American Journal of Plant Sciences
Vol.08 No.03(2017), Article ID:74486,19 pages
10.4236/ajps.2017.83043
The Combined Action Strategy of Two Stresses, Salinity and Cu++ on Growth, Metabolites and Protein Pattern of Wheat Plant
Hamdia M. Abd El-Samad, D. Mostafa, Kholoud N. Abd El-Hakeem
Botany Department, Faculty of Science, Minia University, El-Minia, Egypt

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 5, 2016; Accepted: February 25, 2017; Published: February 28, 2017
ABSTRACT
The response of wheat plants to different osmotic stress levels varied among the different organs root, shoot and spike and the situation of these organs with application of two Cu++ levels 5 mM and 25 mM as CuSO4. The sensitivity of root organ was related with reduction in fresh, dry matter and length. This resulted from reduction of soluble sugar reflected a reduction in water uptake and K+ content of the cell sap. In the moderate organ spike, the reduction in fresh, dry matter and length were concomitant with the accumulation of soluble sugar and a huge accumulation of soluble protein. In the higher organ shoot, this related with more water uptake which in turn induced an accumulation of soluble protein and cofactor K+ content. It can be recorded that shoot was higher Na+ accumulation than root and spike. Data also showed further stimulatory effect on growth parameters by Cu++ applications with either concentration (7.5 mM or 25 mM). Irrigating the soil with either 7.5 or 25 mM CuSO4 induced a huge accumulation in soluble sugar, soluble protein and nitrate reductase. Cupper treatment with either concentration 7.5 mM or 25 mM induced a marked decrease in Na+ content at all OSL and has no significant change in the accumulation of K+ in both shoot and spike whereas induced a huge accumulation in root organ. The synthesis of protein bands with molecular weight 32.3 KDa at −1.5 MPa NaCl level treated with either 7.5 mM or 25 mM Cu++ concentrations was induced. Also the appearance of protein band with molecular weight 37KDa induced only at Cu++ treatments with 25 mM concentration in both control and under different osmotic levels (0.0, −0.3 MPa, −0.9 MPa, −1.5 MPa NaCl).
Keywords:
Osmotic Stress Levels, Cupper, Tolerance, Wheat, Protein Bands

1. Introduction
It is estimated that about 20% of the irrigated land in the present world is affected by salinity that is exclusively classified as arid and desert lands comprising 25% of the total land of our planet [1] [2] . Soil salinity is a major constraint to food production because it limits crop yield and restricts use of land previously uncultivated [3] [4] [5] [6] . Growth and development of glycophytes are negatively affected but halophytes tolerate high salt concentrations [7] [8] . Salinity can be minimized with water and drainage, but the cost of engineering and management is very high [9] [10] [11] . Wheat plant was considered as one of the most important crop plants in Egypt because of its contribution as main nutrient foods for people. Copper deficiency is a significant nutritional disorder of plants grown on alkaline-saline soils of arid and semi-arid climates [12] . Cu is known as an essential micronutrient for the function of copper-zinc superoxide dismutase (SOD) and catalase (CAT) which are the most important ROS scavenger enzymes. Copper plays an important role in the synthesis of phenolic compounds and its deficiency can decrease phenolic in the plants [13] . Mehrizi et al. [14] demonstrated that plant growth is often limited by low levels of soil micronutrients such as copper (Cu), especially in calcareous salt-affected soils of arid and semi-arid regions. The aim of this work was to investigate individual and combined effects of salinity and Cu on growth, leaf relative water content (LRWC), cell membrane permeability, lipid peroxidation, and total phenolic content (TPC) of rosemary (Rosmarinus officinalis L.) in a hydroponic experiment. Thus whether plant adaptation to salinity is accompanied by improved tolerance to heavy metals, to copper in particular, remains an open question, and the mechanisms of plant tolerance to combined action of the two factors remain unstudied. At the same time, to understand the mechanisms of plant tolerance to chloride salinity and heavy metals is really important for both the elucidation of common mechanisms of plant resistance to various extreme factors and agricultural practice [15] [16] [17] .
This study was performed on the wheat plants (cv. Giza 168) a facultative glycophytes capable of completing its life cycle under high concentrations of salinity examined; whether copper effects were manifested under conditions of sodium chloride salinity and the interactions of the two stressors during their combined action on the plant.
2. Materials and Methods
2.1. Experimental Sites
A pot experiment was carried out in open environment at the Botany Garden of the Faculty of Science, Botany and Microbiology Department, Minia University during winter season (from the beginning of November 2014 to the middle of March 2015 until yield production for 120-days). At this period of year temperature was interval between 25˚C to 30˚C, light 12 h and dark 12 h. Grain of cv. Giza 186 was obtained from Beni Suief, Seds Center, Egypt, Agricultural pharmacies in bags, already a factory prepared for sowing. From investigation was carried by Hamdia et al. [11] showed that the salt tolerance (0.0, 20, 50, 100, 150, 200 and 300 mM NaCl levels) of the four wheat cultivars, during vegetative growth and crop yield stages ranked as the following: cv. Sakha 94 > cv. Gimiza11 > cv. Gimiza10 > cv. Giza168. This means that cv. Sakha 94 was the superior and cv. Giza168 was the interior. We select cv. Giza 168 because it was most salt sensitive. Wheat seeds were surface sterilized by immersion in a mixture of ethanol 96% and H2O2 (1:1) for 3 minutes, followed by several washings with sterile distilled water.
2.2. Salinity and Cupper Treatments
The concentrations of NaCl were chosen after preliminary experiments in which the seeds were subjected to different concentrations of NaCl. Eight seeds were sown per pot. Each pot contained 3.8 kg of garden clay soil in three replicates. All pots were irrigated with tap water for two weeks. The seedlings were then treated with five different concentrations of NaCl solutions −0.3 MPa, −0.6 MPa, −0.9 MPa, −1.2 MPa and −1.5 MPa in addition of control irrigated only with water after two weeks from sowing in the first group. In order to maintain the osmotic potential, the soil moisture content was kept near field capacity using tap water. The previous treatments were repeated for irrigation with either 7.5 mM CuSO4 or 25 mM CuSO4 as second and third groups. Three replicate was made for each treatment. Plants were grown in natural conditions for crop yield production.
2.3. Laboratory Analysis for Metabolites
Roots, shoots and spikes dry weight sand length of shoot, root and spike were determined at the end of the experiment. The photosynthetic pigments were determined by Metzner et al. [18] .
Leaf area (Cm2 plant−1) was determined by measuring the leaf length and the maximum width and applying the formula:
Leaf area = k (leaf length × leaf maximum width), where (K = 0.75)
The coefficient k was calculated and assigned different values for different grasses [19] [20] and recently reviewed and given a value of 0.75 for monocot [21] . Water content in plant tissue organs was calculated using fresh and dry weight values [22] . Succulence index, was determined as the water content per unit area of leaves [23] . Harvest index (HI) is an index of shoot dry matter allocated to crop yield production HI = Economic yield/biological yield = (wt. of spike/total wt. of shoot) [24] . The determination of dry matter yields were done after the organs were separately oven-dried at 80˚C. Successive weighting was carried out until the constant dry weight of each sample was reached. Soluble sugars were determined by using anthrone-sulfuric acids method in water extracted sample [25] . Soluble protein contents were measured according to Lowry et al. [26] in also water extracted samples. Nitrate reductase activity was measured as nitrate reductase activity was determined according to Jaworski [27] . One gram fresh 12 qsh leaf sample tissue was incubated for 30 min in assay medium (PH 7), then was boiled at 100˚C for 5 min. The nitrite was then determined calorimetrically using sulphanilic acid and α-naphthylamine solution at 520 nm with a 55B Perking Elemerspectrophotometer (UK). Na+ and K+ were measured Flamphotometeric by Williams and Twin [28] . The electrophoresis was carried out in vertical polyacrylamide gels, using the slab gel apparatus “SE 600, vertical slab gel”. Polyacrylamide gel electrophoresis was carried out according to Laemmli [29] with 12% acrylamide + 1.0% SDS for protein analysis.
3. Statistical Analysis
The experimental data were subjected to the one way analysis of variances (ANOVA test) using the
4. Results
Table 1 and Table 2 shows that fresh, dry matter, of shoot, root and spike under
Table 1. Analysis for fresh and dry matter (g plant−1) shoot and root of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
Table 2. Analysis for fresh and dry matter of spike of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
increasing osmotic stress. They decreased in reference control plants (0.0, −0.3 MPa, −0.6 MPa, −0.9 MPa, −1.2 MPa and −1.5 MPa) as well. The percent of reduction of these parameters at −1.5 MPa was 10.1%, 19.9%, 48%, 48.2%, 30.5% and 27.9% compared with absolute control plants (0.0). Data also showed further stimulatory effect on growth parameters by Cu++ applications with either concentration (7.5 mM or 25 mM). Length of spike generally increases as osmotic stress increased, the maximum percentage values were obtained at −0.3 MPa osmotic stress level (Figures 1(a)-(c)). While shoot and root length tended to decrease as elevating osmotic stress. Cu++ application with either concentration enhanced the length of different three organs of wheat plants. Water content suddenly reduced at −0.3 MPa OSL at different three organs of wheat plants. The percent of reduction was the same value in both shoot and root 57.3% and 42.9% in spike organ (Table 3). After that level water content lower but higher than −0.3 MPa OSL as compared with control plants and this effect was more pronounced at root followed by spike and later shoot organ. Cupper treatment increase water uptake at all OSL, this more detected at pants irrigated with 7.5 mM Cu++ compared with uncupper treatments (Table 3). The percent of increase reach above 2- folds as in −0.9 Mpa and −1.2 MPa OSL in root organs. Leaf area
Figure 1. Analysis for on length (Cm) of shoot (a), root (b), spike (c) and nitrate reductase activity (μ mol g d. m. h−1) (d) of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
Table 3. Analysis for on water content (g plant−1) of shoot, root and spike of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
was elevated as increasing OSL, the high values was recorded at −1.2 MPa OSL, Cu++ treatment with either concentrations 7.5 or 25 mM enlarge the leaf area of wheat plants at all salinization levels (Figure 2). Harvest index was significantly higher as compared with plants irrigated only with water. Harvest index was prominent increase when irrigated the soil with either 7.5 mM or 25 mM osmotic stress level (Figure 2). Succulent index the sign of increasing osmotic stress was markedly elevated as NaCl increase when treated wheat plans with Cu++ (7.5 mM or 25 mM) was generally reduced the succulent index compared with untreated plants (Figure 2). Cholorophyll a and chl. b significantly enhanced with elevating osmotic stress as compared with untreated plants (Figure 3(a) and Figure 3(b)). This effect was more prominent with Chl. b than Chl. a. It is worthy that 7.5 mM Cu++ treatment activate the synthesis of Chl. a more than 25 mM Cu++ treatment, while has no effect on Chl. b and carotenoids (Figure 3(c)) elevated as osmotic stress increased up to −0.9 MPa, obove that a marked lowering was detected reach a maximum values at 1.5 MPa. On the other side applications of Cu++ with either concentrations 7.5 mM or 25 mM was significantly activated the synthesis of carotenoids at all osmotic stress levels. Soluble sugar decreased considerably in shoot while a significant accumulation was recorded in root and spike organs (Table 4). The percent of reduction in shoot at −1.5 MPa OSL was
Figure 2. Analysis for on leaf area (a), harvest index (b) and succulent (c) of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
Figure 3. Analysis for on Chl. a (a), Chl. b (b) and carotenoids (c (mg g−1 d. m.) of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
Table 4. Analysis for on soluble sugar of shoot, root and spike of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
Table 5. Analysis for on soluble protein of shoot, root and spike of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
24.8%, whereas the maximum values were recorded at −1.5 MPa in case of root reach above 2- folds and at −0.9 MPa in case of spike reach 62.2 % above control value. Irrigated the soil with either 7.5 or 25 mM CuSO4 induced a huge accumulation in soluble sugar, this was more observed in root than in shoot and spike reach 4-folds at all OSL compared with untreated plants (Table 3). Soluble protein was considerably increased as increasing OSL, this accumulation was more prominent in spike, root than in shoot (Table 5). The maximum value at −1.5 MPa in shoot, in root and spike at −0.9 MPa OSL, the percent of increase at that level was 42.9%, 77.4% and 278.2%. Cu++ application induced a marked elevating in the protein accumulation especially in spike than in shoot and root and in 7.5 mM Cu++ than 25 mM Cu++ compared with control plants. Nitrate reductase was markedly decreased as increasing salinity levels (Figure 1(d)), there is a sudden drop in the NR activity at −0.3 MPa OSL, the percent of reduction was 18.8% compared with plant irrigated with water. Treatment soil media with either 7.5 mM or 25 mM increased the NR activity. This activation was more obvious at all OS levels. In the reference control sodium increased as increasing OSL in three different organs of wheat plants, the higher value was recorded at highest OSL (Table 6). The percent of increasing at −1.5 MPa was 3-fold in shoot, 2-fold in root and 1.5-fold in spike. Potassium significantly increased in shoot and root while it decreased in spike as OSL increased (Table 3). The highest values were recorded at −0.9 MPa where the percent of increase was 49% and 50% above the value of control plant (Table 3). Whereas the reduction in K+ content in spike at −1.2 MPa was 11.5 below the control value. Cupper treatment with either concentration 7.5 mM or 25 mM induced a marked decrease in Na+ content at all OSL. Cu++ application has no significant change in the accumulation of K+ in both shoot and spike whereas induced a huge accumulation in root organ reach 4-fold
Table 6. Analysis for on Na+ and K+ content of shoot, root and spike of wheat plants Giza 186 grown under OSL alone and with Cu I 7.5 mM or Cu II 25 mM.
at lower and moderate OSL in plant irrigated with 7.5 mM Cu++ and 3-folds in plants irrigated with 25 mM Cu++ (Table 3). The synthesis of protein bands with molecular weight 32.3 KDa at −1.5 MPa NaCl level treated with either 7.5 mM or 25 mM Cu++ concentrations were associated with the accumulation of soluble protein in spike organ at the same level −1.5 MPa with concentrations of both Cu++ treatments (Table 7 and Figure 4). This indicated that this band was induced under the influence of higher salinization levels. Also the appearance of protein band with molecular weight 37 KDa induced only at Cu++ treatments with 25 mM concentration in both control and under different osmotic stress (0.0, −0.3 MPa, −0.9 MPa and −1.5 MPa NaCl).
5. Discussion
The response of wheat plants to different osmotic stress levels varied among the different organs root, shoot and spike. From the present results it can be detected that root organ was the most sensitive organ followed by spike organ and finally the most tolerant organ was shoot to increasing osmotic stress levels. The sensitivity of root organ was related with reduction in fresh, dry matter and length. The percent of reduction at −1.5 MPa OSL was 48%, 48.2% and 11.1% below the control value 100% (i.e. reach lower than 50% of control fresh and dry matter of root). This situation resulted from reduction of soluble sugar reflected a reduction in water uptake and K+ content of the cell sap. The moderate organ of salt tolerance is the spike; the reduction in fresh, dry matter and length (the
Table 7. Analysis for protein pattern of wheat cultivar Giza 168 in response of salinity stress (−0.3 MPa, −0.9 MPa and −1.5 MPa) and interaction with Cu++ treatments (I: 7.5 mM or II: 25 mM) as compared with control plants.
+ Meaning the protein band was found at molecular weight with KDa. -Meaning the protein band was absent at molecular weight with KDa.
Figure 4. Analysis for protein pattern of wheat cultivar Giza 168 in response of salinity stress (−0.3 MPa, −0.9 MPa and −1.5 MPa) and interaction with Cu++ treatments (I: 7.5 mM or II: 25 mM) as compared with control plants.
percent of reduction was 30.5%, 27.9% and 4.3% below the control 100%) was concomitant with the accumulation of soluble sugar and a huge accumulation of soluble protein. The higher organ of salt tolerance was shoot; the percent of reduction in fresh, dry matter and length at −1.5 MPa OSL was 20.2%, 24.4% and 16.7% below control value. This related with more water uptake than root and spike which in turn induced an accumulation of soluble protein and cofactor K+ content. Accordingly one can say that the criteria of the green area could link in some way with the efficiency of photosynthetic apparatus and consequently food manufacturing. This conclusion was greatly confirmed by the differences in the carbohydrate and nitrogen metabolism among the wheat organs. Also, supporting the above view, our results of leaf area and harvest index were elevated while succulent index was lower as osmotic stress level increased. This run paralleled with general tolerance of wheat plants and the production of spike yield.
Hedge and Joshi [31] , Janardan et al. [32] and Hamdia et al. [33] , and Hamdia and Shaddad [11] working with rice, cotton and wheat cultivars, respectively, found that K+/Na+ ratio was high in salt-tolerant than sensitive cultivars and recommended it as a suitable selection criterion for salt tolerance [34] . Al-Alfo- cea et al. and Hamdia et al. [11] [33] and Hamdia and Shaddad (2016) reported that K+ nutrition is not affected by excessive Na+ in salt-tolerant tomato and wheat plants respectively. This situation was interpreted by Garacia et al. [35] who reported that in rice there was no correlation between K+ and Na+ transport and concluded that the genes affecting Na+ uptake had not apparently related with those involved in K+ uptake. However, this situation contrasts with that in triticeae [36] . Antagonistic relations between Na+ and K+ or negative effects of salinity on K+ uptake in different plants were recorded by other authors [11] [33] [37] [38] [39] [40] . The mechanisms of ion distribution increased the osmotic pressure of the shoot which facilitates the steepness of osmoregulation towards the aerial parts which in turn increases the water flow from the root to the shoots which in turn maintained the water status (and the conservation and utilization). Along with this the organic cytosolutes (soluble carbohydrates, soluble proteins and proline) were much higher in shoots than in roots, which again confirmed that broad bean plant considered its strategy of salt tolerance in shoot organ than in root. The overall increase in leaf thickness is a typical response of salt-tolerant plants to salt spray [41] and increased leaf thickness or succulence has been interpreted as an adaptation of plants in terms of conservation of internal water, efficient water storage and dilution of accumulated salts [4] [6] [42] [43] [44] . There is strong evidence that salt affects photosynthetic enzymes, chlorophylls and carotenoids [45] . The decrease in chlorophyll “a” and “b” in the Panicum accessions might have been due to salt induced acceleration of chlorophyll enzymes degradation [46] [47] [48] and/or disorder of chloroplast structure and related proteins [49] [50] . The reduction in leaf area of maize, wheat, cotton, broad bean and parsley tested plants, under saline conditions were also due to reduced growth as a result of decreased water uptake, toxicity of sodium and chloride in the shoot cell as well as reduced photosynthetic pigments recorded by Hamdia [6] similar to work obtained by Ali et al. [51] .
Na+ is the sign of salt toxicity and its accumulation was the sign of sensitivity of wheat organs. In comparison sodium accumulation in three different organs, it can be recorded that the percent of increase in sodium content at −1.5 MPa OSL was 200%, 80% and 133% for shoot, root and spike. This results not related with the previous observation, the lower Na+ content in root as compared with the other two organs shoot and spike this can be said as a sign of the higher Na+ translocation from root to aerial portion of wheat plant. However, the sensitivity in root organ may be due to its efficiency which consumed for Na+ extraction and translocation from it to another parts of wheat plants not to production of growth parameters and reservation of water content. In conformity to the above results wheat plants treated with either Cu++ concentration as:
1) Na+ content retarded considerably in both plant organs.
2) The amount of inorganic cytosolutes (K+) in general increased markedly especially in spike organ..
3) The amount of organic cyto-sloutes (soluble carbohydrates, soluble proteins) also enhanced markedly which in turn could increase the water status and consequently the dry matter yield when compared with the only salinized plants. Nitrate reductase was markedly decreased as increasing OSL this trend correlated with the biosynthesis of pigments
Nitrate reductase was lightly markedly decreased as increasing OSL, this trend correlated with the biosynthesis of pigments Chl. a and Chl. b which effect on the photosynthesis processes that finally affected directly on the production of fresh and dry matter of shoot, root and spike of the tested plants. Application of Cu++ with either concentration accelerate electron transport system in photosynthesis processes which adding further acceleration on nitrate reductase in choloroplast to reduced NO3 to NO2 and finally into NH3 that could be incorporated with carboxylic acid to gain protein this also supported by huge accumulation of protein especially in spike organ the net production of tested wheat plants. Also this supported by the synthesis of protein pattern with molecular weight 37 KDa at Cu++ 25 mM at both control and salinization levels (0.0, −0.3, −0.9 and −1.5 MPa).
Jabeen and Ahmad [52] study the concentration of Na+ and Cl− rapidly increased in the leaves of both the plants under salinity stress. In contrast the nitrate (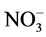 ) and soluble protein concentration were decreased with the increasing salinity. Salinity reduced leaf area, its fresh and dry weight per plant and also inhibited the activity of nitrate reductase (NRA) enzyme. The application of KNO3 significantly reduced the increasing tendency of Na+and Cl−and increased leaf area, its fresh and dry weight per plant,
) and soluble protein concentration were decreased with the increasing salinity. Salinity reduced leaf area, its fresh and dry weight per plant and also inhibited the activity of nitrate reductase (NRA) enzyme. The application of KNO3 significantly reduced the increasing tendency of Na+and Cl−and increased leaf area, its fresh and dry weight per plant,  and soluble protein concentration and NR activity in leaves irrespective to the growth of plant under non saline or saline conditions. Nitrate, taken up by NO3− transporters, is reduced to ammonium by the sequential reaction of nitrate reductase (NR, EC 1・6・6・1) in the cytosol and nitrite reductase (NiR, EC 1・6・6・4) in the plastids/chloroplasts [53] . Debouba et al. [54] showed that NaCl affected these enzyme activities less in the roots than in leaves. This was in accordance with the pronounced decrease of dry weight by salt in leaves compared with that in the roots.
and soluble protein concentration and NR activity in leaves irrespective to the growth of plant under non saline or saline conditions. Nitrate, taken up by NO3− transporters, is reduced to ammonium by the sequential reaction of nitrate reductase (NR, EC 1・6・6・1) in the cytosol and nitrite reductase (NiR, EC 1・6・6・4) in the plastids/chloroplasts [53] . Debouba et al. [54] showed that NaCl affected these enzyme activities less in the roots than in leaves. This was in accordance with the pronounced decrease of dry weight by salt in leaves compared with that in the roots.
The synthesis of protein bands with molecular weight 32.3 KDa at −1.5 MPa OSL treated with either 7.5 mM or 25 mM Cu++ concentrations were associated with the accumulation of soluble protein in spike organ at the same level −1.5 MPa with both Cu++ treatments. This indicated that this band was induced under the influence higher salinization levels. Also the appearance of protein band with molecular weight 37 KDa induced only at Cu++ treatments with 5 mM concentration in both control and under different osmotic stress (0.0, −0.3 MPa, −0.9 MPa and −1.5 MPa NaCl level).This indicated that 7.5 mM Cu++ modified metabolism of wheat plants and related with the accumulation of soluble protein and its expression when compared with the control plants (0.0), which reflected on the increase the production of fresh and dry matter of different wheat organs more than plants treated with 25 mM Cu++ concentration. Maksymiec [55] showed that copper (Cu) is a heavy metal which in recent studies has been attributed an increasing role in metabolic processes of plant cells Hansch and Mendel [56] showed that all organisms have to acquire appropriate amounts of each micronutrient that requires a metal homeostasis network involving mobilization, uptake and distribution within the plant, intracellular trafficking, and storage. Several essential metal ions are redox-active that is the basis for their occurrence as catalytically active cofactors in many metalloenzymes.
Tammam [57] found that salt treatment of broad bean seedling resulted in the disappearance of five polypeptides, while the peptides with molecular mass 26, 18, 14 and 12 KDa increased in their intensity and two peptides 99 and 102 KDa appeared on the gel. Amini and Ehsnapour [58] stated that accumulation of proteins in plant grown under saline condition may provide a storage form of nitrogen that is re-utilized when stress is over and might be due to osmotin like protein in a particular synthesis of those proteins which are involved in modification of cell wall.
Sheldon and Menzies [59] study a solution culture experiment was conducted to examine the effect of Cu toxicity on Rhodes grass (Chlorisgayana Knuth.). Copper toxicity was damaging to plant roots, with symptoms ranging from disruption of the root cuticle and reduced root hair proliferation, to severe deformation of root structure. Eskandari et al. [60] studied different salinization levels 5 levels and four copper on growth and chemical composition Ghazvini, Pistacohio seedlings. Salinity reduced growth parameters, low levels of Cu had no significant effect on leaf area, shoot and root dry matter while decreased stem height. The highest level of Cu++ (7.5 mg kg−1 soil) significantly increased leaf area and shoot dry weight but decreased stem height. Salinity decreased Cu++ and P uptake but increased Na, Cl. Witzel et al., [61] study the root proteome was analyzed based on two-dimensional gel electrophoresis. Hamdia and Shaddad [11] studied four cultivars possessed 17 common protein bands while they different from each other in 6 protein bands. The 14.1 KDa is specific marker for both cultivars Sakha 94 and Giza 168. However, the 33.2 KDa is specific marker for cv. Sakha 94, cv. Gimiza 11 and cv. Giza 168. The 32.3 KDa is specific marker for cv. Sakha 94 and cv. Gimiza 11. The results revealed that three bands at molecular weight 52.1 KDa is induced under salinity stress in four tested cultivars Sakha 94, Gimiza 11, Gimiza 10 and Giza 168, as compared to the control treatment.
Acknowledgements
My great loving remember father, mother Karema Kotob, father Ahmed, sisters Naema and Fatma and all encouragement me from my family and friends.
Cite this paper
Abd El-Samad, H.M., Mostafa, D. and Abd El-Hakeem, K.N. (2017) The Combined Action Strategy of Two Stresses, Salinity and Cu++ on Growth, Metabolites and Protein Pattern of Wheat Plant. American Journal of Plant Sciences, 8, 625-643. https://doi.org/10.4236/ajps.2017.83043
References
- 1. Khan, A.A., Mcneilly, T. and Azhar, F.M. (2001) Stress Tolerance in Crop Plants. International Journal of Agriculture and Biology, 3, 250-255.
- 2. Rasool, S., Hameed, A., Azooz, M.M., Rehman, M., Siddiqi, T.O. and Ahmad, P. (2013) Salt Stress: Causes, Types and Response of Plants. In: Ahmad, P., Azooz, M.M. and Prasad, M.N.V., Eds., Ecophysiology and Response of Plants under Salt Stress, Springer LLC, New York, 1-24.
https://doi.org/10.1007/978-1-4614-4747-4_1 - 3. Eker, S., Comertpay, G., Konuskan, O., Ulger, A.C., Ozturk, L. and Cakmak, I. (2006) Effect of Salinity Stress on Dry Matter Production and Ion Accumulation in Hybrid Maize Varieties. Turkish Journal of Agriculture and Forestry, 30, 65-373.
http://journals.tubitak.gov.tr/agriculture/abstract.htm?id=8523 - 4. Munns, R. and Tester, M. (2008) Mechanisms of Salinity Tolerance. Annual Review of Plant Biology, 59, 651-681.
https://doi.org/10.1146/annurev.arplant.59.032607.092911 - 5. Keshtehgar, A., Rigi, K. and Vazirimehr, M. (2013) Effects of Salt Stress in Crop Plants. International Journal of Agriculture and Crop Sciences, 5, 2863-2867.
http://road.issn.org/issn/2227-670X-international-journal-of-agriculture-and-crop-sciences - 6. Hamdia, M.A. (2016) The Potential Role of Osmotic Pressure to Exogenous Application of Phytohormones on Crop Plants Grown under Different Osmotic Stress. American Journal of Plant Sciences. 7, 937-948.
https://doi.org/10.4236/ajps.2016.76089 - 7. Ribaut. J.M. and Hoisngton, D.A. (1998) Marker-Assisted Selection: New Tools and Strategies. Trends in Plant Science, 3, 236-239.
https://doi.org/10.1016/S1360-1385(98)01240-0 - 8. Parvaiz, A. and Satyawati, S. (2008) Salt Stress and Phyto-Biochemical Responses of Plants—A Review. Plant, Soil and Environment, 54, 88-99.
https://www.researchgate.net/publication/242580345 - 9. Tuna, A.L, Kaya, C., Higgs, D., Murillo-Amador, B., Aydemir, S. and Girgin, A.R. (2008) Silicon Improves Salinity Tolerance in Wheat Plants. Environmental and Experimental Botany, 62, 10-16.
https://doi.org/10.1016/j.envexpbot.2007.06.006 - 10. Turan, S., Cornish, K. and Kumar, S. (2012) Salinity Tolerance in Plants: Breeding and Genetic Engineering. Australian Journal of Crop Science, 6, 1337-1348.
http://www.cropj.com/turan_6_9_2012_1337_1348.pdf - 11. Hamdia, M., Abd, E.-S. and Shaddad, M.A.K. (2016) Mechanisms of Salt Tolerance of Wheat Cultivars. Triticeae Genomics and Genetics, 7, 1-16.
http://biopublisher.ca/index.php/tgg/article/view/2189 - 12. Whitehead, D.C. (2000) Nutrient Elements in Grassland, Soil-Plant-Animal Relationships. CABI Publication, UK University Press, Cambridge, 220-303.
http://www.cabi.org/cabebooks/ebook/20003011639 - 13. Rezazadeh, A., Ghasemnezhad, A., Barani, M. and Telmadarrehei, T. (2012) Effect of Salinity on Phenolic Composition and Antioxidant Activity of Artichoke (Cynarascolymus L.) Leaves. Research Journal of Medicinal Plants, 6, 245-252.
https://doi.org/10.3923/rjmp.2012.245.252 - 14. Mehrizi, M.H., Shariatmadar, H., Khoshgoftarmanesh, A.H. and Dehghani, F. (2012) Copper Effects on Growth, Lipid Peroxidation, and Total Phenolic Content of Rosemary Leaves under Salinity Stress. Agricultural Science and Technology, 14, 205-212.
http://jast.modares.ac.ir/article_4801_b51fe9bbdc09c8197bd783d80603381e.pdf - 15. Lombi, E., Zhao, F.J., Dunham, S.J. and McGrath, S.P. (2001) Phytoremediation of Heavy Metal-Contaminated Soils: Natural Hyperaccumulation versus Chemically Enhanced Phytoextraction. Journal of Environmental Quality, 30, 1919-1926.
https://doi.org/10.2134/jeq2001.1919 - 16. Kholodova, V.P., Netto, D.S., Meshcheryakov, A.B., Borisova, N.N., Aleksandrova, S.N. and Kuznetsov, V.V. (2002) Can Stress-Induced CAM Provide the Performing of the Developmental Program in Mesembryanthemum crystallinum Plants under Long-Term Salinity? Russian Journal of Plant Physiology, 49, 336-343.
- 17. Volkov, K.S., Kholodova, V.P. and Kuznetsov, V.V. (2006) Plant Adaptation to Salinity Reduces Copper Toxicity. Doklady Biological Sciences, 41, 479-481.
https://doi.org/10.1134/S0012496606060159 - 18. Metzner, H., Rau, H. and Senger, H. (1965) Untersuchungen zur Synchronisierbarkeit einzelner Pigmentmangel-Mutanten von Chlorella. Planta, 65, 186-194.
https://doi.org/10.1007/BF00384998 - 19. McKee, G.W. (1974) A Coefficient for Computing Leaf Area in Hybrid Corn. Agronomy Journal, 56, 240-241.
http://scholar.google.co.uk/scholar?q=.+Agronomy+Journal.+56%3A+240-241.&hl=en&as_sdt=0%2C5&as_vis=1 - 20. Bonhomme, R., Varlet, M., Grancher, C. and Chartier, P. (1974) The Use of Hemispherical Photographs for Determining Leaf Index of Young Crops. Photosynthetica, 8, 299-301.
https://en.wikipedia.org/wiki/Hemispherical_photography - 21. Norman, J. and Campbell G.S. (1994) Canopy Structure. In: Pearcy, R.W., Ehleringer, J., Moony, H.A. and Rundel, P.W., Eds., Plant Physiological Ecology, Chapman & Hall, London, 301-326. Physiologia Plantarum, 115, 251-257.
- 22. Lai, K.L. and Lui, L.F. (1988) Increased Plant Regeneration Frequency in Water Stressed Rice Tissue Cultures. Japanese Journal of Crop Science, 57, 553-557.
https://doi.org/10.1626/jcs.57.553 - 23. Romero-Aranda, R. and Syvertsen, J.P. (1996) The Influence of Foliar Applied Urea Nitrogen and Saline Solution on Net Gas Exchange of Citrus Leaves. Journal of the American Society for Horticultural Science, 121, 501-506.
http://journal.ashspublications.org/content/121/3/501.abstract - 24. Beadle, C.L. (1993) Growth Analysis. In: Hall, D.O., Scurlock, J.M.O., Bolharnordenkampfh, R., Leegood, R.C. and Long, S.P., Eds., Photosynthesis and Production in a Changing Environment: A Field and Laboratory Manual, Chapman and Hall, London, 36-46.
http://www.springer.com/gp/book/9780412429002 - 25. Fales, F.W. (1951) The Assimilation and Degradation of Carbohydrates of Yeast Cells. Biological Chemistry, 193, 113-118.
http://www.degruyter.com/view/j/bchm - 26. Lowry, O.H., Roserbrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent. Journal of Biological Chemistry, 193, 265-275.
http://en.wikipedia.org/wiki/Journal_of_Biological_Chemistry - 27. Jaworski, E.G. (1971) Nitrate Reductase Assay in Intact Plant Tissues. Biochemical and Biophysical Research Communications, 43, 1274-1279.
https://doi.org/10.1016/S0006-291X(71)80010-4 - 28. Williams, V. and Twine, S. (1960) Flam Photometric Methods for Sodium, Potassium and Calcium. In: Paech, K. and Tracey, M.V., Eds., Modern Methods of Plants Analysis, Vol. 5, Springer-Verlag, Berlin, 3-5.
https://en.wikipedia.org/wiki/The_Williams_Brothers - 29. Laemmli, U.K. (1970) Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature, 227, 680-685.
https://doi.org/10.1038/227680a0 - 30. Steel, R.G. and Torrie, J.H. (1960) Principles and Procedures of Statistics. McGraw-Hill Book Co., New York.
http://garfield.library.upenn.edu/classics1977/A1977DU23500002.pdf - 31. Hedge, B.A. and Joshi, G.C. (1975) Mineral Salt Absorption in Saline Rice Irrigation on Growth and Photosynthetic Pigments of Safflower and Sunflower Plants. Bulletin of the Faculty of Science, 4, 29-39.
https://archive.org/stream/mineralsaltsabso00sutc/mineralsaltsabso00sutc_djvu.txt - 32. Janardan, K.V., Murtay, K., Girira, J. and Panchaksharais, S. (1976) Salt Tolerance of Cotton and Potential Use of Saline Water for Irrigation. Current Science, 45, 334-336.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1065823/pdf/plntphys00553-0131.pdf - 33. Hamdia, M., Abd, E.-S. and Shaddad, M.A.K. (2014) The Exogenous Amelioration Roles of Growth Regulators on Crop Plants Grow under Different Osmotic Potential. Journal of Stress Physiology & Biochemistry, 10, 203-213.
http://www.jspb.ru/ - 34. Alfocea, F.P., Estan, M.T., Caro, M. and Bolarin, M.C. (1993) Response of Toma to Cultivars to Salinity. Plant Soil, 150, 203-211.
https://doi.org/10.1007/BF00013017 - 35. Garacia, A., Rizzo, C.A., UdDin, J., Bartos, S.L., Senadhira, D., Flowers, T.J. and Yeo, A.R. (1997) Sodium and Potassium Transport to the Xylem Are Inherited Independently in Rice, and the Mechanism of Sodium: Potassium Selectivity between Rice and Wheat. Plant, Cell & Environment, 20, 1167-1174.
https://doi.org/10.1046/j.1365-3040.1997.d01-146.x - 36. Gorham, J., Bristol, A., Young, E.M., Wyn Jones, E.G. and Kashour, G. (1990) Salt Tolerance in Triticea: K/Na Discrimination in Barley. Journal of Experimental Botany, 41, 1095-1101.
- 37. Hamdia, M.A. and Shadad, M.A.K. (1996) Salt Tolerance of Soybean Cultivars. Biologia Plantarum, 39, 263-269.
http://scholar.google.com.eg/citations?view_op=view_citation&hl=en&user=qUM54T8AAAAJ&citation_for_view=qUM54T8AAAAJ:0EnyYjriUFMC - 38. Carjaval, M., Cerda, A. and Martinez, V. (2000) Modification of the Response of Saline Stressed Tomato Plants by the Correction of Cation Disorders. Plant Growth Regulation, 30, 37-47.
http://link.springer.com/article/10.1023/A:1006359503099 - 39. Grieve, C.M. and Poss, J.A. (2000) Wheat Response to Interactive Effects of Boron and Salinity. Journal of Plant Nutrition, 23, 1217-1226.
https://www.jstor.org/stable/42951377
https://doi.org/10.1080/01904160009382095 - 40. Hamdia, M.A. and Barakat, N.A. (2013) The Physiological Mechanisms of Calcium Chloride Application on Broad Bean Plants Grown under Salinity Stress. Journal of Ecology and Natural Environment, 5, 371-377.
https://doi.org/10.5897/JENE10.089 - 41. Rozema, J.T., Duech, H. and Wesselman, B.F. (1983) Nitrogen Dependent Growth Stimulation by Salt Stand-Line Species. Oecologia Plantarum, 4, 41-52.
http://www.resecol.wur.nl/publ/1993_Bakker,Leeuw,Dijkema,Leendertse,Prins_SaltMarshesAlongTheCoastOfTheNetherlands.pdf - 42. Breckle, S.W. (2002) Salinity, Halophytes and Salt Affected Natural Ecosystems Affected. Department of Ecology, Faculty of Biology University of Bielefeld, Bielefeld.
http://scholar.google.co.uk/scholar?q=Breckle,+S.W.+(2002)+Salinity,+Halophytes&hl=en&as_sdt=0&as_vis=1&oi=scholart - 43. Munns, R. (2005) Genes and Salt Tolerance: Bringing Them Together. New phytologist, 167, 645-663.
https://doi.org/10.1111/j.1469-8137.2005.01487.x - 44. Hamdia, M.A.E.-S. (2013) The Physiological Response of Wheat Plants to Exogenous Application of Gibberellic Acid (GA3) or Indole-3-Acetic Acid (IAA) with Endogenous Ethylene under Salt Stress Conditions. International Journal of Plant Physiology and Biochemistry, 5, 58-64.
https://www.google.co.uk/search?q=Hamdia%2C+M.+Abd+El-Samad+%282013%29 - 45. Stepien, P. and Klobus, G. (2006) Water Relations and Photosynthesis in Cucumissativus L. Leaves under Salt Stress. Biologia Plantarum, 50, 610.
https://doi.org/10.1007/s10535-006-0096-z - 46. Hernandez, J.A., Olmos, E., Corpas, F.J., Sevilla, F. and De1, R.L.A. (1995) Salt-Induced Oxidative Stress in Chloroplasts of Pea Plants. Plant Science, 105, 151-167.
- 47. Hernandez, J., Jimenez, A., Mullineaux, P. and Sevilla, F. (2000) Tolerance of Pea Plants (Pisum sativum) to Long-Term Salt Stress Is Associated with Induction of Antioxidant Defences. Plant, Cell & Environment, 23, 853-862.
https://doi.org/10.1046/j.1365-3040.2000.00602.x - 48. Hernandez, J.A. and Almansa M.S. (2002) Short-Term Effects of Salt Stress on Antioxidant Systems and Leaf Water Relations of Pea Leaves. Physiologia Plantarum, 115, 251-257.
http://www.ncbi.nlm.nih.gov/pubmed/12060243 - 49. Singh, A.K. and Dubey, R.S. (1995) Changes in Chlorophyll a and b Contents and Activities of Photosystems 1 and 2 in Rice Seedlings Induced by NaCl. Photosynthetica, 31, 489-499.
http://science.report/pub/37918935 - 50. Sabir, P., Ashraf, M., Hussain, M. and Jamil, A. (2009) Relationship of Photosynthetic Pigments and Water Relations with Salt Tolerance of Proso Millet (Panicum Miliacum L.) Accessions. Pakistan Journal of Botany, 41, 2957-2964.
https://www.google.co.uk/webhp?sourceid=navclient&oq - 51. Ali, Y., Aslam, Z., Ashraf, M.Y. and Tahir, G.R. (2004) Effect of Salinity on Chlorophyll Concentration, Leaf Area, Yield and Yield Components of Rice Genotypes Grown under Saline Environment. International Journal of Environmental Science& Technology, 1, 221-225.
https://doi.org/10.1007/BF03325836 - 52. Jabeen, N. and Ahmad, R. (2011) Foliar Application of Potassium Nitrate Affects the Growth and Nitrate Reductase Activity in Sunflower and Safflower Leaves under Salinity. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 39, 172-178.
- 53. Ireland, R.J. and Lea, P.J. (1999) The Enzymes of Glutamine, Glutamate, Asparagines and Aspartate Metabolism. In: Singh, B.K., Ed., Plant Amino Acids: Biochemistry and Biotechnology, Marcel Dekker, New York, 49-109.
- 54. Debouba, M., Maaroufi-Dghimi, H., Ghorbel, M.H. and Gouia, H. (2007) Changes in Growth and Activity of Enzymes Involved in Nitrate Reduction and Ammonium Assimilation in Tomato Seedlings in Response to NaCl Stress. Annals of Botany, 99, 1143-1151.
https://doi.org/10.1093/aob/mcm050 - 55. Maksymiec, W. (1998) Effect of Copper on Cellular Processes in Higher Plants. Photosynthetica, 34, 321-342.
https://doi.org/10.1023/A:1006818815528 - 56. Hansch, R. and Mendel, R.R. (2009) Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Current Opinion in Plant Biology, 12, 259-266.
https://doi.org/10.1016/j.pbi.2009.05.006 - 57. Tammam, A.A. (2003) Response of Viciafaba Plants to the Interactive Effect of Sodium Chloride Salinity and Salycilic Acid Treatment. Acta Agronomica Hungarica, 51, 239-248.
https://doi.org/10.1556/AAgr.51.2003.3.1 - 58. Amini, F. and Ehasapour, A.A. (2005) Soluble Proteins, Proline, Carbohydrates and Na+/K+ Changes in Two Tomato (Lycopersicon esculentimil L.) Cultivars under in Vitro Salt Stress. American Journal of Biochemistry and Biotechnology, 1, 212-216.
http://thescipub.com/issue-ajbb/1/4 - 59. Sheldon, A.R. and Menzies, N.W. ( 2005) The Effect of Copper Toxicity on the Growth and Root Morphology of Rhodes Grass (Chloris gayana Knuth.) in Resin Buffered Solution Culture. Plant and Soil, 278, 341-349.
https://doi.org/10.1007/s11104-005-8815-3 - 60. Eskandari, S., Mozaffari, V. and Pour, A.T. (2014) Effects of Salinity and Copper on Growth and Chemical Composition of Pistachio Seedlings. Journal of Plant Nutrition, 37, 1063-1079.
https://doi.org/10.1080/01904167.2014.881862 - 61. Witzel, K., Matros, A., Strickert, M., Kaspar, S., Peukert, M., Muhling, K.H., Borner, A. and Mock, H.P. (2014) Salinity Stress in Roots of Contrasting Barley Genotypes Reveals Time-Distinct and Genotype-Specific Pattern for Defined Proteins. Molecular Plant, 7, 336-355.






