS is accurate, non-destructive, and usually requires only a minimum of sample preparation. The aim of the present investigation was to evaluate the lipoprotein-cholesterol and lipoprotein-triglyceride fractions and subfractions in hyperlipidemia induced by a single dose of poloxamer 407 to mice. Our overarching goal was to quantify the changes in the serum lipopro- tein levels after poloxamer 407 treatment by utilizing a novel technique; specifically, small-angle X-ray scatter- ing (SAXS). 2. MATERIALS AND METHODS 2.1. Materials Male CBA/C57BL mice (breeding station of the Institute of Cytology and Genetics, Russian Academy of Sciences, Novosibirsk, Russia) having a body mass of 20-25 g were used. Poloxamer P-407 (Pluronic F-127, Sigma) was administered to mice, as a single, i.p. injection, in a dose of 1000 mg/kg. The animals were decapitated at 3, 24, 48 hrs, and then at 4, 5, 7, 12, 14 days after a single dose of poloxamer 407. All experiments followed the official “Rules for the work involving experimental animals” and “Ethical Committee Recommendations of working with laboratory animals”. Serum was obtained after centrifugation of blood sam- ples at 3000 × g for 15 min at 4C (Eppendorf Centri- fuge 5415R, Germany) and stored at –70C until analy- sis of total cholesterol (C), triglycerides (TG) of lipo- proteins (LP): C-LP, TG-LP, their fractions, and subfrac-  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 724 tions. 2.2. Small-Angle X-Ray Scattering (SAXS) According to Otvos et al. (2002) [15], LP fractions were divided into the following 4 main classes: high density LP (HDL), low-density LP (LDL), very-low-density LP (VLDL), and chylomicrons (the last ones were not de- termined by the method used) or 7 subfractions: HDL3, HDL2, LDL, Intermediate-Density LP–IDL, VLDL3-5, VLDL1-2, chylomicrons. Interval borders of fractions and subfractions (according to scale of sizes, ro) were the same as was indicated by Otvos (2002) [15]. A novel method for determination of fractional com- position of LPs using the small-angle X-ray scattering approach (SAXS) was used [4]. This method is inexpen- sive, quick, and capable of determining the relative con- tent of different LP fractions, both as a size distribution of various LP particles and as absolute units of the total concentration of lipid in LP fractions. At the same time, clinical diagnostic laboratories usually determine the concentrations of individual lipids in LP fractions, mainly TG and free cholesterol (FC), and often the total concentration of TG, FC and high density lipoproteins (HDL) free cholesterol. Measurement of fractional and subfractional composi- tion of serum LP fractions and subfractions.was pro- vided according to a method described earlier [4]. SAXS roentgenograms were obtained using a Siemens diffrac- tometer (Germany) by the method of step-by-step scan- ning with use of a goniometer and X-ray scintillation detector. Small-angle roentgenograms were measured in the angular range: h = 0.013 ÷ 0.22 A-1, where h = 4π · sin(θ)/λ; 2θ-scattering angle. A special thermostatted (20oC) quartz capillary cuvette (0.6 mm in diameter), having a wall thickness of 0.01 mm was used. The radia- tion wavelength (λ) was 1.54 Е. The small-angle X-ray roentgenograms were corrected by taking into account background scattering, adsorption, and collimation, after which the X-ray data became smoothed. The first step of mathematical processing of the SAXS data and compu- tation checks of functions for size distribution of spheri- cal particles were executed using a special computer program and algorithms described earlier, and also by use of optimization programs [4]. The results are reported as the mean standard devia- tion of at least 3 different experiments for each sample analyzed. The differences between samples were ana- lyzed by the Student’s t-test, and a P 0.05 was consid- ered statistically significant. 2.3. Morphological Study of Liver For morphometrical study of liver tissue, samples were first fixed in a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde on 0.1 M phosphate buffer, post- fixed in 1% osmium tetroxide solution, and then em- bedded in an Epon-Araldit mixture. Semithin, 1 micron tissue sections were obtained using the Ultramicrotome LKB-8800-V (Bromma, Vallingby, Sweden), stained with toludine blue and examined under a STAR Zeiss light microscope (Germany). The numeric density of macro- phage cells was calculated as the number of cells per 1 мм2 of tissue at a final magnification of 640. Not less than 50 fields of view were studied for every series of experiments. The cell measurements were made with the Motic Images 2000 Program. Ultrastructural changes of liver cells were investigated with an electron microscope, JEM 1400 (Jeol, Japan). Quantitative data were then processed using the Statistica-4 program, and the reli- ability of distinctions judged by the Student’s t-test. 3. RESULTS The relative weight of liver and spleen increased 48 hrs after poloxamer 407 administration (when the most sig- nificant hyperlipidemia was noted) (Figure 1). In pe- ripheral blood, there was an increased number of mono- cytes observed at day 12 (Figure 2). 3.1. Morphological Studies Light and electron microscopic studies demonstrated that liver sinusoids were considerably extended 1 day after poloxamer 407 administration, and that the numeric density of macrophages was significantly increased (al- most twice) (Table 1). The heterogeneity of macrophage dimensions was strongly increased. Very large cells, with X-axis: time after the single administration of poloxamer 407 (1000 mg/kg) to mice Y-axis: Organ: Body weight ratio of liver and spleen, %. The relative weight was expressed as the organ: body weight ratio Control group n = 26, poloxamer-treated groups the mean± SEM rep- resent a minimum of 7 mice at each time point. The data are shown as mean ± SEM (*p < 0.01 vs control) Figure 1. Relative liver and spleen weight of mice during sin- gle administration of poloxamer 407 (1000 mg/kg) to mice.  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 725 725 X-axis: Time after poloxamer 407 administration to mice. Y-axis: The number of PMN, monocytes and lymphocytes is expressed as % from the total amount of leukocytes. Poloxamer was administered i.p. as a single dose (1000 mg/kg). The number of animals in each group is 5. The data are shown as mean ± SEM (*p < 0.05 vs control). Figure 2. Influence of poloxamer 407 on leukocytes composi- tion of the peripheral blood of mice (%). Table 1. The numerical density of liver macrophages during single administration of poloxamer 407 to mice. Groups, the number of mice The numeric density of liver macrophages, per mm2 1. Control (intact) (5) 1277.4 ± 36.86 2. Poloxamer, 24 hrs (5) 2002.6 ± 36.58 P < 0.01 3. Poloxamer, 5 days after (5) 844.6 ± 28.74 P < 0.01 The data are shown as mean ± SEM. The number of mice is in the parentheses. a cross-sectional area more than 90-100 square microns, were observed among macrophages, which comprised about 40% of the macrophage population. These mac- rophages were filled with granular material, so their cy- toplasm had a foamy appearance (Figure 3). Five days after poloxamer 407 administration, mor- phometric data for the liver demonstrated that the spe- cific numerical density of macrophages was decreased, not only in comparison with the previous value, but in relation to the controls as well (Table 1). As before, large macrophages with a “foamy” cytoplasm containing electron light (transparent) material (possibly, of lipid origin) were observed, but the fraction of these cells was considerably reduced compared to the number of these cells in the previous experiment (Table 1). The cyto- plasm of hepatocytes in most cases was loose and mostly crumbly, with several large vacuoles, and their size was moderately increased. Electron micrograph of liver cells. In sinusoid lumen a large liver macrophage with foamy cytoplasm, filled by numerous electron light granules. Denotes: Mph–macrophage; Hep–hepatocyte; S-sinusoid *-electron light granules of macrophages Poloxamer was administered i.p. as a single dose (1000 mg/kg). Study was performed at the 1st day after administration of a single dose of poloxamer 407. Figure 3. Influence of poloxamer 407 on ultrastructure of liver cells. 3.2. Effect of Poloxamer 407 on Fractional and Subfractional Composition of Serum lipoproteins 3.2.1. Lipoprotein-Cholesterol (C-LP) Compared to intact (Control) mice, there was a signifi- cant (6-fold) increase in the serum total C-LP at 24 and 48 hrs, as well as day 4, in mice which were adminis- tered poloxamer 407 (Table 2). As shown in Table 2, among the total C-LP, the greatest increase (50-fold) was observed with the pro-atherogenic C-VLDL fraction (both C-VLDL1-2 and C-VLDL3-5 subfractions), as well as with intermediate density lipoproteins (C-IDL) (20- fold). However, it should be noted that serum concentra- tions of each fraction begin to decrease by day 4. The C-LDL concentration increased about twice, relative to controls, and continued to remain elevated at day 4. C-LDL1-3 was moderately (3- to 5-fold) increased at 48 hrs and at day 4 (Table 2). The C-IDL and TG-IDL sub- fractions were increased significantly (20-30-fold), while the C-LDL1-3 subfraction was moderately in- creased (3- to 5-fold) at 48 hrs and at day 4. Among anti-atherogenic fractions and subfractions, only a moderate increase of C-HDL was noted (2- to  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 726 Table 2. Concentration of C-LP fractions and subfractions (mg/dl) in murine serum during administration of poloxamer 407 (1000 mg/kg). LP Control, intact (n = 10) Poloxamer, 24 hrs (n = 10) Poloxamer, 48 hrs (n = 8) Poloxamer, 4 days (n = 5) C-LP (All) 107.4 ± 8.84 662.9 ± 82.40*** 599.7 ± 104.11** 727.2 ± 87.2* C-VLDL 6.5 ± 1.85 345.8 ± 46.90*** 271.3 ± 73.02** 134.4 ± 46.23** C-VLDL1-2 0.3 ± 0.07 16.7 ± 2.13*** 12.8 ± 4.70** 8.9 ± 4.11 C-VLDL3-5 6.0 ± 1.71 329.1 ± 45.54*** 258.5 ± 70.44** 125.5 ± 42.43** C-LDL 43.1 ± 7.67 106.9 ± 24.15** 201.0 ± 34.38*** 247.3 ± 31.52*** C-IDL 1.9 ± 1.61 40.3 ± 11.13** 62.6 ± 14.88*** 34.9 ± 13.32* C-LDL1-3 41.2 ± 8.61 66.6 ± 25.54 146.1 ± 32.68** 212.4 ± 35.56** C-HDL 57.9 ± 9.75 210.2 ± 48.36** 127.4 ± 41.93 45.4 ± 13.30 C-HDL2 43.1 ± 10.02 166.5 ± 38.70** 91.9 ± 47.31 16.9 ± 8.55 C-HDL3 14.7 ± 3.55 43.7 ± 20.21 35.5 ± 8.94 * 28.5 ± 9.23 The data are shown as mean ± SEM (*p< 0.05; **p < 0.01; ***р < 0.001 vs control) The number of animals is in the parentheses. Abbreviations: C-LP–cholesterol of lipoproteins; C-HDL–cholesterol of high density lipoproteins; C-LDL–cholesterol of low density lipoproteins; C-IDL–cholesterol of intermediate density lipoproteins; C-VLDL–cholesterol of very low density lipoproteins. 4-fold), with a return to baseline by day 4 (primarily due to a decrease in the concentration of the C-HDL2 sub- fraction), while the C-HDL3 subfraction showed a mod- erate increase (Table 2). 3.2.2. Lipoproteins-TG (TG-LP) In general, the total amount of TG-LP increased more significantly as compared to C-LP (20-fold), with a tendency to return to normal levels by day 4 (Tables 2 and 3). TG-VLDL “behavior” was similar to C-VLDL (increased 20- to 40-fold), and both subfractions (TG- VLDL1-2 and TG-VLDL3-5) were responsible for the elevation of the TG-VLDL fraction (Tables 2 and 3). Similar to C-LDL, an increase in TG-LDL was observed (6- to 8-fold). The level of C-IDL (Table 2) and TG-IDL (Table 3) was shown to be increased (~18- to 28-fold, respectively) with a significant difference at 24, 48 hrs, and on day 4, as compared to the controls (Ta- bles 2 and 3). A moderate elevation was also observed for C-HDL and TG-HDL (2- to 3-fold) by 24 hrs, but serum levels were not significantly greater than controls from 24 hrs onward (Tables 2 and 3). The TG-HDL2 subfraction was elevated only at 24 hrs after poloxamer 407 administration when compared to controls (Table 3). There were no significant changes of the TG-HDL3 sub- fraction (Table 3). 4. DISCUSSION Atherosclerosis is a complex disorder of lipid metabo- lism and a chronic inflammatory disease [16]. Changes in lipoprotein metabolism and hyperlipidemia play an important role in the progression of atherosclerosis. The initial period of atherosclerosis development and the interaction of lipoproteins with cells involved in the dis- lipidemic state merits further investigation. Mouse mod- els of hyperlipidemia and atherosclerosis are useful tools with which to study the role of “individual” lipoproteins of different classes. Lipoproteins are divided into 4 main fractions (classes) —HDL, LDL, VLDL, and chylomicrons, or 7 subfrac- tions (HDL3, HDL2, LDL, IDL, VLDL3-5, VLDL1-2, chy- lomicrons), or 16 sub/subfractions [15,17,18]. Most se- rum cholesterol, about 60-70% of total serum cholesterol, is known to be contained in LDL-C as the major athero- genic lipoprotein fraction [1]. During the last decade, several new methods, including the analytical ultracen- trifugal micromethod, were developed for identification of atherogenic LDL, and their subclasses, without their preparative isolation [19]. At the same time, it is important to investigate other lipoprotein fractions—namely, VLDL and HDL, as well as their subfractions. VLDL, which is a precursor of LDL, and some forms of VLDL, is atherogenic, especially their remnant forms. VLDL contains most of the serum triglycerides, their role, of which, is now thought to be an independent and important risk factor in atherosclero- sis [20]. HDL is known as an antiatherogenic fraction of lipoproteins [21]. With the assistance of the new physico- chemical SAXS method, it is possible to simultaneously 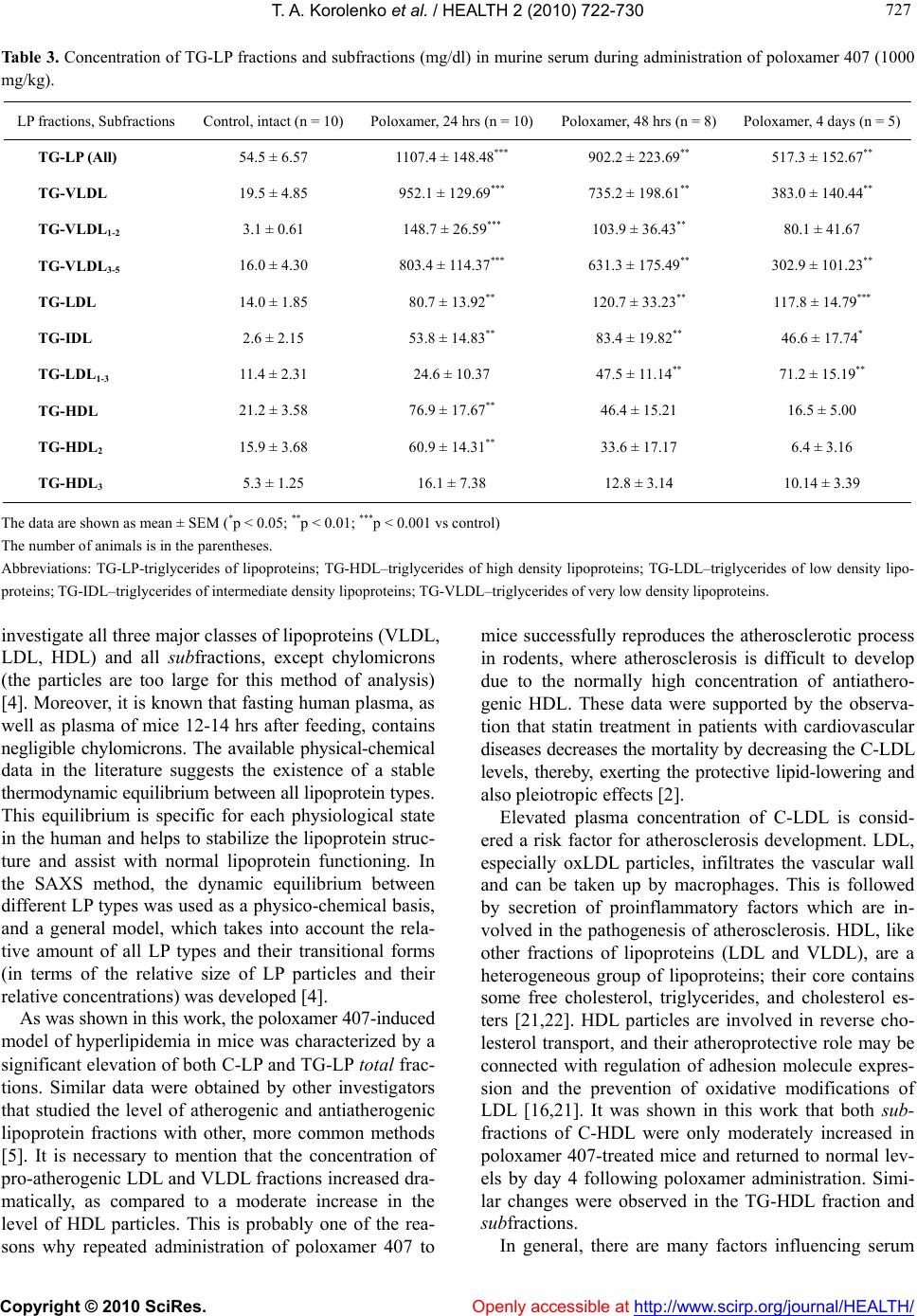 T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 727 727 Table 3. Concentration of TG-LP fractions and subfractions (mg/dl) in murine serum during administration of poloxamer 407 (1000 mg/kg). LP fractions, Subfractions Control, intact (n = 10) Poloxamer, 24 hrs (n = 10)Poloxamer, 48 hrs (n = 8) Poloxamer, 4 days (n = 5) TG-LP (All) 54.5 ± 6.57 1107.4 ± 148.48*** 902.2 ± 223.69** 517.3 ± 152.67** TG-VLDL 19.5 ± 4.85 952.1 ± 129.69*** 735.2 ± 198.61** 383.0 ± 140.44** TG-VLDL1-2 3.1 ± 0.61 148.7 ± 26.59*** 103.9 ± 36.43** 80.1 ± 41.67 TG-VLDL3-5 16.0 ± 4.30 803.4 ± 114.37*** 631.3 ± 175.49** 302.9 ± 101.23** TG-LDL 14.0 ± 1.85 80.7 ± 13.92** 120.7 ± 33.23** 117.8 ± 14.79*** TG-IDL 2.6 ± 2.15 53.8 ± 14.83** 83.4 ± 19.82** 46.6 ± 17.74* TG-LDL1-3 11.4 ± 2.31 24.6 ± 10.37 47.5 ± 11.14** 71.2 ± 15.19** TG-HDL 21.2 ± 3.58 76.9 ± 17.67** 46.4 ± 15.21 16.5 ± 5.00 TG-HDL2 15.9 ± 3.68 60.9 ± 14.31** 33.6 ± 17.17 6.4 ± 3.16 TG-HDL3 5.3 ± 1.25 16.1 ± 7.38 12.8 ± 3.14 10.14 ± 3.39 The data are shown as mean ± SEM (*p < 0.05; **p < 0.01; ***р < 0.001 vs control) The number of animals is in the parentheses. Abbreviations: TG-LP-triglycerides of lipoproteins; TG-HDL–triglycerides of high density lipoproteins; TG-LDL–triglycerides of low density lipo- proteins; TG-IDL–triglycerides of intermediate density lipoproteins; TG-VLDL–triglycerides of very low density lipoproteins. investigate all three major classes of lipoproteins (VLDL, LDL, HDL) and all subfractions, except chylomicrons (the particles are too large for this method of analysis) [4]. Moreover, it is known that fasting human plasma, as well as plasma of mice 12-14 hrs after feeding, contains negligible chylomicrons. The available physical-chemical data in the literature suggests the existence of a stable thermodynamic equilibrium between all lipoprotein types. This equilibrium is specific for each physiological state in the human and helps to stabilize the lipoprotein struc- ture and assist with normal lipoprotein functioning. In the SAXS method, the dynamic equilibrium between different LP types was used as a physico-chemical basis, and a general model, which takes into account the rela- tive amount of all LP types and their transitional forms (in terms of the relative size of LP particles and their relative concentrations) was developed [4]. As was shown in this work, the poloxamer 407-induced model of hyperlipidemia in mice was characterized by a significant elevation of both C-LP and TG-LP total frac- tions. Similar data were obtained by other investigators that studied the level of atherogenic and antiatherogenic lipoprotein fractions with other, more common methods [5]. It is necessary to mention that the concentration of pro-atherogenic LDL and VLDL fractions increased dra- matically, as compared to a moderate increase in the level of HDL particles. This is probably one of the rea- sons why repeated administration of poloxamer 407 to mice successfully reproduces the atherosclerotic process in rodents, where atherosclerosis is difficult to develop due to the normally high concentration of antiathero- genic HDL. These data were supported by the observa- tion that statin treatment in patients with cardiovascular diseases decreases the mortality by decreasing the C-LDL levels, thereby, exerting the protective lipid-lowering and also pleiotropic effects [2]. Elevated plasma concentration of C-LDL is consid- ered a risk factor for atherosclerosis development. LDL, especially oxLDL particles, infiltrates the vascular wall and can be taken up by macrophages. This is followed by secretion of proinflammatory factors which are in- volved in the pathogenesis of atherosclerosis. HDL, like other fractions of lipoproteins (LDL and VLDL), are a heterogeneous group of lipoproteins; their core contains some free cholesterol, triglycerides, and cholesterol es- ters [21,22]. HDL particles are involved in reverse cho- lesterol transport, and their atheroprotective role may be connected with regulation of adhesion molecule expres- sion and the prevention of oxidative modifications of LDL [16,21]. It was shown in this work that both sub- fractions of C-HDL were only moderately increased in poloxamer 407-treated mice and returned to normal lev- els by day 4 following poloxamer administration. Simi- lar changes were observed in the TG-HDL fraction and subfractions. In general, there are many factors influencing serum  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 728 concentration of lipoproteins (biosynthesis, uptake by endocytosis, secretion, and degradation). The liver is the main source of VLDL. LDL catabolism occurs primarily in the liver and in most extrahepatic tissues [2]. Different types of cells have been shown to have LDL receptors on the plasma membrane [6]. Catabolism of LDL has been shown to occur inside of lysosomes [23,24]. Me- tabolism of HDL is closely related to homeostasis of lipids in the intact vertebrate, with lipoproteins residing in both the serum and in tissues [2]. Poloxamer 407 appears to act as a general lipase in- hibitor. Mammalian lipases include three main lipase members; namely, lipoprotein lipase, hepatic lipase, and endothelial lipase, acting in vitro and in vivo on lipopro- teins [23]. Lipoprotein lipase has a high triglyceride li- pase activity, while endothelial lipase exerts phospholi- pase activity. Endothelial lipase is able to hydrolyze lip- ids from HDL, while hepatic lipase exerts functional activity on all classes of lipoproteins [23]. Lipoprotein lipase (EC 3.1.1.34) (LPL) is a key enzyme in lipopro- tein metabolism. LPL belongs to the class of enzymes known as hydrolases, which catalyze the degradation of glycerides, including triglycerides. Poloxamer 407 (as well as the nonionic detergent Triton WR 1339) has been shown to inhibit LPL, and this effect has been suggested as playing a crucial role in disturbances of lipoprotein transport and a significant elevation in the serum levels of LP-TG. There are some similarities in the models of polox- amer and Triton WR 1339-induced hyperlipidemia fol- lowing a single intraperitoneal administration of each agent. Both models were characterized by dramatic in- creased serum concentrations of cholesterol and, espe- cially triglycerides, in rats and mice. As mentioned above, the resultant hypertriglyceridemia occurs due to surfactant-mediated inhibition of LPL’s enzymatic activ- ity [25,26]. Moreover, Triton WR 1339 is a well-known lysosomotropic agent, accumulating inside of lysosomes of liver cells; specifically, hepatocytes and Kupffer cells [27]. In both species of rodents, Triton-induced hyper- lipidemia occurred 24 hrs after detergent administration, and was followed by a dramatic increase in both lipo- protein cholesterol and, especially, of lipoprotein triglyc- eride concentrations [26,27]. We have recently shown significant increases in the concentration of atherogenic C-LDL, C-VLDL (due to an increase of the VLDL3-5 subfraction), and IDL in mice, and even more profound elevations in rats (Korolenko et al., submitted In Press). These hyperlipidemic animal models can be used to study the role of lipoproteins, especially lipoprotein triglycerides, (but also C-LDL and C-VLDL) in the pathogenesis of atherosclerosis, as well as for testing the efficacy of new hypolipidemic drugs. The poloxamer 407-induced mouse model of hyperlipidemia would ap- pear to resemble a Type 2a/2b or 3 dislipidemia in hu- mans. Mice and rat models of hyperlipidemia are used more often now because of their convenience, the ability to genetically alter the animals to evaluate the effect of a particular gene on lipid metabolism, and because they are cost-effective. The poloxamer 407-induced mouse model of dyslipidemia and atherosclerosis reliably re- produces the hyperlipidemic state, and, if administered on a repetitive basis (or, if dosed continuously with the aid of an implanted osmotic pump), causes formation of fibrofatty aortic lesions in the same size and number as classic diet-induced or gene-knockout mouse models [28]. Poloxamer 407 has been used in several fields of medi- cine as a nano-carrier for different drugs for the treat- ment of several inflammatory diseases and tumors [29]. However, when used to induce dyslipidemia and athero- sclerosis in C57BL/6 mice, it must be emphasized that these are supraphysiologic doses of P-407 that would never be utilized in any commercial formulation em- ploying its sustained-release or reverse-thermal gelation properties. Additionally, most applications of poloxamer 407 in the pharmaceutical literature deal with non-par- enteral routes of delivery, unlike the intraperitoneal ad- ministration of poloxamer 407 to intentionally induce dyslipidemia in mice [30-34]. In conclusion, we have identified changes in the lipid fractions and subfractions following a single dose of poloxamer 407 to mice, as well as characterized the rela- tive spleen and liver weights, and the effects on blood leukocytes. Additionally, we have pioneered the use of SAXS as a method to determine the concentrations of the serum lipids and lipid subfractions. This new ana- lytical approach can now be successfully employed to determine the concentrations of lipoprotein fractions, and their subfractions, in either mouse or human plasma samples for a more complete picture of the hyperlipi- demic state. 5. ACKNOWLEDGEMENTS This work was partially supported by integrated grant of Siberian Branch of the Russian Academy of Medical Sciences and Far East Division of Russian Academy of Sciences on natural immunomodula- tors study (2006-2008). Authors are grateful to engineer M.Tuzikov for help in Small-angle X-ray scattering, Dr. Monoszon A., Dr. Kisarova Ya., Dr. Sukhovershin R., Dr. K. Loktev for technical help during preparing of the manuscript. REFERENCES [1] Anderson, L. (2005) Candidate-based proteomics in the  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 729 729 search for biomarkers of cardiovascular disease. The Journal of Physiology, 563(1), 23-60. [2] Gotto, A.M.Jr, and Farmer, J.A. (2006) Drug insight: The role of statins in combination with ezetimide to lower LDL cholesterol. Nature Clinical Practice Cardiovascu- lar Medicine, 3(12), 664-672. [3] Ugawa, T., Kakuta, H., Moritani, H., Inagaki, O. and Shikama, H. (2003) YM-53601, a novel squalene syn- thase inhibitor, suppresses lipogenic biosynthesis and lipid secretion in rodents. British Journal of Pharmacol- ogy, 139(1), 140-146. [4] Tuzikov, F.V., Tuzikova, N.A., Galimov, R.V., Panin, L.E. and Nevinsky, G.A. (2002) General model to describe the structure and dynamic balance between different human serum lipoproteins and its practicl application. Medical Science Monitor, 8(6), pp. 79-88. [5] Dumortier, G., Grossiord, J.L., Agnely, F. and Chaumeil, J.C. (2006) A review of poloxamer 407 pharmaceutical and pharmacological Characteristics. Pharmaceuticals Research, 23(12), 2709-2728. [6] Leon, C., Wasan, K.M., Sachs-Barrable, K. and Johnston, T.P. (2006) Acute P-407 administration to mice causes hypercholesterolemia by inducing cholesterolgenesis and down-regulating low-density lipoprotein receptor expres- sion. Pharmaceuticals Research, 23(7), 1597-1607. [7] Kim, H.Y., Jeong, D.M., Jung, H.J., Jung, H.J., Yozo- kawa, T. and Choi, J.S. (2008) Hypolipidemic effects of Sophora Flavescens and its constituents in poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Biological & Pharmaceutical Bulletin, 31(1), 73-78. [8] Johnston, T.P. and Palmer, W.K. (1997) The effect of pravastatin on hepatic 3-hydroxy-3-methylglutaryl CoA reductase obtained from poloxamer 407-induced hyper- lipidemic rats. Pharmacotherapy, 17(2), 342-347. [9] Johnston, T.P., Baker, J.C., Hall, D., Jamal, S., Palmer, W.K. and Emeson, E.E. (2000) Regression of poloxamer 407-induced atherosclerotic lesions in C57BL/6 mice using atorvastatin. A therosclerosis, 149(2), 303-313. [10] Johnston, T.P., Nguyen, L.B., Chu, W.A. and Shefer, S. (2001) Potency of select statin drugs in a new mouse model of hyperlipidemia and atherosclerosis. Interna- tional Journal of Pharmaceutics, 229(1-2), 75-86. [11] Nash, V.J., Johnston, T.P. and Palmer, W.K. (1996) Ef- fects of nicotinic acid on poloxamer 407-induced hyper- lipidemia. Pharmacotherapy, 16(1), 10-15. [12] Johnston, T.P., Li, Y., Jamal, A.S., Stechschulte, D.J. and Dileepan, K.N. (2003) Poloxamer 407-induced athero- sclerosis in mice appears to be due to lipid derangements and not due to its direct effects on endothelial cells and macrophages. Mediators of Inflammation, 12(3), 147- 155. [13] Johnston, T.P. (2009) Poloxamer 407 increases soluble adhesion molecules, ICAM-1, VCAM-1, and E-selectin, in C57BL/6 Mice. Journal of Pharmacy and Pharma- cology, 61(12), 1681-1688. [14] Johnston, T.P. and Waxman, D.J. (2008) The induction of atherogenic dyslipidemia in poloxamer 407-treated mice is not mediated through PPARα. Journal of Pharmaceu- tical Sciences, 60(6), 753-759. [15] Otvos, J.D. (2002) Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clinical Laboratory, 48(3-4), 171-180. [16] Pelton, P.D, Patel, M. and Demarest, K.T. (2005) Nuclear receptors as potential targets for modulating reverse cho- lesterol transport, Current Topics in Medicinal Chemistry, 5(3), 265-282. [17] Jeyarajah, E.J., Cromwell, W.C. and Otvos, J.D. (2006) Lipoprotein particle analysis by nuclear magnetic reso- nance spectroscopy. Clinics in Laboratory Medicine, 26(4), 847-870. [18] Cromwell, W.C. and Otvos, J.D. (2006) Heterogeneity of low-density lipoprotein particle number in patients with type 2 diabetes mellitus and low-density lipoprotein cho- lesterol <100 mg/dl. American Journal of Cardiology, 98(12), 1599-1602. [19] Bozóky, Z., Fülöp, L. and Köhidai, L. (2001) A short run new analytical ultracentrifugal micromethod for deter- mining low-density lipoprotein sub-fractions using Sch- lieren refractometry. European Biophysics Journal, 29(8), 621-627. [20] Barcia, A.M. and Harris, H.W. (2005) Triglyceride-rich lipoproteins as agents of innate immunity, Clinical Infec- tious Diseases, 41(10), 498-503. [21] Tanaka, H., Ishida, T., Johnston, T.P., Yasuda, T., Ueyama, T., Kojima, Y., Kundu, R.K., Quertermous, T. and Hirata, K.-I. (2009) Role of endothelial lipase in plasma HDL levels in a murine model of hypertriglyceridemia. Jour- nal of Atherosclerosis and Thrombosis, 16(4), 327-338. [22] Gatica, L.V., Vega, V.A., Zirulnik, F., Oliveros, L.B. and Gimenez, M.S. (2006) Alterations in the lipid metabo- lism of rat aorta: Effects of vitamin A deficiency. Journal of Va scu lar Research , 43(6), 602-610. [23] Brown, R.J. and Rader, D.J. (2007) Lipases as modula- tors of atherosclerosis in murine models. Current Drug Targets, 8(12), 1307-1319. [24] Ballabio, A. and Gieselmann, V. (2009) Lysosomal dis- orders: From storage to cellular damage. Biochimica et Biophysica Acta, 1793(4), 684-696. [25] Johnston, T.P. and Palmer, W.K. (1993) Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochemical Pharmacology, 46(6), 1037-1042. [26] Millar, J.S., Cromley, D.A, McCoy, M.G., Rader, D.J. and Billheimer, J.T. (2005) Determining hepatic triglyc- eride production in mice: Comparison of poloxamer 407 with triton WR 1339. Journal of Lipid Research, 46(9), 2023-2028. [27] Schneider, P., Korolenko, T.A. and Busch, U. (1997) A review of drug-induced lysosomal disorders of the liver in man and laboratory animals. Microscopy Research and Technique, 36(4), 253-275. [28] Johnston, T.P. (2004) The P-407-induced murine model of dose-controlled hyperlipidemia and atherosclerosis: A review of findings to date. Journal of Cardiovascular Pharmacology, 43(4), 595-606. [29] Kuzman, D., Fon Tacer, K., Cerne, M., Rezen, T., Aci- movic, J., Cegovnik, U., Kocjan, D., Urleb, U. and Roz- man, D. (2009) Modulation of hepatic transcriptome in the poloxamer P-407 hyperlipidemia mouse model. Acta Chimica Slovenica, 56(1), 262-269. [30] Fruchart, J.C. and Duriez, P. (2006) Mode of action of fibrates in the regulation of triglyceride and HDL-cho- lesterol metabolism. Drugs Today (Barc), 42(1), 39-64. [31] Butler, J.A., Hagen, T.M. and Moreau, R. (2009) Lipoic acid improves hypertriglyceridemia by stimulating tria-  T. A. Korolenko et al. / HEALTH 2 (2010) 722-730 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 730 cylglycerol clearance and downregulating liver triacyl- glycerol secretion. Archives of Biochemistry and Bio- physics, 485(1), 63-71. [32] Mora, S., Otvos, J.D., Rifai, N., Rosenson, R.S., Buring, J.E. and Ridker, P.M. (2009) Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardio- vascular disease in women. Circulation, 119(17), 931- 939. [33] Zhou, X., Johnston, T.P., Johansson, D., Parini, P., Funa, K., Svensson, J. and Hansson, G.K. (2009) Hypercholes- terolemia leads to elevated TGF-beta 1 activity and T helper 3-dependent autoimmune responses in atheroscle- rotic mice, A therosclerosis, 204(2), 381-387. [34] Gotto, A.M. and Farmer, J.A. (2007) Atherosclerosis: Pathogenesis, morphology, and risk factors. In: Willerson J.T., Wellens H., Cohn J.N. and Holmes D.R.Jr. Eds., Cardiovascular Medicine, 3rd Edition, Springer, London, 1593-1613. ABBREVIATIONS C–cholesterol TG–triglycerides LP–lipoproteins C (TG)-HDL–cholesterol (triglycerides) of high density lipoproteins C (TG)-LDL–cholesterol (triglycerides) of low density lipoproteins C (TG)-IDL–cholesterol (triglycerides) of intermediate density lipoproteins C (TG)-VLDL–cholesterol (triglycerides) of very low density lipoproteins SAXS-Small-angle X-ray scattering |

