Advances in Microbiology
Vol.2 No.4(2012), Article ID:25948,6 pages DOI:10.4236/aim.2012.24073
Evaluation of Potential for Translocation of Listeria monocytogenes from Floor Drains to Food Contact Surfaces in the Surrounding Environment Using Listeria innocua as a Surrogate
Food Science Institute, Department of Animal Sciences and Industry, Kansas State University, Manhattan, US.
Email: jasdeep@ksu.edu
Received August 28, 2012; revised September 23, 2012; accepted October 10, 2012
Keywords: Listeria monocytogenes; Listeria innocua; Drain; Translocation; Stainless Steel Coupons
ABSTRACT
Floor drains in processing environments harbor Listeria spp. due to continuous presence of humidity and organic substrates. Cleaning and washing activities in food-processing facilities can translocate the bacterial cells from the drain to the surrounding environment, thus contaminating food products still in production. This study evaluated the potential for translocation of Listeria monocytogenes from drains to food contact surfaces in the surrounding environment using Listeria innocua as a surrogate. A 7 × 7 × 8-foot polycarbonate flexi-glass chamber with a 10-inch-diameter drain mounted on an aluminum cabinet was used. Stainless steel coupons (6.4 × 1.9 × 0.1 cm, 12 per height) were hung at 1, 3, and 5 feet inside the chamber. Four treatment sets; non-inoculated, non-treated; non-inoculated, treated; inoculated, treated; inoculated non-treated; and two subtreatments of 8 h and 48 h were performed. For the inoculated sets, meat slurry (10 g of ground beef in 900 mL water) and a four-strain cocktail of Listeria innocua at 7 - 8 log CFU/mL were used. For the treated sets, in addition, a commercial cleaner and sanitizer was applied. The drain was cleaned using a pressure hose (40 - 50 psi) after 8 h and 48 h. Coupons were then removed and enriched in listeria enrichment broth to establish if any cell translocated from the drain onto the stainless steel coupons via aerosols generated during washing. Confirmation was done using VIP Listeria rapid test kits. Results indicated translocation at all three heights ranging from 2% - 25%. Significantly higher translocation (p < 0.05) was found at 1 foot (up to 25%), followed by 3 feet (up to 11%) and 5 feet (up to 2.7%). This research indicated that translocation of Listeria spp. from drains to food contact surfaces does occur and increases with increased proximity to the drain.
1. Introduction
Bacteria have been shown to enter foods as a result of contact with contaminated surfaces [1], but contamination of commercially processed food products with Listeria monocytogenes and other Listeria spp. occurs in post-processing environments rather than as a result of organisms surviving the processing operation. L. monocytogenes is also known to be associated frequently with raw materials used in food processing facilities, which may constantly reintroduce the organism to the plant environment [2]. Pulsed-field gel electrophoresis (PFGE) typing of Listeria strains isolated from a meat-processing plant in a 2-year period showed the persistence of closely related Listeria strains in the plant environment [3]. Listeria monocytogenes can cause mild (listerial gasteroentritis) to severe, life-threatening illnesses (invasive listeriosis) [4].
The foods that have been commonly implicated in invasive listeriosis outbreaks are ready-to-eat (RTE) foods. RTE foods can be contaminated if the ingredients are contaminated with L. monocytogenes and are not sufficiently processed to destroy viable cells of this pathogen, or if introduction of L. monocytogenes occurs because of improper sanitary conditions or practices [5].
Several factors contribute to the growth of microorganisms in food-processing environments, including moisture, nutrients, pH, oxidation-reduction potential, temperature, presence or absence of inhibitors, microbial interactions, and time. Moisture plays an increasingly important role and promotes the survival of bacterial cells on different surfaces. Processing plant structures, including equipment, as well as maintenance, repair, and practices that entrap moisture often result in microbial niche development [6]. Numerous sampling studies have been conducted to assess the prevalence of Listeria spp. in different food production and processing facilities. Samples were taken from the floor, drains, processing equipment, food contact surfaces, and environment. Significant findings included the recovery of L. monocytogenes from the floor drains from 2% - 50% in all tested food establishments [7].
Floor drains in processing environments harbor Listeria spp. due to the continuous presence of humidity and organic substrates. Listeria adhere to, colonize, and become entrapped on the drain surface in a slimy mucilaginous coating of colonizing bacterial cells and associated polymers called biofilm. This biofilm coating protects the bacterial cells against environmental stress, offers resistance to cleaning and disinfection, and is difficult to eradicate or remove compared with free, living cells [2,8]. The time available for biofilm formation depends on the frequency of cleaning activities in a processing unit. Food contact surfaces typically may be cleaned several times a day or at the end of each shift; however, environmental surfaces such as walls and drains may be cleaned only once per week. Biofilm clearly has more time to develop on environmental surfaces [9]. A study found that although bacterial cells readily attached to food contact surfaces in processing facilities, extensive surface colonization and biofilm formation occurred only on environmental surfaces [10]. Several studies carried out in fish-processing plants have shown a correlation between the presence of L. monocytogenes in drains and on food contact surfaces, hence on the finished product [11]. Microbial cells may be transferred to the food product by vectors such as air, personnel, and cleaning systems [12,13]. The open nature of drains means that they are continuously challenged by a wide range of microbes, which vary depending on the site of the drain. Listeria spp., if present in the drains, may transfer from drains onto food contact surfaces, thus contaminating the food being processed. In dairy processing, an outbreak of Listeria associated with chocolate milk, which sickened 45 people, was traced to a drain that contaminated the milk filler above it [14]. Migration of the organism may occur from drains to food through workers and food handlers, contaminated equipment, and high-pressure cleaning and scrubbing in food-processing environments. Because aerosols generated as a result of high-pressure cleaning and washing activities (40 - 60 psi) may translocate bacterial cells, our study was designed to evaluate the potential for translocation of L. monocytogenes from drains onto food contact surfaces in the surrounding environment using L. innocua as a surrogate [15].
2. Materials and Methods
2.1. Bacterial Cultures and Inoculum Preparation
The bacterial cultures used in this study included four strains of Listeria innocua (ATCC 33091, 51742, 49595, and 33090) which were obtained from the American Type Culture Collection (ATCC). The lypholized microorganisms were individually transferred to 9 mL tryptic soy broth (TSB, Difco, Franklin Lakes, NJ, USA), vortexed to mix the suspension well, and incubated at 35˚C for 24 h. Each strain was then combined into a single mixed culture suspension to obtain a four-strain cocktail of L. innocua. A 7 - 8 log CFU/mL culture suspension was used for inoculation purposes. The cell density of this suspension was determined by serially diluting the pure culture that was grown in TSB, and plating in duplicate onto modified oxford medium agar (MOX, Difco, Franklin Lakes, NJ, USA). The bacterial cell counts were obtained after incubating the plates at 35˚C for 24 h.
2.2. Preparation of Drain Surface
A 10-inch-diameter, circular, painted cast iron drain, mounted onto a 2 × 3-feet “090” with a two-part white epoxy finish aluminum cabinet was used. The drain was placed in a 316 stainless bowl and a schedule 40 PVC male 4-inch adapter was screwed into the drain and was fitted with a 40 PVC pipe (manufactured by RGF Pvt. Ltd., West Palm Beach, FL, USA). A 5-gallon polyethylene bucket was used to collect the drain wash water.
2.3. Preparation of Surfaces
Stainless steel is a surface finish commonly found in food-processing environments. A research study showed that Listeria grew on stainless steel, teflon, nylon, and polyester for 7 to 18 d, whereas its biofilm formation was supported at 21˚C but was reduced at 10˚C [16]. Hence, stainless steel coupons were hung inside the chamber at three different heights and used for sampling to test translocation. Polished stainless steel coupons (6.4 × 1.9 × 0.1 cm) were washed with Fisherband Sparkleen (Fisher Scientific, Hampton, New Hampshire, USA) detergent and autoclaved for use.
2.4. Preparation of Meat Slurry
For the preparation of the meat slurry, 10 g of ground beef 80:20 (All Natural Ground Beef Chuck) was placed into a stomacher bag. To this, 100 mL of distilled water was added, then stomached for 1 min; 900 mL of distilled water was added to this mixture to make it 1 liter. 10 mL of bacterial cocktail (7 - 8 Log CFU/mL) was then added to the meat slurry for the inoculated sets.
2.5. Inoculation of the Drain
The drain was inoculated with meat slurry at regular intervals, as described further, to simulate the normal conditions of drain surfaces in a food-processing facility.
2.6. Cleaning and Washing Activities
Commercial cleaning and washing operations are done with a water pressure hose from 40 - 50 psi. Such high pressure generates aerosols. In this study, a commercial cleaner (alkaline-sodium hypochlorite 0.1% - 0.5%) and sanitizer (chlorinated ammonium compound consisting of N-alkyl dimethyl benzyl ammonium chlorides, Nalkyl dimethyl ethylbenzyl ammonium chlorides, and ethyl alcohol) were used. The sampling was performed at the end of 8 h based on the usual duration of a shift in a typical production facility. The time period for development of biofilms, 48 h, was also evaluated.
2.7. VIP for Listeria
VIP for Listeria (BioControl Systems. Inc., AOAC approved 997.03) was used for confirmation. If Listeria is present, an antigen-antibody-chromogen complex is formed that is visually read on the kit as a band formation.
2.8. Procedure
Autoclaved stainless steel coupons with binder clips were passed through 1-mL pipettes and placed on cooling racks were hung at 1, 3, and 5 feet with nylon thread strings inside the chamber. A total of 12 racks (4 per height) were used. On each of these racks, a set of 3 coupons was placed; making a total of 12 coupons per height. This study was performed for 8-h and 48-h time periods, each consisting of 4 sets; Non-Inoculated, NonTreated; Non-Inoculated, Treated; Inoculated, NonTreated; and Inoculated, Treated. The term inoculated refers to use of bacterial cocktail whereas treated refers to use of a commercial cleaner and sanitizer.
2.8.1. Non-Inoculated, Treated and Non-Inoculated, Non-Treated
The drain was inoculated with meat slurry at 0, 4, and 8 h. The prepared slurry was poured into the drain at 0 h. The drain was washed with a high-pressure water hose (40 psi) and poured again with slurry at 4 h. The process was repeated at 8 h. The drain was then allowed to sit for 30 min and washed with water (40 psi). The commercial cleaner was then applied and allowed to sit for 60 sec before the sanitizer was used, per manufacturer’s instructions, in the treated set whereas no cleaner or sanitizer was used in the non-treated set. The coupons hung inside the chamber during cleaning were then collected in individual sterile plastic bags.
2.8.2. Inoculated, Treated and Inoculated, Non-Treated
The drain was inoculated with meat slurry at 0, 4, and 8 h. The slurry with 10 mL L. innocua cocktail was poured into drain at 0 h. The drain was washed with a highpressure water hose (40 psi) and again poured with slurry at 4 h. The process was repeated at 8 h. The drain was then allowed to sit for 30 min and washed with water (40 psi). The commercial cleaner was then applied and allowed to sit for 60 sec before sanitizer was used, per manufacturer’s instructions, in the treated set whereas no cleaner or sanitizer was applied in the non-treated set. The coupons hung inside the chamber during cleaning were then collected in individual sterile plastic bags.
For each of these sets, after collection of coupons, 100 mL of listeria enrichment broth (LEB, Difco, Franklin Lakes, NJ, USA) was added to each of the bags containing stainless steel coupons. The coupons with LEB were incubated at 35˚C for 48 h. After 48 hours of incubation, the turbid broths were streaked onto the prepoured MOX plates, then incubated at 35˚C for 48 h. If black colonies were seen on the MOX plates, those were recorded as presumptive positive for Listeria. Typical Listeria colonies from the MOX plates were isolated and grown in 9 mL TSB test tubes for 48 h at 35˚C. To confirm the presence of Listeria spp., in the turbid TSB test tubes, the rapid VIP Listeria Test was performed. Positive test kits were used as confirmation of the presence of Listeria in the samples.
The same procedure was repeated for 48 h to study the translocation of bacterial cells when biofilms have been developed in the drain surface. The drain was inoculated with an L. innocua cocktail in meat slurry, as described previously, at 0, 8, 12, 24, 36, and 48 h.
For sampling the drain, sponge method was used. Three drain sites were sampled; drain surface (197.98 cm2), drain crate (278.07 cm2), and drain pipe (335.98 cm2). Sampled sponges (18 oz. “Speci Sponge”, 3.8 × 7.6 cm; Nasco Laboratory, Fort Atkinson, WI, USA) were placed in sterile bags with 20 mL letheen broth (Difco, Franklin Lakes, NJ, USA). Serial dilutions were made and spread plated on tryptic soy agar (TSA, Difco), MOX, and thin agar layer MOX (TALMOX) [17]. The wash water from the drain collected in a bucket was also plated for enumeration for each set. The plates were incubated at 35˚C for 48 h. Bacterial counts were taken and reported as CFU/area.
Three replications of each of the experimental sets for both 8 h and 48 h were performed.
2.9. Statistical Analysis
For statistical analysis, Single Factor Model with binomial distribution was used, and data were analyzed using the GENMOD procedure (SAS 9.1.2, 2004, Cary, NC, USA). The analysis was performed to find the probability for positive test coupons obtained as a result of translocation of bacterial cells from the drain to the stainless steel coupons. The experimental sets—Inoculated, Treated; and Inoculated, Non-Treated for both 8-h and 48-h periods—were observed to fit adequately into the model. The height at which the coupons were hung inside the chamber had a significant effect (p < 0.05) on the number of positive coupons obtained due to cell translocation from the drain to the coupons.
3. Results and Discussion
Bacterial populations enumerated from sponge sampling of the drain ranged between 3.5 - 4 log CFU/area in the inoculated treated sets in the 8-h set while counts were 6 - 8 log CFU/area in the 48-h of the inoculated sets. The treatment with commercial cleaner and sanitizer reduced bacterial population in the drain only by 0.5 log CFU/area. However, samples obtained from wash waters showed 3 log CFU/mL and 4 log CFU/mL reduction in bacterial population in 8-h and 48-h sets, respectively in the treated sets. Previous prevalence studies have shown a reduction in the prevalence of Listeria monocytogenes over 50% in slaughterhouse and upto 16% in smokehouse due to cleaning activities undertaken [18].
Tables 1 and 2 show the percentage of positive samples obtained for the different experimental sets. If there was no contamination in the drain to begin with, as indicated by non-inoculated sets for both 8-h and 48-h, no translocation of bacterial cells occurred from the drain onto the coupons and the surrounding environment.
In the 8-h set, translocation of bacterial cells was seen at all three heights. The percentage of positive samples was from 2% - 17%. Higher translocation was seen at 1 foot, followed by 3 feet and 5 feet, respectively, indicating that the closer the proximity from the drain, the greater the number of bacterial cells that transfer from the drain to the surrounding surfaces.
The translocation at 1 foot for the Inoculated, NonTreated set was 16.6%, whereas the Inoculated, Treated set was 13.8%. These percentage figures based on the experimental set further indicate that when a cleaning and sanitizing treatment is applied to control or eliminate the bacterial cells in the drain, the number of cells that translocate is fewer compared with the untreated drain. The translocation at 3 feet for the Inoculated, NonTreated set was 11.1%, compared with 5/5% for the Inoculated, Treated set. At 5 feet, the translocation for the Inoculated, Non-Treated set was 2.7% but 0% for the Inoculated, Treated Set. These percentages further reinforce the need for cleaning and sanitizing treatments to floor drains, because the number of cells translocated from the non-treated drain is higher than from the treated drain.
In the 48-h set, the coupons were found positive for translocation at 1, 3 and 5 feet. The range of percentage positives in this case was higher compared with the 8-h set, 2% - 25%. This may be attributed to the longer time available for the bacterial cells to grow and proliferate in the drain and also to form a biofilm as a protection against environmental stress. The average translocation at 1 foot was the highest, which was 25%, compared with 6.9% at 3 feet and 1.8% at 5 feet.
At the height of 1 foot, the percentage translocation for both Inoculated, Non-Treated and Inoculated, Treated sets was found to be 25%. At 3 feet, 8.3% positive coupons were obtained from the Inoculated, Non-Treated set whereas 5.5% were seen in the Inoculated, Treated set. At 5 feet, 2.7% positive samples were seen in the Inoculated, Treated set.
This study agrees with previous research findings that indicate using high pressure hoses can discharge Listeria spp. to unreachable areas and food contact surfaces [19]; and suggests that optimization is required in cleaning and washing steps to limit the generation of viable aerosols. Similar suggestion was seen in a study by that found that in the fish-processing plants that did not use high-pressure sprayers for cleaning, L. monocytogenes was overall
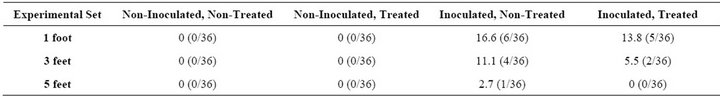
Table 1. Percentage of positive samples (coupons) during 8 h for Listeria spp. due to translocation from the drain into the surrounding environment.
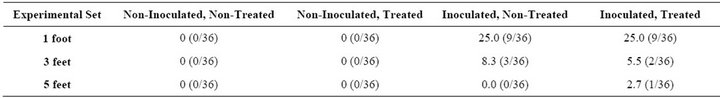
Table 2. Percentage of positive samples (coupons) during 48 h for Listeria spp. due to translocation from the drain into the surrounding environment.
infrequently isolated from food contact surfaces even when a high number of positive samples were obtained from the drains [20]. Studies on guidelines to control L. monocytogenes in small to medium scale fresh cut and packaging operations have also indicated modification the cleaning and sanitizing procedures as one of the means of pathogen control [21]. A higher degree of mechanical action and the use of detergents may play a role in the reduction in the spread of contamination by aerosols.
Because of the ubiquitous nature of Listeria monocytogenes in the general environment, minimizing its presence throughout food production and processing environments is vital. Effective and reliable personnel practices and hygiene are required in addition to the application of effective cleaning procedures to the manufacturing equipment and the food-processing environment itself.
4. Acknowledgements
The authors like to thank the Kansas Beef Council (KBC) and the Food Safety Consortium (FSC, USDA) for funding the project. This is contribution No. 12-013-J from the Kansas Agricultural Experiment Station.
REFERENCES
- P. J. Eginton, H. Gibson, J. T. Holah, P. S. Handley and P. Gilbert, “The Influence of Substratum Properties on Attachment of Bacterial Cells,” Colloids and Surfaces B: Biointerfaces, Vol. 5, No. 3-4, 1995, pp. 153-159. doi:10.1016/0927-7765(95)01219-9
- E. A. Zotolla, “Microbial Attachment and Biofilm Formation: A New Problem in Food Industry?” Food Technology, Vol. 48, 1994, pp. 107-114.
- D. Senczek, R. Stephan and F. Untermann, “Pulsed-Field Gel Electrophoresis (PFGE) Typing of Listeria Strains Isolated from a Meat Processing Plant over a 2-Year Period,” International Journal Food Microbiology, Vol. 62, 2000, pp. 155-159. doi:10.1016/S0168-1605(00)00395-0
- P. Berche, J. L. Gaillard and S. Richard, “Invasiveness and Intracellular Growth of Listeria monocytogenes,” Infection, Vol. 16, No. 2, 1988, pp. 145-148.
- USFDA/CFSAN and CDC, “Executive Summary, Reducing the Risk of Listeria monocytogenes FDA/CDC 2003 Update of the Listeria Action Plan, 2003. http://www.cfsan.fda.gov/~dms/lmr2plan.html
- M. L. Gray and A. H. Killinger, “Listeria monocytogenes and Listeria Infections,” Bacteriology Reviews, Vol. 30, 1966, pp. 309-382.
- E. T. Ryser and E. H. Marth, “Listeria, Listeriosis and Food Safety, Incidence and Control of Listeria in Food Processing Facilities,” CRC Press, Taylor and Francis Group, 2007, pp. 681-766.
- T. Zhao, M. P. Doyle and P. Zhao, “Control of Listeria monocytogenes in a Biofilm by Competitive-Exclusion Microorganisms,” Applied Environmental Microbiology, Vol. 72, 2004, pp. 3996-4003. doi:10.1128/AEM.70.7.3996-4003.2004
- I. C. Blackman and J. F. Frank, “Growth of Listeria monocytogenes as a Biofilm on Various Food Processing Surfaces,” Journal of Food Protection, Vol. 59, 1996, pp. 827-831.
- H. Gibson, J. H. Taylor, K. E. Hall and J. T. Holah, “Biofilms and Their Detection in the Food Industry,” R&D Report No. 1 Chipping Campden, Campden and Chorleywood Food Research Association, 1994.
- A. D. Hoffman, K. L. Gall, D. M. Norton and M. Wiedmann, “Listeria monocytogenes Contamination Patterns for the Smoked Fish Processing Environment and for Raw Fish,” Journal of Food Protection, Vol. 66, 2003, pp. 52-60.
- J. T. Holah, “Industrial Monitoring: Hygiene in Food Processing,” In: L. F. Melo, T. R. Bott, M. Fletcher and B. Capdeville, Eds., Biofilms: Science and Technology, Kluwer Academic Publishers, Dordrecht, 1992, pp. 645- 660.
- J. T. Holah, R. P. Betts and R. H. Thorpe, “The Use of Direct Epiflourescent Microscopy (DEM) and the Epifluorescent filter technique (DEFT) to Assess Microbial Populations of food contact surfaces,” Journal of Applied Bacteriology, Vol. 65, 1988, pp. 215-221. doi:10.1111/j.1365-2672.1988.tb01888.x
- C. B. Dalton, C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor and P. M. Griffin, “An Outbreak of Gastroenteritis and Fever Due to L. monocytogenes in Milk,” New England Journal of Medicine, Vol. 336, 1997, pp. 100-105. doi:10.1056/NEJM199701093360204
- G. Zhang, L. Ma, O. A. Oyarzabal and M. P. Doyle, “Aerosol Studies with Listeria innocua and Listeria monocytogenes,” Journal of Food Protection, Vol. 70, 2007, pp. 1857-1865.
- I. C. Blackman and J. F. Frank, “Growth of Listeria monocytogenes as a Biofilm on Various Food Processing Surfaces,” Journal of Food Protection, Vol. 59, 1996, pp. 827-831.
- D. H. Kang and D. Y. C. Fung, “Thin Agar Layer Method for Recovery of Heat-Injured Listeria monocytogenes,” Journal of Food Protection, Vol. 62, 1999, pp. 1346- 1349.
- G. Wulff, L. Gram, P. Ahrens and B. F. Vogel, “One Group of Genetically Similar Listeria monocytogenes Strains Frequently Dominate and Persist in Several Fish Slaughter and Smokehouses,” Applied and Environmental Microbiology, Vol. 72, 2006, pp. 4313-4322. doi:10.1128/AEM.02288-05
- J. L. K. Kornacki, “Detecting Sources of Listeria monocytogenes in Ready-to-Eat Food Processing Environments,” 2012. http://www.fsis.usda.gov/PDF/Seminar_Detecting_Sources_of_LM.pdf
- J. Thimothe, K. K. Nightingale, K. Gall, V. N. Scott and M. Wiedmann, “Tracking of Listeria monocytogenes in Smoked Fish Processing Plants,” Journal of Food Protection, Vol. 67, 2004, pp. 328-341.
- T. Suslow and L. Harris, “Guidelines for Control of Listeria monocytogenes in Small to Medium Scale Packaging and Fresh-Cut Operations,” 2000. http://anrcatalog.ucdavis.edu/pdf/8015.pdf

