American Journal of Plant Sciences
Vol.4 No.1(2013), Article ID:27662,5 pages DOI:10.4236/ajps.2013.41022
Do Really Close Stomata by Soil Drying ABA Produced in the Roots and Transported in Transpiration Stream?
![]()
The Department of Biology Education, Chungbuk National University, Cheongju, Korea.
Email: jslee0318@chungbuk.ac.kr
Received October 7th, 2012; revised November 8th, 2012; accepted December 8th, 2012
Keywords: Abscisic Acid; Commelina communis L.; Signal Transduction Pathway; Water Stress
ABSTRACT
Stomatal aperture responses of Commelina communis L. between well watered plants and water stressed plants were investigated. To see the very rapid response to water stress, the plants were directly rooted out from the soil and exposed to the air immediately. Stomata, rooted out from the soil, were totally closed within 10 minutes without any detention time while the stomata of the plants in the soil had been kept opening. These results suggest that stomatal response to the abrupt water stress is very rapid indicating that guard cell itself or leaves could sense water status in the plants.
1. Introduction
The basic role of the stomata is to regulate transpiration and photosynthesis. Therefore, an understanding of the response to water stress is critical to any discussion of how plant senses the signal [1]. Abscisic acid (ABA) is a ubiquitous hormone in vascular plants. Because of its main role in moderating a plant’s response to water stress, ABA has been characterized as a stress hormone. Under drought conditions, leaf-ABA concentration can increase up to 40 times, which is the most dramatic change in concentration reported for any hormone in response to an environmental signal [2]. Mac Robbie [3] has presented a plausible explanation for ABA-induced stomatal closure. It is clear that ABA plays a critical role in stomatal closing [4,5]. What is not clear is how ABA reaches in the guard cells under water stress. Stomata appear to respond to purgation of many aspects of the soil-plant-atmosphere hydraulic continuum, but there is little agreement regarding the mechanism by which stomata sense such perturbations. During the last 30 years, evidence has accumulated to support the view that in the early stages of soil drying ABA produced in the roots and transported in the transpiration stream can function as a physiological signal in the regulation of gas exchange [6]. As water stress begins, some of the ABA carried by the xylem stream is synthesized in roots that are direct contact with the drying soil. Because this transport can occur before the low water potential of the soil causes any measurable change in water status of the leaves, ABA is believed to be a signal that helps reduce the transpiration rate in the leaves by closing stomata [6].
Is it true that ABA transportation through xylem can function as a physiological signal in the stomatal closing under the water stress? Most of the results to support the above theory have been accumulated and conducted by the researchers who were supervised by Davies [6-10].
In some plant’s responses to the environmental factors are quite surprising in terms of time as it is very fast as much as an animal neuron system. There are very symbolic plants which are Mimosa (Mimosa pudica L.), Drosera rotundifolia L. and Flytrap (Dionaea muscipula) as a showing of thigmotropism and insectivorous plants. Their leaves fold immediately as like as animal neuron signal transduction reaction when they are touched by the hands or the insects. Almost all of stomatal responses about water drought have been performed throughout long-terms and to see the effect of water stress in well watered plants, it will take times. The reduction in stomatal aperture due to ABA is extremely variable and seems to be highly dependent on the method used for analysis of the response [10]. Therefore, in this experiment, to see the very rapid response to water stress, the plants were rooted out from the soil and exposed to the air immediately. Epidermis was taken from the leaf according to the minutes and stomatal apertures were measured.
2. Materials and Method
The experiments were carried out on the abaxial surface of leaves of Commelina communis. The plants were potted in John Innes No. 2 compost supplemented with Phostrogen and watered every morning. They were grown in a glass house (minimum temperature of 20˚C during the day and 15˚C at night) under a light regime of 18 hours day and 6 hours night (natural daylight supplemented by high pressure sodium lighting: 150 μmole m−2·s−1). Three or four week old fully grown Commelina communis were placed in the dark for 1 hour before the experiments in order to close the stomata. After various intervals, intact segments were transferred into liquid paraffin and epidermal strips were peeled. Strips of lamina between the major parallel veins on either side of the midrib were removed by cutting with a razor blade on a glass slide. A cut was made through the upper epidermis at one end of the lamina strips, taking care not to cut the lower epidermis. When the tissue was inverted, the “tab” of lamina formed could be lifted with forceps and pulled back for a few mm when the lower epidermis could be readily separated from the spongy mesophyll cells. The epidermis was peeled away from the mesophyll by pulling gently on the tab. A 90˚ peeling angle was used. The peeling angle of 90˚ represents a compromise between high cell mortality at obtuse angles and excessive contamination with mesophyll at acute angles [11]. Stomatal apertures of epidermal strips from the intact leaves were measured under a microscope with a calibrated ocular micrometer disc. Measurements of 20 stomata took within 2 min, and a strict timetable was employed during experiment. Each experiment was repeated at least twice and started approximately at the same time in each morning.
3. Results and Discussion
Light is the most important environmental factor stimulating stomatal opening. Stomata usually open when leaves are transferred from the darkness to the light [12]. Stomatal opening and closing response in intact leaves was quite fast as it was shown in Figure 1 [13]. On transfer to the light, the stomata in the intact leaf, floated in water in an enclosed Petri dish, opened to about 4.5 μm within about 10 min. After 1.5 h, when the leaves were returned to the dark, the stomata in the intact leaf started to close right away. On transfer to the dark, Stomatal aperture under the dark was decreased to 8 μm at 20 min. This was the half of when stomatal opening was maximal.
Figure 2 shows how stomata close when they were exposed to abrupt water stress under the sun. Most experiments dealing with stomatal closing by water stress took the relatively long terms. When we study water stress, we use very well watered plants in the very beginning, and stop watering to give water stress. Therefore, stomatal conductance in the condition of water stress could be measurable through several days or weeks. However, in this experiment, to see the stomatal response by the level of minutes, the plants were rooted out from the soil and exposed to the air immediately. In this study stomata, rooted out from the soil, were totally closed within 10 minutes without any detention time while the stomata of the plants in the soil had been kept opening until the end of the experiments. Even in this case, stomatal closing under the water stress was much faster than stomatal closing in the dark. To the plants, severe water stress could be much more dangerous as stomata loss much water in the very short time, but during the dark, stomata are not exposed to emergency situation in terms of water status.

Figure 1. Opening and closing of stomata of Commelina communis in intact leaves and isolated epidermis. Leaves were kept in the dark for 1 h, exposed to light for 90 min and then returned to the dark. Epidermis was taken from the leaf at the end of the dark period. Each point is the mean of two replicate experiments and 40 stomatal apertures were measured. Bar indicates maximum standard error (±0.89). (●) Intact leaves in distilled water; (○) isolated epidermis in 10mM MES-KOH buffer (pH 6.15) plus 100mM KCl; (□) isolated epidermis in buffer plus 10 mM KCl [13].
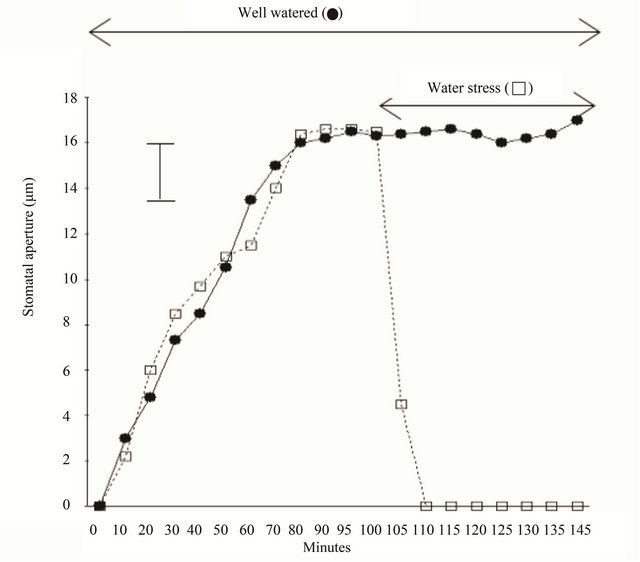
Figure 2. Opening and closing of stomata of Commelina communis in intact leaves. The plants were kept in the dark for 1 h, exposed to light for 150 min. in situations with well watered status (●) and abrupt water stress (□). In this experiment to see the very rapid response to water stress, the plants were rooted out from the soil and exposed to the air immediately. Epidermis was taken from the plants according to the minutes. Each point is the mean of two replicate experiments and 40 stomatal apertures were measured. Bar indicates maximum standard error (±2.4).
Gowing [14] found that guard cells did not react concededly according to the concentration of ABA in the xylem and the determination of ABA concentration around guard cells was not necessarily similar to the xylem ABA concentration. Some plant reacts to the environmental factors quite quickly. It was thought that it would be interesting to see the changes of potential difference (PD) of guard cells in the intact leaves in response to light and CO2. Fast hyperpolarization of guard cell membrane PD reaching from −9 mV to −12 mV both for T. virginiana and C. communis in response to light, blue light and red light has been observed [15-17]. The initial responses were the fastest and the saturation point of hyperpolarization was reached within 30 sec. At the onset of CO2, the PD showed a dramatic hyperpolarizetion between 10 and 15 mV in T. virginiana or 42 mV in C. communis. The saturation point of hyperpolarization was reached with a lag time between 1 and 6 sec. A wave of depolarization has been reported following from the localized wounding or burning in many species [18]. These waves were observed in the apoplast of the epidermis in various species. The transmitted signal was termed “Ricca’s factor” after its proposer [19-21]. Malone and Stankovic [22] suggested the arrival of the wave alter leaf water potential and thereby induces stomatal activities. The key points of “Ricca’s factor” represent that leaves and stem have a potential to sense the wounding or burning stress. Stomata normally adjust to water stress by closing in response to declining leaf water potential [23]. Hsiao’ view suggests a possibility showing that stomata respond directly to the leaf water status. Tardieu and Davies [9] have got the contradictory results as xylem ABA concentrations are relatively constant throughout the day. This result could represent that ABA did not act as a signal arriving in the transpiration stream. Burkely [24] suggested that stomatal guard cells responded to leaf water potential indicating that the leaf itself could sense the different water status. The most easily observed stomatal response to perturbation of leaf water balance is the response to humidity [24]. When the humidity around a leaf is reduced, stomatal conductance (gs) typically increases for 5 - 15 min. and then declines for another 20 - 75 min., ultimately approaching a steady state of gs that is lower than the initial value [25-30].
Much of the ABA in the transpiration stream is taken up and metabolized by the mesophyll cell. During the early stages of water stress, however, the pH of the xylem sap becomes more alkaline, increasing from about pH 6.3 to about pH 7.2 [31]. They suggested that during water stress, the slight alkaline xylem sap favors the dissociation of ABAH to ABA−. Therefore, ABA− does not easily pass through membranes, under condition of water stress, more ABA− reaches guard cells. This anion trap concept seems to be weak as guard cell also has membrane and mesophyll is relatively close to xylem, but guard cells are located at the end of lower epidermis.
For a better understanding of the regulatory role of ABA as an adaptive signal during drought stress, information about the dynamics of generation and distribution of physiologically active ABA pools is necessary. Generation of active pools of ABA revealed by in vivo imaging of water-stressed Arabidopsis [32]. They found that water stress applied to the root system resulted in the generation of ABA pools in the shoot but not in the root. Hence, water stress recognized by the root system predominantly results in shoot-localized ABA action that culminates in a focused response in guard cells. ABA is transported by both the xylem and the phloem, but it is normally abundant in phloem sap [33].
In Arabidopsis, numerous genes that respond to dehydration stress have been identified and categorized as responses to dehydration and early response to dehydration genes. There are at least four independent regulatory systems for gene expression in response to water stress. Two of them are ABA-dependent, other are ABA-independent [34]. Both ABA-dependent and independent osmotic stress signaling first modify constitutively expressed transcription factors, leading to expression of early response transcriptional activators, which then activates downstream stress tolerance effector genes [35]. Therefore, it could be suggested that there are many possible adapt pathways how plant react to the water stress.
4. Acknowledgements
This work was supported by the research grant of Chungbuk National University in 2012.
REFERENCES
- D. J. Kim and J. S. Lee, “Current Theories for Mechanism of Stomatal Opening: Influence of Blue Light Mesophyll Cells, and Sucrose,” Journal of Plant Biology, Vol. 50, No. 5, 2007, pp. 523-526. doi:10.1007/BF03030704
- K. Raschke, “Action of Abscisic Acid on Guard Cells,” In: E. Zeiger, G. D. Farquhar and I. R. Cowan, Eds., Stomatal Function, Stanford University Press, Stanford, 1987, pp. 253-279.
- E. A. C. MacRobbie, “Calcium and ABA-Induced Stomatal Closure,” Philosophical Transactions of the Royal Society of London B, Vol. 338, No. 1283, 1992, pp. 5-18. doi:10.1098/rstb.1992.0124
- J. S. Lee, “The Effects of Two Abscisic Acid Analogues, WL19224 and WL19377, on Stomatal Closure,” Journal of Plant Biology, Vol. 43, No. 1, 2000, pp. 56-59. doi:10.1007/BF03031037
- Y. Lee, Y. B. Choi, S. Sur, J. S. Lee, S. M. Assmann, C. O. Joe, J. F. Kellerher and R. C. Crain, “Abscisic AcidInduced Phosphoinositide Turnover in Guard Cell Protoplasts of Vicia faba,” Plant Physiology, Vol. 110, No. 3, 1996, pp. 986-996.
- W. J. Davies and Z J. hang, “Root Signals and the Regulation of Growth and Development of Plants in Drying Soil,” Annual Review of Plant Physiology & Plant Molecular Biology, Vol. 42, 1991, pp. 55-76. doi:10.1146/annurev.pp.42.060191.000415
- J. Zhang and W. J. Davies, “Sequential Response of Whole Plant Water Relations to Prolonged Soil Drying and the Involvement of Xylem Sap ABA in the Regulation of Stomatal Behaviour of Sunflower Plants,” New phytologist, Vol. 113, No. 2, 1989, pp. 167-174. doi:10.1111/j.1469-8137.1989.tb04703.x
- J. Zhang and W. J. Davies, “Changes in the Concentration of ABA in the Xylem Sap as a Function of Changing Soil Water Status Can Account for Changes in Leaf Conductance and Growth,” Plant Cell & Environment, Vol. 13, No. 3, 1990, pp. 277-285. doi:10.1111/j.1365-3040.1990.tb01312.x
- F. Tardieu and W. J. Davies, “Stomatal Response to Abscisic Acid Is a Function of Current Plant Water Status,” Plant Physiology, Vol. 98, No. 2, 1992, pp. 540-545. doi:10.1104/pp.98.2.540
- C. L. Trejo, W. J. Davies and L. M. P. Ruiz, “Sensitivity of Stomata to Abscisic Acid,” Plant Physiology, Vol. 102, 1993, pp. 497-502.
- J. D. B. Weyers and A. J. Travis, “Selection and Preparation of Leaf Epidermis for Experiments on Stomatal Physiology,” Journal of Experimental Botany, Vol. 32, No. 4, 1981, pp. 837-850. doi:10.1093/jxb/32.4.837
- J. S. Lee, “Stomatal Opening Mechanism of CAM Plants,” Journal of Plant Biology, Vol. 53, No. 1, 2010, pp. 19-23. doi:10.1007/s12374-010-9097-8
- J. S. Lee and D. J. F. Bowling, “Effect of the Mesophyll on Stomatal Opening in Commelina communis,” Journal of Experimental Botany, Vol. 43, No. 7, 1992, pp. 951- 957. doi:10.1093/jxb/43.7.951
- D. J. G. Gowing, H. G. Jones and W. J. Davies, “XylemTransported Abscisic Acid: The Relative Importance of Its Mass and Its Concentration in the Control of Stomatal Aperture,” Plant Cell and Environment, Vol. 16, No. 4, 1993, pp. 453-459. doi:10.1111/j.1365-3040.1993.tb00892.x
- J. S. Lee and D. J. F. Bowling, “Influence of the Mesophyll on the Change of Electrical Potential Difference of Guard Cells Induced by Red Light and CO2 in Commelina communis L. and Tradescantia virginiana L.,” Korean Journal of Plant Biology, Vol. 36, No. 4, 1993, pp. 383-389.
- J. S. Lee and D. J. F. Bowling, “The Effect of a Mesophyll Factor on the Swelling of Guard Cell Protoplasts of Commelina communis L.,” Journal of Plant Physiology, Vol. 142, No. 2, 1993, pp. 203-207. doi:10.1016/S0176-1617(11)80964-8
- J. S. Lee, “The Relationship between Stomatal Opening and Photosynthetic Activity of the Mesophyll in Commelina communis L.,” Korean Journal of Environmental Science, Vol. 15, No. 12, 2006, pp. 1109-1117.
- C. Wildon, H. M. Doherty, G. Eagles, D. J. Bowles and J. F. Thain, “Systemic Responses Arising from Localized Heat Stimuli in Tomato Plants,” Annals of Botany, Vol. 64, No. 6, 1989, pp. 691-695.
- J. W. Van Sambeek and B. G. Pickard, “Mediation of Rapid Electrical, Metabolic, Transpirational, and Photosynthetic Changes by Factors Released from Wounds. I. Variation Potential and Putative Action Potentials in Intact Plants,” Canadian Journal of Botany, Vol. 54, No. 23, 1976, pp. 2642-2650. doi:10.1139/b76-284
- J. S. Lee and D. J. F. Bowling, “Influence of the Mesophyll on Stomatal Opening,” Australian Journal of Plant Physiology, Vol. 22, No. 3, 1995, pp. 356-363. doi:10.1071/PP9950357
- U. Ricca, “Solution d’un Probleme de Physiologie: La Propagation de Stimulus Dans la Sensitive,” Archives Italiennes de Biologie, Vol. 65, 1916, pp. 219-232.
- M. Malone and B. Stankovic, “Surface Potentials and Hydraulic Signals in Wheat Leaves Following Localized Wounding by Heat,” Plant Cell and Environment, Vol. 14, No. 4, 1991, pp. 431-436. doi:10.1111/j.1365-3040.1991.tb00953.x
- T. C. Hsiao, “Plant Response to Water Stress,” Annual Review of Plant Physiology, Vol. 24, 1973, pp. 519-520. doi:10.1146/annurev.pp.24.060173.002511
- T. N. Burkley, “The Control of Stomata by Water Balance,” New Phytologist, Vol. 168, No. 2, 2005, pp. 275- 292. doi:10.1111/j.1469-8137.2005.01543.x
- I. R. Cowan and G. D. Farquhar, “Stomatal Function in Relation to Leaf Metabolism and Environment,” Symposium Society of Experimental Biology, Vol. 31, 1977, pp. 471-505.
- L. Kappen, G. Andresen and R. Losch, “In Situ Observations of Stomatal Movements,” Journal of Experimental Biology, Vol. 38, No. 1, 1987, pp. 126-141.
- D. A. Grantz, “Plant Response to Atmosphere Humidity,” Plant Cell and Environment, Vol. 13, No. 7, 1990, pp. 667- 679. doi:10.1111/j.1365-3040.1990.tb01082.x
- K. A. Mott and D. F. Parkhurt, “Stomatal Response to Humidity in Air and Helix,” Plant Cell and Environment, Vol. 14, No. 5, 1991, pp. 509-512. doi:10.1111/j.1365-3040.1991.tb01521.x
- J. L. Monteith, “A Reinterpretation of Stomatal Responses to Humidity,” Plant Cell and Environment, Vol. 18, No. 4, 1995, pp. 357-364. doi:10.1111/j.1365-3040.1995.tb00371.x
- R. Oren, J. S. Sperry, G. G. Katul, D. E. Pataki, B. E. Ewers, N. Phillips and K. V. R. Schafer, “Survey and Synthesis of Intraand Interspecific Variation in Stomatal Sensitivity to Vapour Pressure Deficit,” Plant Cell and Environment, Vol. 22, No. 12, 1999, pp. 1515-1526. doi:10.1046/j.1365-3040.1999.00513.x
- S. Wilkinson and W. J. Davies, “Xylem Sap pH Increase: A Drought Signal Received at the Apoplastic Face of the Guard Cell That Involves the Suppression of Saturable Abscisic Acid Uptake by the Epidermal Symplast,” Plant Physiology, Vol. 113, No. 2, 1997, pp. 559-573.
- A. Christmann, T. Hoffmann, L. Teplova, E. Grill and A. Muller, “Generation of Active Pools of Abscisic Acid Revealed by in Vivo Imaging of Water-Stressed Arabidopsis,” Plant Physiology, Vol. 137, No. 1, 2005, pp. 209- 219. doi:10.1104/pp.104.053082
- M. Seo and T. Koshiba, “Complex Regulation of ABA Biosynthesis in Plants,” Trends of Plant Science, Vol. 7, No. 1, 2002, pp. 41-48. doi:10.1016/S1360-1385(01)02187-2
- G. T. Huang, S. L. Ma, L. P. Bai, L. Zhang, H. Ma, P. Jia, Z. Liu, M. Zhong and Z. F. Guo, “Signal Transduction during Cold, Salt, and Drought Stresses in Plants,” Molecular Biology of Republic, Vol. 39, No. 2, 2012, pp. 969-987. doi:10.1007/s11033-011-0823-1
- J. K. Zhu, “Salt Drought Stress Signal Transduction in Plants,” Annual Review of Plant Biology, Vol. 53, 2002, pp. 247-273. doi:10.1146/annurev.arplant.53.091401.143329

