Paper Menu >>
Journal Menu >>
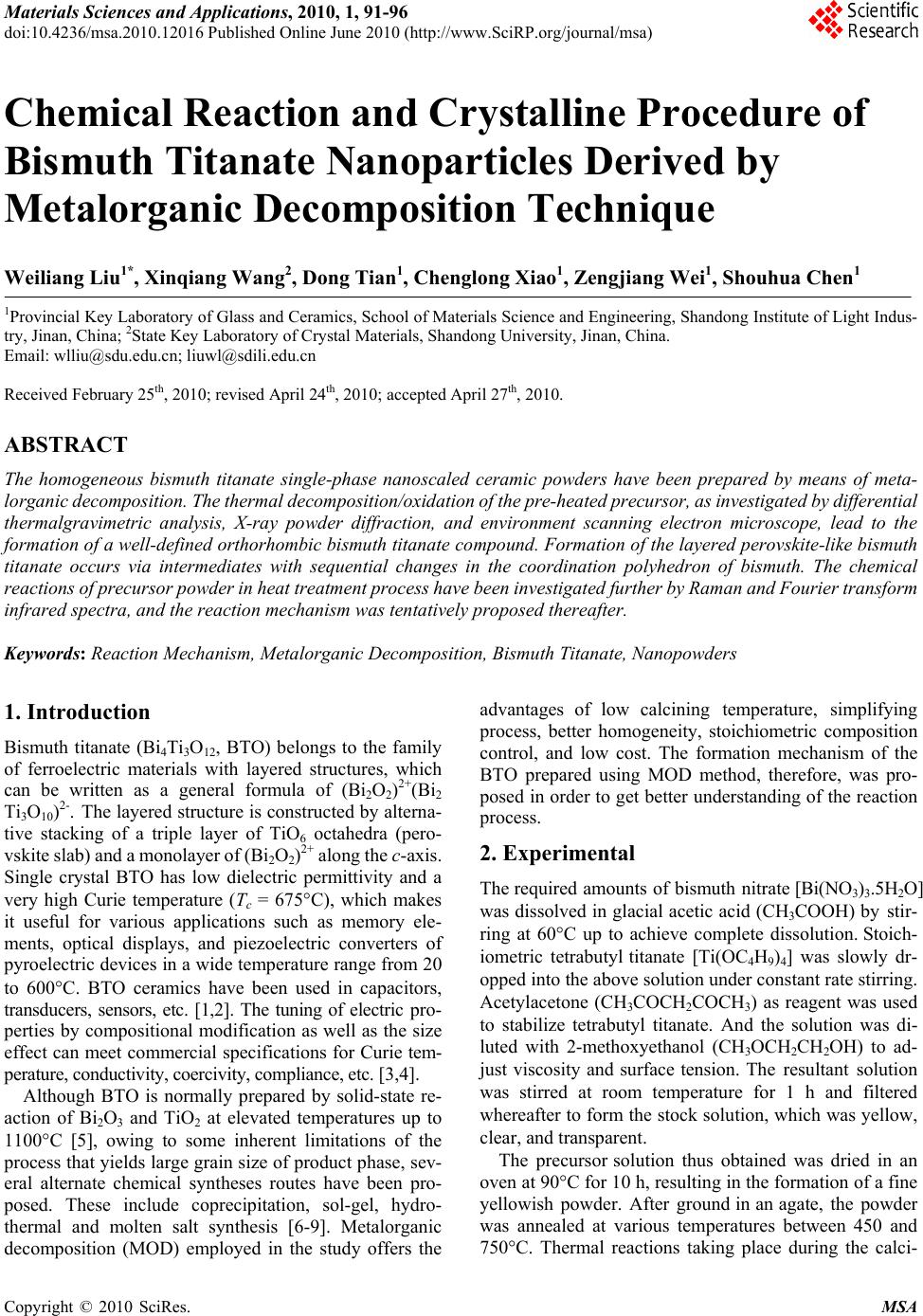 Materials Sciences and Applicatio ns, 2010, 1, 91-96 doi:10.4236/msa.2010.12016 Published Online June 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Chemical Reaction and Crystalline Procedure of Bismuth Titanate Nanoparticles Derived by Metalorganic Decomposition Technique Weiliang Liu1*, Xinqiang Wang2, Dong Tian1, Chenglong Xiao1, Zengjiang Wei1, Shouhua Chen1 1Provincial Key Laboratory of Glass and Ceramics, School of Materials Science and Engineering, Shandong Institute of Light Indus- try, Jinan, China; 2State Key Laboratory of Crystal Materials, Shandong University, Jinan, China. Email: wlliu@sdu.edu.cn; liuwl@sdili.edu.cn Received February 25th, 2010; revised April 24th, 2010; accepted April 27th, 2010. ABSTRACT The homogeneous bismuth titanate single-phase nanoscaled ceramic powders have been prepared by means of meta- lorganic decomposition. The thermal decomposition/oxidation of the pre-heated precursor, as investigated by differential thermalgravimetric analysis, X-ray powder diffraction, and environment scanning electron microscope, lead to the formation of a well-defined orthorhombic bismuth titanate compound. Formation of the layered perovskite-like bismuth titanate occurs via intermediates with sequential changes in the coordination polyhedron of bismuth. The chemical reactions of precursor powder in heat treatment process have been investigated further by Raman and Fourier transform infrared spectra, and the reaction mechanism was tentatively proposed thereafter. Keywords: Reaction Mechanism, Metalorganic Decomposition, Bismuth Titanate, Nanopowders 1. Introduction Bismuth titanate (Bi4Ti3O12, BTO) belongs to the family of ferroelectric materials with layered structures, which can be written as a general formula of (Bi2O2)2+(Bi2 Ti3O10)2-. The layered structure is constructed by alterna- tive stacking of a triple layer of TiO6 octahedra (pero- vskite slab) and a monolayer of (Bi2O2)2+ along the c-axis. Single crystal BTO has low dielectric permittivity and a very high Curie temperature (Tc = 675C), which makes it useful for various applications such as memory ele- ments, optical displays, and piezoelectric converters of pyroelectric devices in a wide temperature range from 20 to 600C. BTO ceramics have been used in capacitors, transducers, sensors, etc. [1,2]. The tuning of electric pro- perties by compositional modification as well as the size effect can meet commercial specifications for Curie tem- perature, conductivity, coercivity, compliance, etc. [3,4]. Although BTO is normally prepared by solid-state re- action of Bi2O3 and TiO2 at elevated temperatures up to 1100C [5], owing to some inherent limitations of the process that yields large grain size of product phase, sev- eral alternate chemical syntheses routes have been pro- posed. These include coprecipitation, sol-gel, hydro- thermal and molten salt synthesis [6-9]. Metalorganic decomposition (MOD) employed in the study offers the advantages of low calcining temperature, simplifying process, better homogeneity, stoichiometric composition control, and low cost. The formation mechanism of the BTO prepared using MOD method, therefore, was pro- posed in order to get better understanding of the reaction process. 2. Experimental The required amounts of bismuth nitrate [Bi(NO3)3.5H2O] was dissolved in glacial acetic acid (CH3COOH) by stir- ring at 60°C up to achieve complete dissolution. Stoich- iometric tetrabutyl titanate [Ti(OC4H9)4] was slowly dr- opped into the above solution under constant rate stirring. Acetylacetone (CH3COCH2COCH3) as reagent was used to stabilize tetrabutyl titanate. And the solution was di- luted with 2-methoxyethanol (CH3OCH2CH2OH) to ad- just viscosity and surface tension. The resultant solution was stirred at room temperature for 1 h and filtered whereafter to form the stock solution, which was yellow, clear, and transparent. The precursor solution thus obtained was dried in an oven at 90°C for 10 h, resulting in the formation of a fine yellowish powder. After ground in an agate, the powder was annealed at various temperatures between 450 and 750°C. Thermal reactions taking place during the calci- 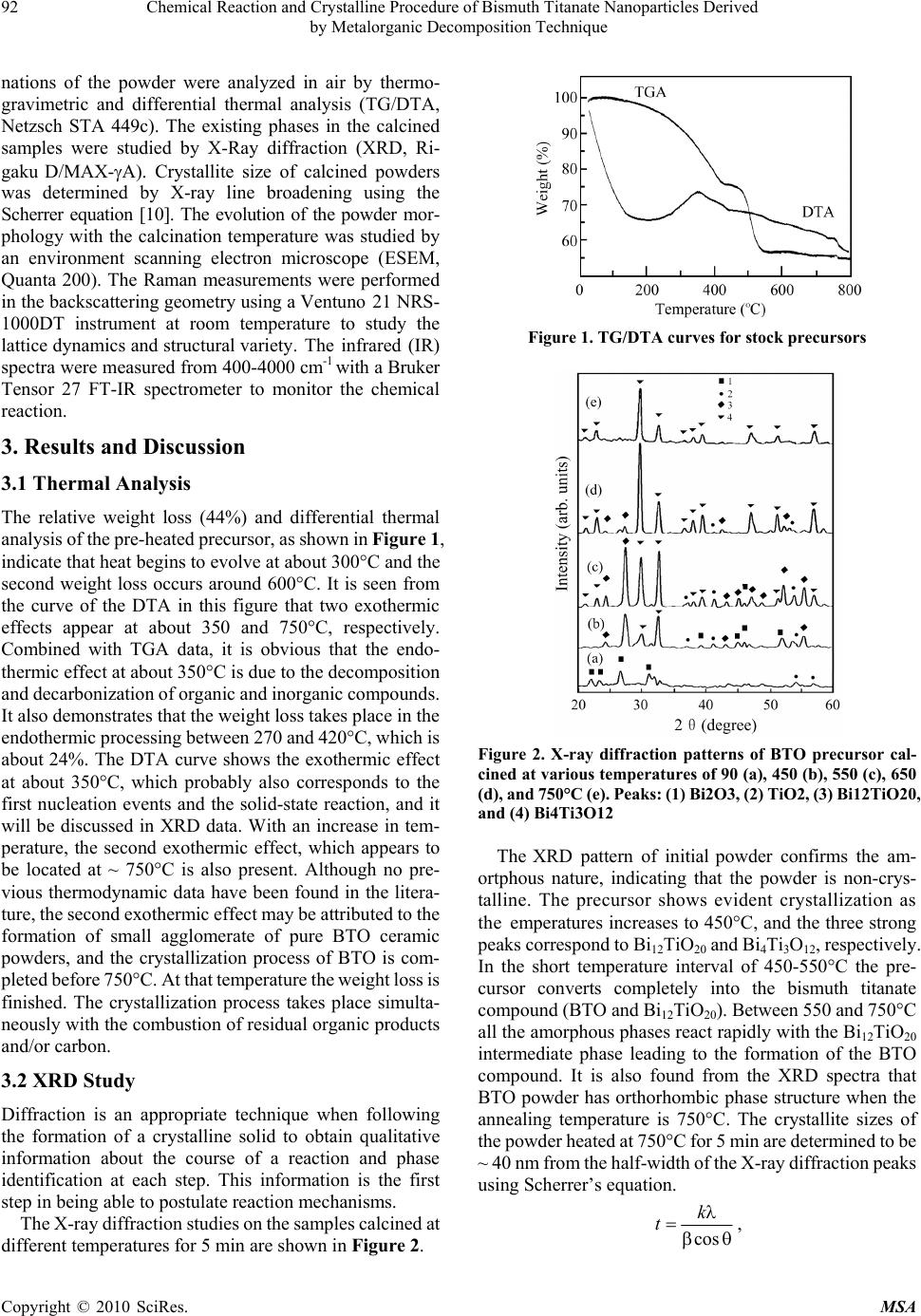 92 Chemical Reaction and Crystalline Procedure of Bismuth Titanate Nanoparticles Derived by Metalorganic Decomposition Technique nations of the powder were analyzed in air by thermo- gravimetric and differential thermal analysis (TG/DTA, Netzsch STA 449c). The existing phases in the calcined samples were studied by X-Ray diffraction (XRD, Ri- gaku D/MAX-A). Crystallite size of calcined powders was determined by X-ray line broadening using the Scherrer equation [10]. The evolution of the powder mor- phology with the calcination temperature was studied by an environment scanning electron microscope (ESEM, Quanta 200). The Raman measurements were performed in the backscattering geometry using a Ventuno 21 NRS- 1000DT instrument at room temperature to study the lattice dynamics and structural variety. The infrared (IR) spectra were measured from 400-4000 cm-1 with a Bruker Tensor 27 FT-IR spectrometer to monitor the chemical reaction. 3. Results and Discussion 3.1 Thermal Analysis The relative weight loss (44%) and differential thermal analysis of the pre-heated precursor, as shown in Figure 1, indicate that heat begins to evolve at about 300°C and the second weight loss occurs around 600°C. It is seen from the curve of the DTA in this figure that two exothermic effects appear at about 350 and 750°C, respectively. Combined with TGA data, it is obvious that the endo- thermic effect at about 350°C is due to the decomposition and decarbonization of organic and inorganic compounds. It also demonstrates that the weight loss takes place in the endothermic processing between 270 and 420°C, which is about 24%. The DTA curve shows the exothermic effect at about 350°C, which probably also corresponds to the first nucleation events and the solid-state reaction, and it will be discussed in XRD data. With an increase in tem- perature, the second exothermic effect, which appears to be located at ~ 750°C is also present. Although no pre- vious thermodynamic data have been found in the litera- ture, the second exothermic effect may be attributed to the formation of small agglomerate of pure BTO ceramic powders, and the crystallization process of BTO is com- pleted before 750°C. At that temperature the weight loss is finished. The crystallization process takes place simulta- neously with the combustion of residual organic products and/or carbon. 3.2 XRD Study Diffraction is an appropriate technique when following the formation of a crystalline solid to obtain qualitative information about the course of a reaction and phase identification at each step. This information is the first step in being able to postulate reaction mechanisms. The X-ray diffraction studies on the samples calcined at different temperatures for 5 min are shown in Figure 2. Figure 1. TG/DTA curves for stock precursors Figure 2. X-ray diffraction patterns of BTO precursor cal- cined at various temperatures of 90 (a), 450 (b), 550 (c), 650 (d), and 750°C (e). Peaks: (1) Bi2O3, (2) TiO2 , (3) Bi12TiO20, and (4) Bi4Ti3O12 The XRD pattern of initial powder confirms the am- ortphous nature, indicating that the powder is non-crys- talline. The precursor shows evident crystallization as the emperatures increases to 450C, and the three strong peaks correspond to Bi12TiO20 and Bi4Ti3O12, respectively. In the short temperature interval of 450-550°C the pre- cursor converts completely into the bismuth titanate compound (BTO and Bi12TiO20). Between 550 and 750°C all the amorphous phases react rapidly with the Bi12TiO20 intermediate phase leading to the formation of the BTO compound. It is also found from the XRD spectra that BTO powder has orthorhombic phase structure when the annealing temperature is 750°C. The crystallite sizes of the powder heated at 750°C for 5 min are determined to be ~ 40 nm from the half-width of the X-ray diffraction peaks using Scherrer’s equation. cos k t , Copyright © 2010 SciRes. MSA 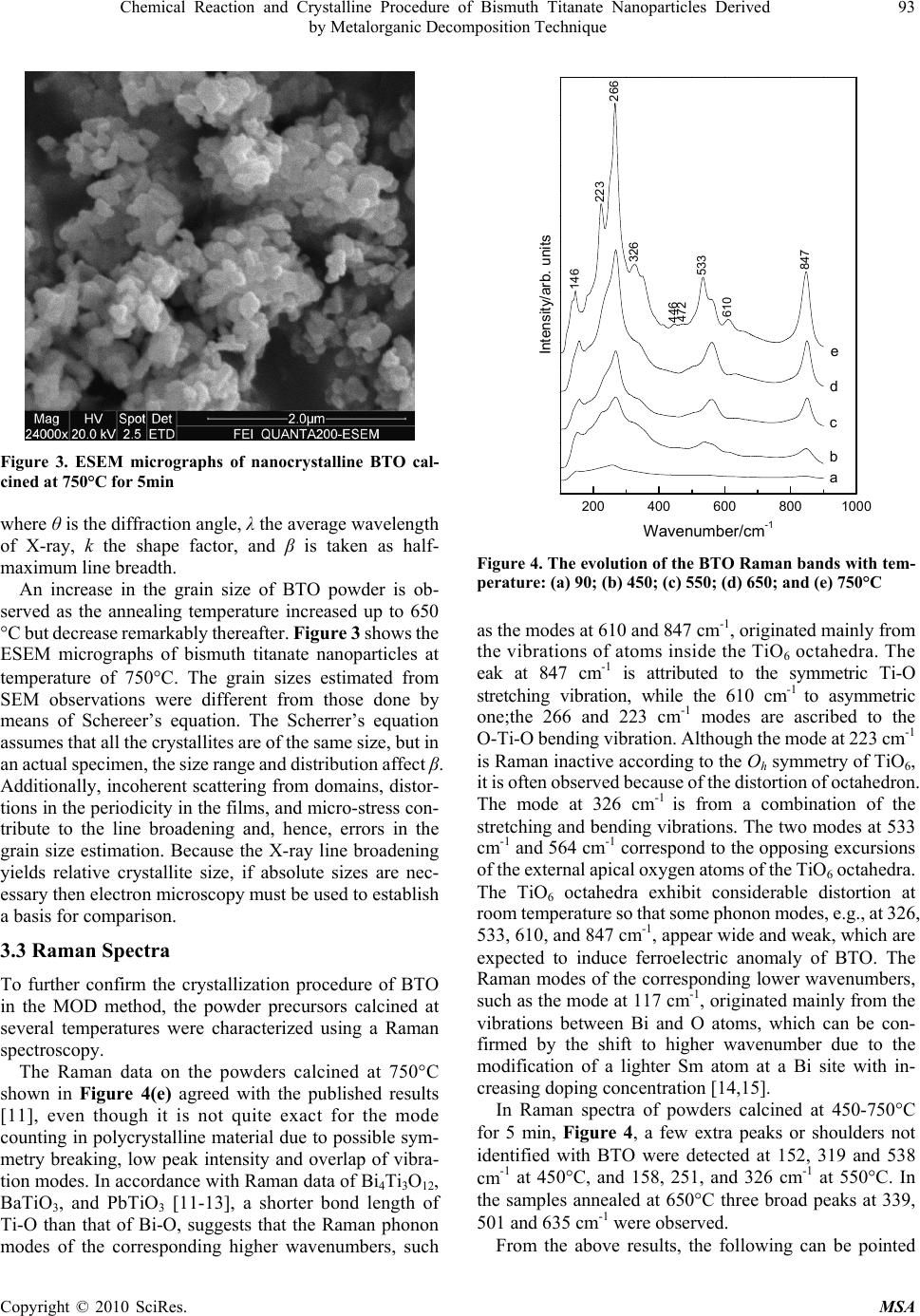 Chemical Reaction and Crystalline Procedure of Bismuth Titanate Nanoparticles Derived 93 by Metalorganic Decomposition Technique Figure 3. ESEM micrographs of nanocrystalline BTO cal- cined at 750°C for 5min where θ is the diffraction angle, λ the average wavelength of X-ray, k the shape factor, and β is taken as half- maximum line breadth. An increase in the grain size of BTO powder is ob- served as the annealing temperature increased up to 650 °C but decrease remarkably thereafter. Figure 3 shows the ESEM micrographs of bismuth titanate nanoparticles at temperature of 750C. The grain sizes estimated from SEM observations were different from those done by means of Schereer’s equation. The Scherrer’s equation assumes that all the crystallites are of the same size, but in an actual specimen, the size range and distribution affect β. Additionally, incoherent scattering from domains, distor- tions in the periodicity in the films, and micro-stress con- tribute to the line broadening and, hence, errors in the grain size estimation. Because the X-ray line broadening yields relative crystallite size, if absolute sizes are nec- essary then electron microscopy must be used to establish a basis for comparison. 3.3 Raman Spectra To further confirm the crystallization procedure of BTO in the MOD method, the powder precursors calcined at several temperatures were characterized using a Raman spectroscopy. The Raman data on the powders calcined at 750°C shown in Figure 4(e) agreed with the published results [11], even though it is not quite exact for the mode counting in polycrystalline material due to possible sym- metry breaking, low peak intensity and overlap of vibra- tion modes. In accordance with Raman data of Bi4Ti3O12, BaTiO3, and PbTiO3 [11-13], a shorter bond length of Ti-O than that of Bi-O, suggests that the Raman phonon modes of the corresponding higher wavenumbers, such 200 400 600 8001000 146 223 266 326 446 472 533 610 847 e d c b a Intensity/arb. units Wavenumber/cm-1 Figure 4. The evolution of the BTO Raman bands with tem- perature: (a) 90; (b) 450; (c) 550; (d) 650; and (e) 750°C as the modes at 610 and 847 cm-1, originated mainly from the vibrations of atoms inside the TiO6 octahedra. The eak at 847 cm-1 is attributed to the symmetric Ti-O stretching vibration, while the 610 cm-1 to asymmetric one;the 266 and 223 cm-1 modes are ascribed to the O-Ti-O bending vibration. Although the mode at 223 cm-1 is Raman inactive according to the Oh symmetry of TiO6, it is often observed because of the distortion of octahedron. The mode at 326 cm-1 is from a combination of the stretching and bending vibrations. The two modes at 533 cm-1 and 564 cm-1 correspond to the opposing excursions of the external apical oxygen atoms of the TiO6 octahedra. The TiO6 octahedra exhibit considerable distortion at room temperature so that some phonon modes, e.g., at 326, 533, 610, and 847 cm-1, appear wide and weak, which are expected to induce ferroelectric anomaly of BTO. The Raman modes of the corresponding lower wavenumbers, such as the mode at 117 cm-1, originated mainly from the vibrations between Bi and O atoms, which can be con- firmed by the shift to higher wavenumber due to the modification of a lighter Sm atom at a Bi site with in- creasing doping concentration [14,15]. In Raman spectra of powders calcined at 450-750°C for 5 min, Figure 4, a few extra peaks or shoulders not identified with BTO were detected at 152, 319 and 538 cm-1 at 450°C, and 158, 251, and 326 cm-1 at 550°C. In the samples annealed at 650°C three broad peaks at 339, 501 and 635 cm-1 were observed. From the above results, the following can be pointed Copyright © 2010 SciRes. MSA 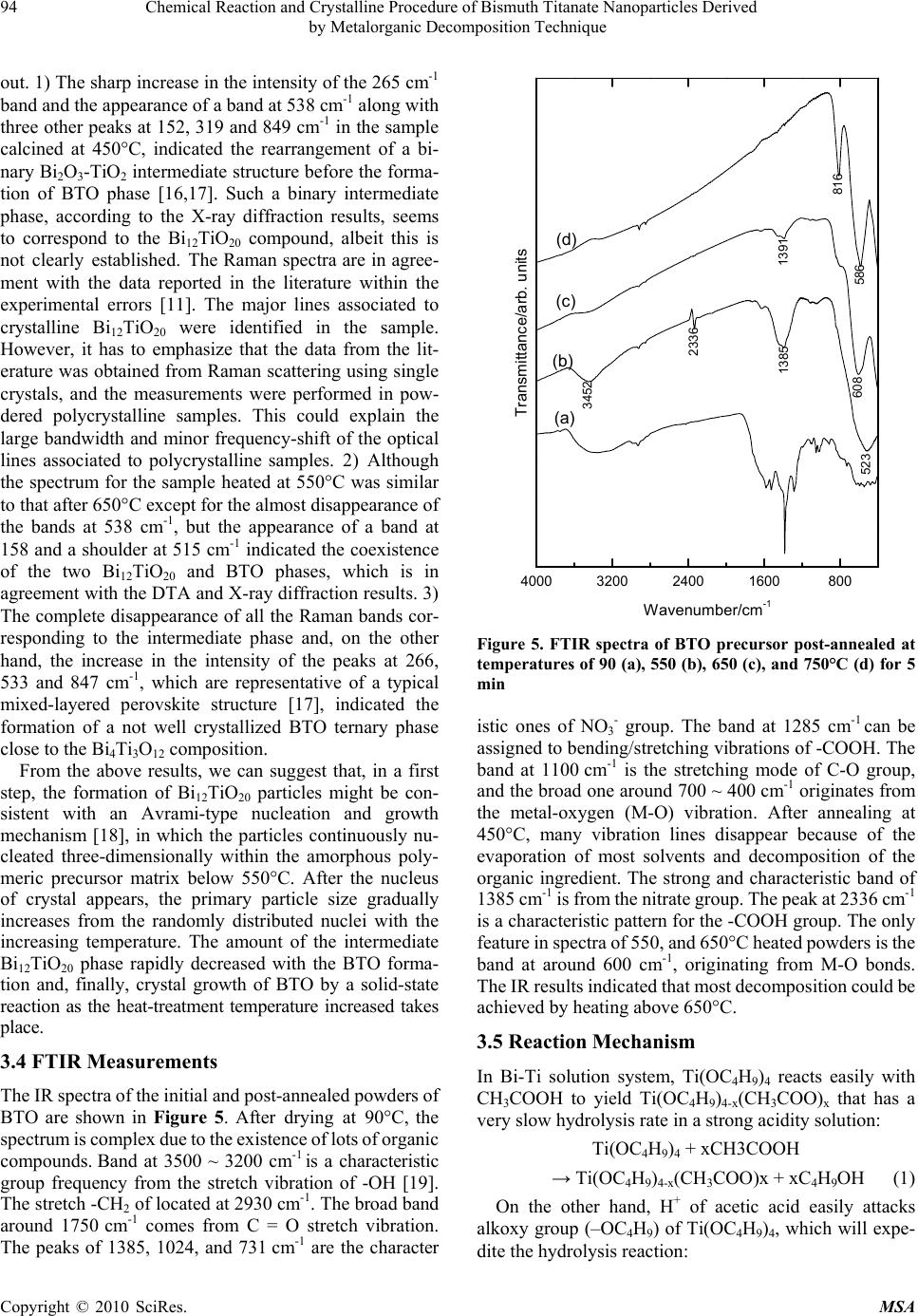 94 Chemical Reaction and Crystalline Procedure of Bismuth Titanate Nanoparticles Derived by Metalorganic Decomposition Technique out. 1) The sharp increase in the intensity of the 265 cm-1 band and the appearance of a band at 538 cm-1 along with three other peaks at 152, 319 and 849 cm-1 in the sample calcined at 450°C, indicated the rearrangement of a bi- nary Bi2O3-TiO2 intermediate structure before the forma- tion of BTO phase [16,17]. Such a binary intermediate phase, according to the X-ray diffraction results, seems to correspond to the Bi12TiO20 compound, albeit this is not clearly established. The Raman spectra are in agree- ment with the data reported in the literature within the experimental errors [11]. The major lines associated to crystalline Bi12TiO20 were identified in the sample. However, it has to emphasize that the data from the lit- erature was obtained from Raman scattering using single crystals, and the measurements were performed in pow- dered polycrystalline samples. This could explain the large bandwidth and minor frequency-shift of the optical lines associated to polycrystalline samples. 2) Although the spectrum for the sample heated at 550°C was similar to that after 650°C except for the almost disappearance of the bands at 538 cm-1, but the appearance of a band at 158 and a shoulder at 515 cm-1 indicated the coexistence of the two Bi12TiO20 and BTO phases, which is in agreement with the DTA and X-ray diffraction results. 3) The complete disappearance of all the Raman bands cor- responding to the intermediate phase and, on the other hand, the increase in the intensity of the peaks at 266, 533 and 847 cm-1, which are representative of a typical mixed-layered perovskite structure [17], indicated the formation of a not well crystallized BTO ternary phase close to the Bi4Ti3O12 composition. From the above results, we can suggest that, in a first step, the formation of Bi12TiO20 particles might be con- sistent with an Avrami-type nucleation and growth mechanism [18], in which the particles continuously nu- cleated three-dimensionally within the amorphous poly- meric precursor matrix below 550°C. After the nucleus of crystal appears, the primary particle size gradually increases from the randomly distributed nuclei with the increasing temperature. The amount of the intermediate Bi12TiO20 phase rapidly decreased with the BTO forma- tion and, finally, crystal growth of BTO by a solid-state reaction as the heat-treatment temperature increased takes place. 3.4 FTIR Measurements The IR spectra of the initial and post-annealed powders of BTO are shown in Figure 5. After drying at 90°C, the spectrum is complex due to the existence of lots of organic compounds. Band at 3500 ~ 3200 cm-1 is a characteristic group frequency from the stretch vibration of -OH [19]. The stretch -CH2 of located at 2930 cm-1. The broad band around 1750 cm-1 comes from C = O stretch vibration. The peaks of 1385, 1024, and 731 cm-1 are the character 4000 3200 2400 1600800 523 1385 2336 3452 586 816 608 1391 (d) (c) (b) (a) Transmittance/arb. units Wavenumber/cm -1 Figure 5. FTIR spectra of BTO precursor post-annealed at temperatures of 90 (a), 550 (b), 650 (c), and 750°C (d) for 5 min istic ones of NO3 - group. The band at 1285 cm-1 can be assigned to bending/stretching vibrations of -COOH. The band at 1100 cm-1 is the stretching mode of C-O group, and the broad one around 700 ~ 400 cm-1 originates from the metal-oxygen (M-O) vibration. After annealing at 450°C, many vibration lines disappear because of the evaporation of most solvents and decomposition of the organic ingredient. The strong and characteristic band of 1385 cm-1 is from the nitrate group. The peak at 2336 cm-1 is a characteristic pattern for the -COOH group. The only feature in spectra of 550, and 650°C heated powders is the band at around 600 cm-1, originating from M-O bonds. The IR results indicated that most decomposition could be achieved by heating above 650°C. 3.5 Reaction Mechanism In Bi-Ti solution system, Ti(OC4H9)4 reacts easily with CH3COOH to yield Ti(OC4H9)4-x(CH3COO)x that has a very slow hydrolysis rate in a strong acidity solution: Ti(OC4H9)4 + xCH3COOH → Ti(OC4H9)4-x(CH3COO)x + xC4H9OH (1) On the other hand, H+ of acetic acid easily attacks alkoxy group (–OC4H9) of Ti(OC4H9)4, which will expe- dite the hydrolysis reaction: Copyright © 2010 SciRes. MSA  Chemical Reaction and Crystalline Procedure of Bismuth Titanate Nanoparticles Derived 95 by Metalorganic Decomposition Technique 3 (–OC4H9) of Ti(OC4H9)4, which 49 49 TiOC HHHOH TiOHC HHOH 49 TiOHC H OHH (2) 49 Ti OHTi OCHTi O Ti (3) 492 492 Ti OTiTi OH TiOTi(OCH) OTi TiO[Ti(OC H)]nOTi (4) 33322 32 32 Bi(NO)CH OCHCH OH CH OCHOB(NO)HNO (5) 49432232 4932 232493 Ti(OCH)CHOCH CHOBi(NO) Ti(OC H) OCH CHOBi(NO )C HOCH (6) According to the above-mentioned analyses and the experimental results, the following reaction scheme is the most reasonable to describe the decomposition process: 4932 232 232222 Ti(OCH)OCHCHOBi(NO) Bi OTiOCOH ONO (7) Bismuth titanate Bi12TiO20 is crystallochemically re- lated to γ-Bi2O3; these compounds have similar structural frameworks and almost identical cubic cell parameters [20]. In view of this fact, formation of Bi12TiO20 in the Bi2O3-TiO2 system can be considered as a phase transition from α-Bi2O3 to γ-Bi2O3 initiated by incorporation of a titanium dioxide admixture into the Bi2O3 structure. The solid-phase synthesis of Bi4Ti3O12 involves oc- currence of both rearrangement and transport processes; thus, formation of Bi4Ti3O12 occurs by the mechanism of successive rearrangements: 23212 20 Bi OTiOBiTiO (8) 1220243 12 BiTiOTiOBiTi O (9) This transformation pattern reflects the crystallochem- ical genesis of the structure in the course of the solid- phase reaction. The structure formation involves a suc- cessive increase in the number of the nearest atoms adja- cent to Bi, with the last, high-temperature stage being accompanied by a considerably larger change in the bis- muth coordination number than the first, low-temperature stage [20]. 4. Conclusions In summary, homogeneous and fine BTO ceramic pow- ders have been prepared by metalorgainc decomposition method. Based on TGA/DTA, and XRD results, we con- clude that the synthesis of the BTO compound takes place through the formation of an intermediate phase of com- position Bi12TiO20, which is formed during the heating between 350 and 550°C. Prolonged heat treatment be- tween 550 and 750°C promote a rapid consumption by solid-state reaction of the intermediate phase with the formation of BTO, without any indication on the forma- tion of other different phases or segregation of the indi- vidual metal oxides. These results support the contention for the metalorganic precursor synthesis method as useful to prepare ceramics with complex composition such as those of bismuth titanates. The postulated mechanisms are further confirmed due to the structural variety as shown in Raman and FTIR studies. 5. Acknowledgements This work is supported by the Youth Scientist Fund of Shandong Province (2007BS04007), the Doctoral Startup Foundation of Shandong Institute of Light Industry, and National Natural Science Foundation of China (50772 059). REFERENCES [1] P. Siriprapa, A. Watcharapasorn and S. Jiansirisomboon, “Electrical and Mechanical Characteristics of (Bi4-x Lax) Ti3O12 Ceramics,” Ferroelectrics, Vol. 382, No. 1, 2009, pp. 160-165. [2] I. L. Trubnikov, S. N. Svirskaya, A. A. Zubkov and I. N. Toguleva, “Possible Ways to Obtain Materials Based on Bismuth Titanate Bi4Ti3O12,” Russian Journal of Applied Chemistry, Vol. 82, No. 11, 2009, pp. 1911-1914. [3] A. Moure, A. Castro and L. Pardo, “Aurivillius-Type Ceramics, A Class of High Temperature Piezoelectric Materials: Drawbacks, Advantages and Trends,” Progress in Solid State Chemistry, Vol. 37, No. 1, 2009, pp. 15-39. [4] M. Villegas, A. C. Caballero, T. Jardiel, C. Arago and J. Maudes, “Evaluation of Piezoelectric Properties of Bi4Ti3O12 Based Ceramics at High Temperature,” Ferroelectrics, Vol. 393, 2009, pp. 44-53. [5] A. Watcharapasorn, P. Siriprapa, and S. Jiansirisomboon, “Grain Growth Behavior in Bismuth Titanate-Based Ceramics,” Journal of the European Ceramic Society, Vol. 30, No. 1, 2010, pp. 87-93. [6] P. Pookmanee and S. Phanichphant, “Characterization of Lead-Free Bismuth Titanate (Bi4Ti3O12) Synthe-Sized by a Modified Oxalate Co-Precipitation Method,” Journal of Ceramic Processing Research, Vol. 10, No. 4, 2009, pp. 448-452. [7] H. Ke, W. Wang, L. Chen, J. Xu, D. Jia, Z. Lu and Y. Zhou, “Crystallization Process of Lanthanum-Substituted Bismuth Titanate Synthesized by a Facile Sol-Gel Method,” Journal of Sol-Gel Science and Technology, Vol. 53, No. 1, 2010, pp. 135-140. [8] Y. Wang, Y. Wen, H. Ding and Y. Shan, “Improved Structural Stability of Titanium-Doped Beta-Bi2O3 during Copyright © 2010 SciRes. MSA  96 Chemical Reaction and Crystalline Procedure of Bismuth Titanate Nanoparticles Derived by Metalorganic Decomposition Technique Copyright © 2010 SciRes. MSA Visible-Light-Activated Photocatalytic Processes,” Journal of Materials Science, Vol. 45, No. 5, 2010, pp. 1385-1392. [9] A. Watcharapasorn, P. Siriprapa and S. Jiansirisomboon, “Grain Growth Behavior in Bismuth Titanate-Based Ceramics,” Journal of the European Ceramic Society, Vol. 30, No. 1, 2010, pp. 87-93. [10] B. D. Stojanovic, A. Z. Simoes, C. O. Paiva-Santos, C. Quinelato, E. Longo and J. A. Varela, “Effect of Processing Route on the Phase Formation and Properties of Bi4Ti3O12 Ceramics,” Ceramics International, Vol. 32, No. 6, 2006, pp. 707-712. [11] H. Idink, V. Srikanth, W. B. White and E. C. Subbarao “Raman Study of Low Temperature Phase Transitions in Bismuth Titanate, Bi4Ti3O12,” Journal of Applied Physics, Vol .76, No. 3, 1994, pp. 1819-1823. [12] P. R. Graves, G. Hua, S. Myhra and J. G. Thompson, “The Raman Modes of the Aurivillius Phases: Temperature and Polarization Dependence,” Journal of Solid State Chemistry, Vol. 114, No. 1, 1995, pp. 112-122. [13] P. S. Dobal and R. S. Katiyar, “Studies on Ferroelectric Perovskites and Bi-Layered Compounds Using Micro- Raman Spectroscopy,” Journal of Raman Spectroscopy, Vol. 33, No. 6, 2002, pp. 405-423. [14] W. L. Liu, H. R. Xia, H. Han and X. Q. Wang, “Structural and Electrical Characteristics of Bi3.5SM0.5Ti3O12 Thin Films on Si(100),” Journal of Crystal Growth, Vol. 264, No. 1-3, 2004, pp. 351-356. [15] W. L. Liu, H. R. Xia, H. Han and X. Q. Wang, “Structural, Morphology and Electrical Studies of Sm-Modified Bismuth Titanate thin Films on Si(100),” Journal of Solid State Chemistry, Vol. 177, No. 9, 2004, pp. 3021-3027. [16] J. F. Meng, P. S. Dobal, R. S. Katiyar and G. T. Zou, “Optical Phonon Modes and Phase Transition in the Bi4Ge3-Xtixo12 Ceramic System,” Journal of Raman Spec- troscopy, Vol. 29, No .12, 1998, pp. 1003-1008. [17] J. Meng, R. S. Katiyar and G. T. Zou, “Micro-Raman Scattering of Bismuth Titanate at Low Temperature,” Journal of Raman Spectroscopy, Vol. 28, No. 6, 1997, pp. 797-801. [18] W. Li, J. Gu, J. Ma, X. M. Lu and J. S. Zhu, “Investigation on Effective Dimensionality of Domain Growth in Bi4Ti3O12 Films,” Integrated Ferroelectrics, Vol. 79, No. 1, 2006, pp. 63-70. [19] X. Jing, S. Chen and E. Yao, “Introduction of IR Spectra,” Tianjian Technology Press, Tianjin, 1992, pp. 99-136. [20] M. I. Morozov, L. P. Mezentseva and V. V. Gusarov, “Mechanism of Formation of Bi4Ti3O12,” Russian Journal of General Chemistry, Vol. 72, No. 7, 2002, pp. 1038-1040. |

