Journal of Cancer Therapy
Vol.4 No.1(2013), Article ID:28152,8 pages DOI:10.4236/jct.2013.41036
Endoscopic Mucosal Resection: Therapy for Early Colorectal Cancer
![]()
Department of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, USA.
Email: *kondalkyanam@gmail.com
Received October 27th, 2012; revised November 26th, 2012; accepted December 5th, 2012
Keywords: EMR; Endoscopic Mucosal Resection; ESD; Endoscopic Submucosal Dissection; Early Colon Cancer; Colon Cancer Therapy
ABSTRACT
We review the use of Endoscopic Mucosal Resection in the treatment of early colorectal cancer. Newer endoscopic imaging modalities have lead to earlier detection of advanced lesions thus enabling endoscopic curative therapy of lesions that would otherwise need surgery. Early outcomes data suggest promising results. But further long term prospective studies are needed.
1. Introduction
Colorectal cancer (CRC) is the third most common new cancer diagnosis and cause of cancer related death among both men and women [1]. The incidence and prevalence of CRC has been declining in part due to the effect of screening colonoscopy leading to early detection and also secondary prevention through polypectomy [2]. Recently published long term data also demonstrates a reduction in mortality from colon cancer due to colonoscopic polypectomy [3]. Improvements in preparation for colonoscopy, quality of colonoscopy, techniques of adenoma detection, newer endoscopic imaging methods have led to earlier detection of advanced neoplasia [4]. Newer and improved techniques of polyp removal have in turn facilitated effective endoscopic removal of flat and lateral spreading tumors (LST) and early carcinomas of the colorectum.
Current approaches for the treatment of LST and early colon cancer include surgical resection, laparoscopic colectomy, transanal endoscopic micro-surgery (TEMS) for rectal lesions, endoscopic mucosal resection (EMR) and endoscopic mucosal dissection (ESD).
In this review article we will discuss the use of EMR and ESD in the treatment of LST early colon cancer. These two modalities serve as staging tools for early colon cancer and can also be curative [5].
1.1. Recognition of Colon Lesions
1.1.1. Standard Techniques
Most colonic neoplasia, including subtle flat polyps, and sessile serrated polyps can be recognized with careful inspection methods including careful washing of any adherent stool or mucous, tip deflection to ensure examination behind folds and flexures, and recognition of subtle mucosal patterns for flat and serrated polyps.
1.1.2. New Techniques
Cap fitted colonoscopy uses the aid of a plastic cap fitted to the end of the colonoscope. Its use does not increase the adenoma detection rate (ADR) [6-8], but has been shown to reduce polyp miss rate [9].
Chromoendoscopy, the application of topical dye to the colon surface, improves detection of flat dysplastic lesions in IBD surveillance when compared to standard light [10]. It is now recommended as and acceptable standard practice in IBD surveillance [11]. This technique has shown only a marginal increase in ADR at the cost of increased procedure time in average risk screening/surveillance colonoscopy [12,13]. It may however prove useful in detecting the subset of flat and/or serrated adenomas [13].
Randomized trials of high definition colonoscopy and standard definition colonoscopy have either shown a modest improvement [14] in adenoma detection rate or no improvement [15,16]. An RCT done at our center combining high definition, NBI and wide angle endoscopy demonstrated a decreased miss rate [17] and a retrospective controlled study demonstrated a small but significant increase in ADR [18].
Narrow band imaging (NBI) has been used to classify lesions endoscopically using the Sano classification system [19]. NBI has not been shown prospectively to improve adenoma detection rate [20]. In a randomized controlled trial of standard definition, high definition and NBI colonoscopy, there was no difference in the proportion of subjects found to have adenomas (or adenoma detection rate) but the number of adenomas detected per subject was higher with NBI and high definition colonoscopy [16]. NBI was shown to be more accurate in predicting polyp histology [16]. Meta-analyses of NBI have shown no improvement or mixed results in adenoma detection [21-23].
1.1.3. Classification of Colon Neoplasia
In vivo classification of colonic neoplasia is important for several reasons:
1) Distinguish subtle flat or sessile polyps from inflammatory or otherwise altered mucosa;
2) Distinguish low grade adenomas for routine removal versus advanced adenoma and early carcinoma for advanced endoscopic resection such as EMR and ESD, versus deeply invasive carcinoma for surgical resection;
3) Identify low risk (small distal) hyperplastic polyps that do not require removal;
4) Correct classification of low risk adenoma followed by “resect and discard” without histological evaluation.
The Paris classification (Figure 1) is a consensus classification of superficial lesions of the gastrointestinal tract that describes the shape (sessile, penduculated, flat, etc) of neoplastic lesions [24]. This was first devised in 2002 by a multidisciplinary group of experts including, gastroenterologists, surgeons and pathologists. This is a widely accepted and well validated classification of superficial lesions of the gastrointestinal tract. It is now an accepted method of classification of colon lesions that can be used to determine the mucosal depth of a particular lesion and also whether therapy of lesion can be undertaken endoscopically. In general, lesions that are flat with central depression below the mucosal surface or ulceration, are most likely to harbour invasive neoplasia. A high degree of correlation has been found between the Paris classification and final histopathology [25].
The Kudo classification (Figure 2) primarily uses chromoendoscopy and classifies surface vascular patterns as normal, faint, network, dense, irregular, and sparse patterns (Figure 2, left to right, bottom row shows magnified image) [26]. This classification has been well validated and is able to facilitate endoscopic differentiation of neoplastic and non-neoplastic lesions, as well as to identify lesions at risk for invasive neoplasia [27]. Accurate distinction of invasive vs. non-invasive patterns is facilitated by magnification endoscopy.
The Sano classification (Figure 3) utilizes narrow band imaging to evaluate the capillary pattern (CP) and classifies the lesions into 1, 2, 3A and 3B (Figure). A further
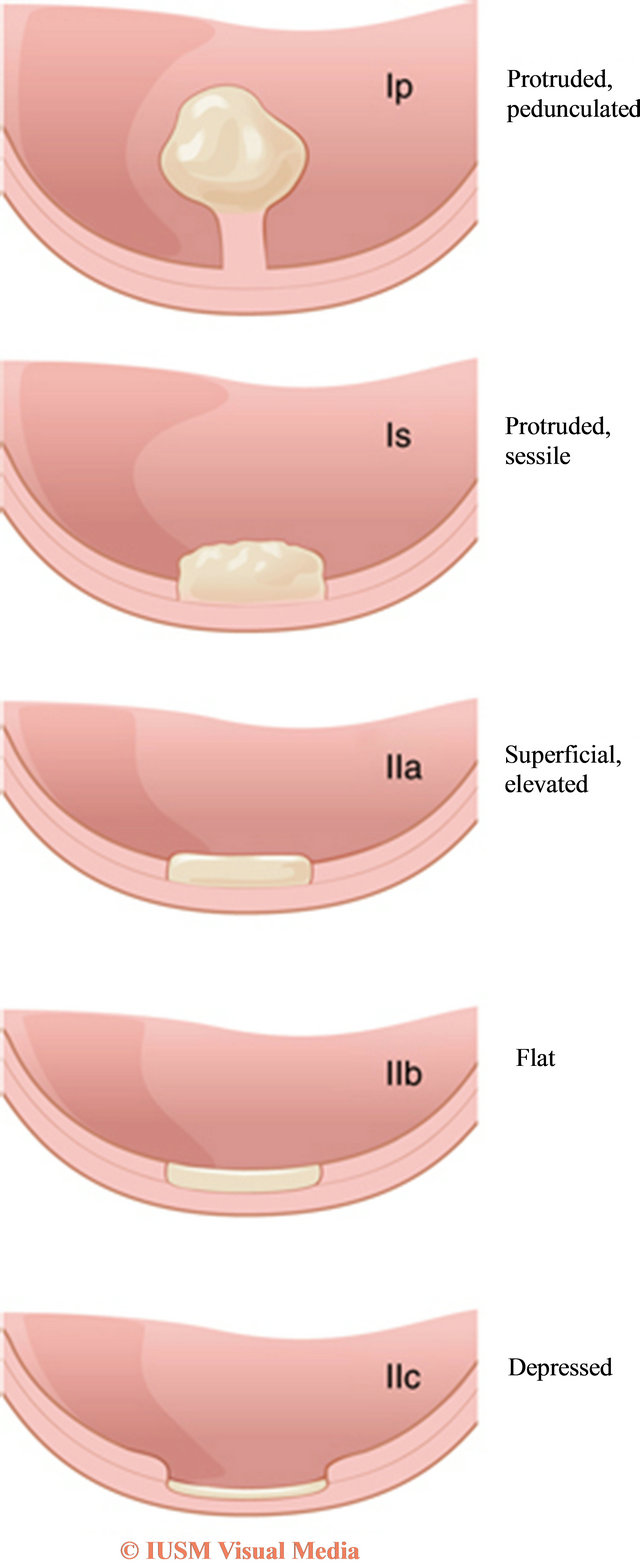
Figure 1. Paris Classification (Reproduced with permission from Nature Publishing and Kahi et al. [12]).
modification involves the identifying meshed capillary vessels (MCVs) [27]. Multiple prospective studies have validated the Sano classification. There is a high degree of correlation between the Sano classification of neoplasia and final histopathology and it is useful in differentiating hyperplastic (CP1), adenomatous (CP2), advanced adenomatous (CP3A), and invasive lesions (CP3B) requiring surgical therapy [27].
More recently, as a result of better technology and
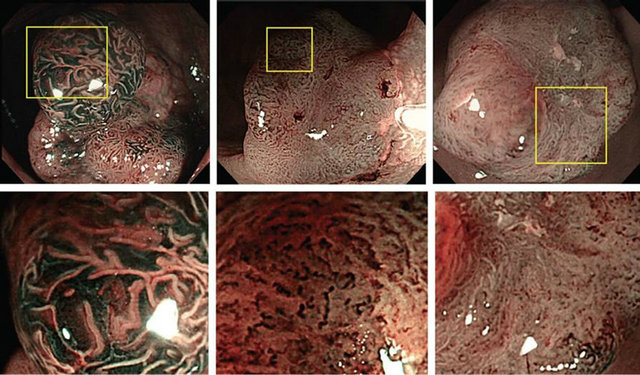
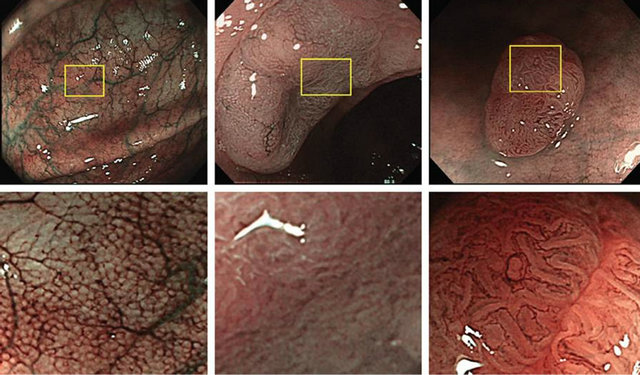
Figure 2. Kudo Classification (Reproduced with permission from Wada et al. [39]).
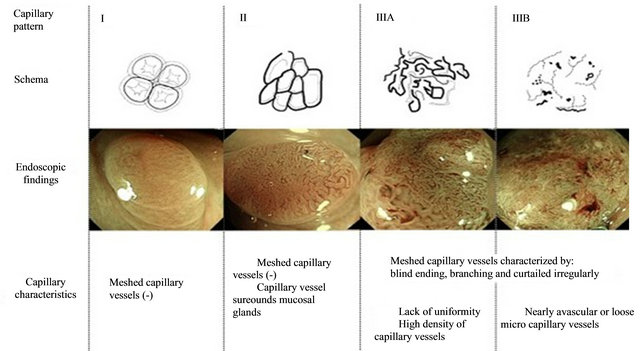
Figure 3. Sano Classification (Reproduced from Uraoka et al, permission requested [27]).
newer processors, the Sano and Kudo systems have been used in a tandem fashion and their combined use has shown an increase in specificity and sensitivity [27]. The use of magnifying NBI to evaluate pit patterns (Sano) and vascular patterns (Kudo) without chromoendoscopy has been suggested and preliminary studies have shown promising results [27].
A simplified classification schema was recently developed and validated using NBI International Colorectal Endoscopic (NICE) classification based on lesion color, brightness, and capillary/pit patterns [28].
Confocal laser endomicroscopy (CLE) provides endoluminal imaging similar in resolution and magnification to histopathology in diagnosing colon neoplasia and may be a valuable tool in “diagnose and discard” strategy [29-31]. There may be synergistic benefit in combining CLE and NBI in assessing polyps [31]. In the context of EMR for early colon cancer, CLE may be useful in discriminating lesions in vivo that are amenable to endoscopic therapy from those that require surgery.
1.1.4. Endoscopic Mucosal Resection Techniques
EMR is a modified and enhanced technique of polypectomy using injection and snare cautery. A solution is injected into the submucosa to raise the lesion and provide a cushion for electrocautery. A snare is used to strangulate the selected area so that the mucosa is separated from the submucosa and the muscularis is excluded from the snare. Electrocautery is then applied to resect the selected area. Depending on the size of the lesion en bloc resection or piecemeal resection can be performed. Various techniques can be used to maximize the effectiveness of EMR.
1.1.5. Types of EMR [5]
Inject and cut: The polyp is removed by snare cautery after saline/fluid injection.
Inject, lift and cut: A grasping forceps is used to lift the lesion before snare cautery excision the lesion.
Cap assisted EMR: A snare is positioned around the opening of a cap fitted to the endoscope. The lesion is suctioned into the cap and resected by snare cautery.
Ligation assisted EMR: A variceal banding kit modified for EMR is used to deploy a band in the standard fashion across the lesion of interest. The tension in the band excludes to muscularis mucosa. A snare is then deployed above or below the band to resect lesion.
1.1.6. EMR Steps
The ideal position for the lesion is generally at the 6 o’clock position while also ensuring a stable position with the least amount of torque in the endoscope to allow visualization, injection and resection. A flat adenoma is shown in Figure 4. The next step is injection. Choice of injection solution is largely arbitrary and is dictated by familiarity. The most commonly used solution is saline dyed with methylene blue or indigo carmine. Methylcellulose and other bulking agents have also been used as an injectate to increase the lifting duration. Epinephrine 1:10,000 - 100.000 may also be added to the solution to prevent immediate bleeding and facilitate visualization during resection. Multiple different agents, combinations of agents are being studied in an attempt to identify the optimal lifting solution (Figure 5). The lesion margin can be marked in two ways: (A) In subtle flat lesions, the circumference of lesion can be marked with superficial electrocautery such as the tip of a snare before EMR. After initiation of EMR the effect of cautery can diminish the ability to delineate abnormal mucosa (This image
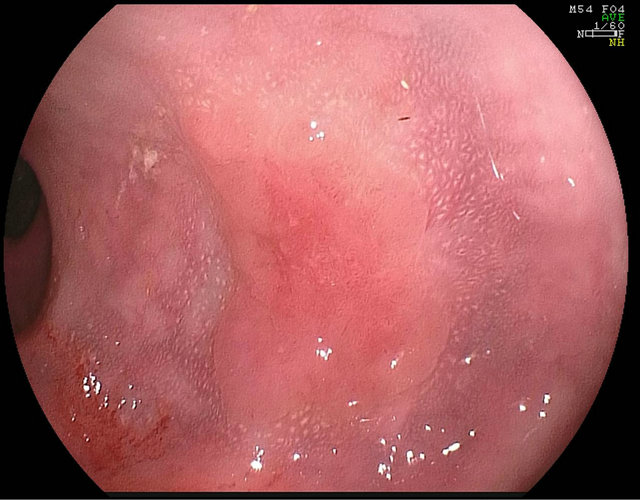
Figure 4. Example of a flat adenoma.
also shows incision around mucosa). (B) Chromoendoscopy may be performed to enhance the earance of the lesion, diagnose the vascular pattern and to determine the extent of the lesion.
The choice of snare is also arbitrary with no data to support superiority of one snare over the other. Multiple snares are available such as thin, ultrathin, braided (Boston Scientific, Natick, MA), iSnare (US Endoscopy, Mentor, OH), spiral (Olympus, Center Valley, PA) etc. Typically a stiff snare facilitates pushing the snare into the saline cushion with catheter below the level of the polyps. As the snare is closed, the lumen is partially deflated to allow the polyp to collapse into the snare lumen. A small margin of adjacent normal mucosa should be ensnared to ensure a complete resection (Figure 6). Ideal electrocautery settings are unknown with some expert centers using predominantly coagulation and others pure cutting current.
After completion of EMR, argon plasma coagulation of the margins of lesion may be performed, particularly if there is suspicion of residual neoplasia at the margin of
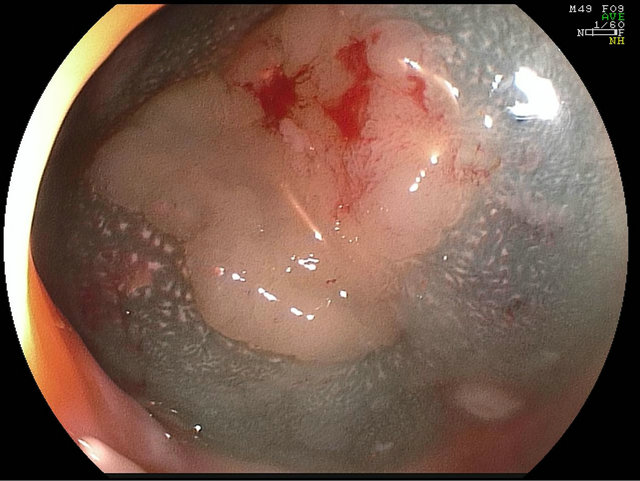
Figure 5. Saline and indigo carmine lift.
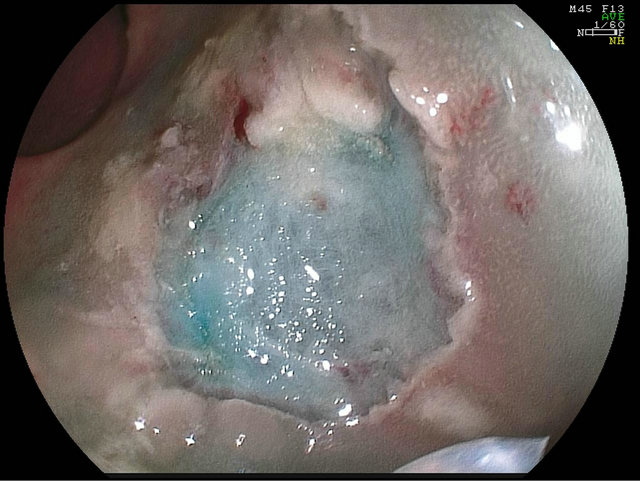
Figure 6. Post EMR appearance.
the resection (Figure 7). There is some evidence to suggest APC is effective in recurrence [32]. Prophylactic closure of the defect created by EMR defect with clips may prevent bleeding delayed bleeding and theoretically delayed perforation due to cautery effect and may be appropriate in high risk lesions such as large right colonic LST (Figure 8).
Polyp retrieval is accomplished by using various commercially available retrieval tools such as Roth Net (US endoscopy, Mentor, OH). The polyp is then pinned on a paraffin or cork block to provide accurate orientation for histo-pathologic evaluation of deep margins which is an important step especially when carcinoma is suspected (Figure 9). In the setting of piecemeal resection, the largest polypoid region of the polyp is most likely to harbour invasion and should be pinned for careful pathological assessment.
1.1.7. ESD Technique
Polyp positioning, lesion marking and injection solution
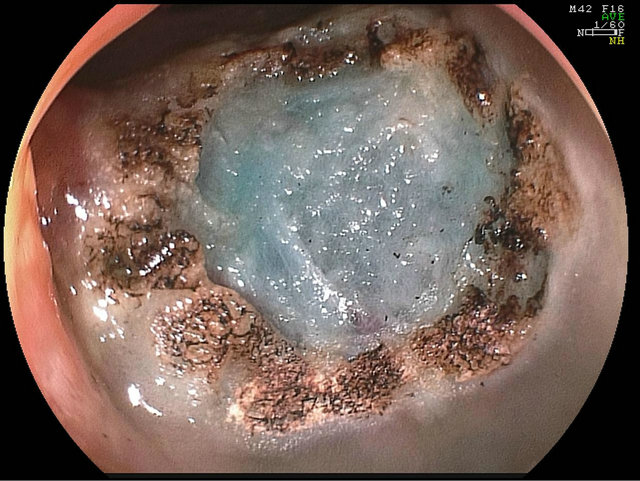
Figure 7. APC around resection margins.
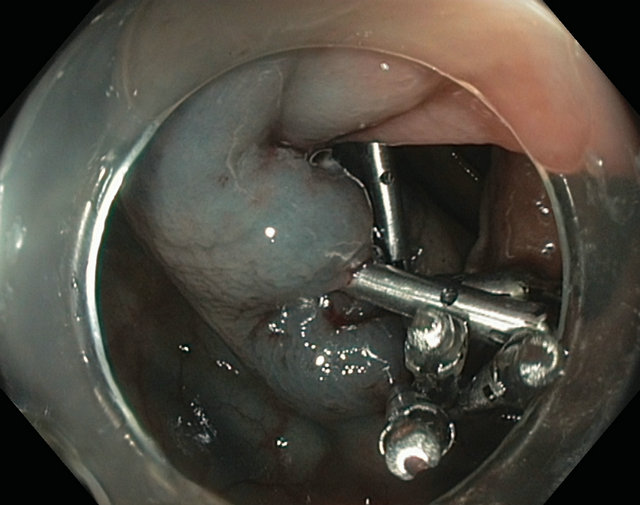
Figure 8. Clip closure of EMR defect.
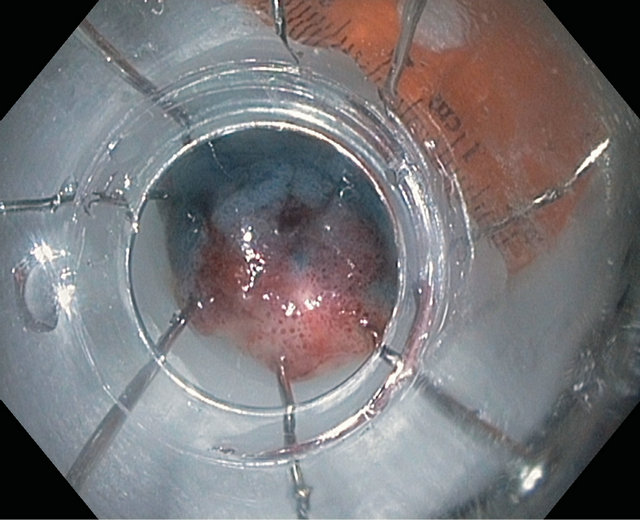
Figure 9. Pinning of EMR polyp specimen.
for ESD are similar to the steps described above in EMR section however the initial injection is usually at the perimeter of the lesion. A circumferential incision is then made around the lesion within the submucosa with electrocautery knives that have been specially adapted to allow precise control of incision depth. The lesion is then dissected along its deep margins within the submucosal space underneath the lesion using a knife to obtain an “en bloc” specimen. Retrieval and processing of the specimen is similar to steps described in EMR section.
1.2. Complications of EMR and ESD
1.2.1. Immediate
Active bleeding after EMR can be treated with coagulation and clipping. Oozing can be treated by APC. Coagulation should be performed with specialized coagulation forceps to reduce the risk of full thickness thermal injury and minimal thermal settings must be used. Peforation can usually be recognized by a “target sign” which represents two rings of tissue that have been cauterized. The first is the mucosa-submucosa interface, and the second, the submucosa-mucularis propria interface (Figure 10). Perforations can be safely and effectively closed by “through the scope” clips. A newer “over the scope” clip (OVESCO, Tubingen, Germany) can be used to close larger perforations. The patient should be admitted, placed on antibiotics and bowel rest and a surgery consult should be obtained. Abdominal pain (without perforation) and abdominal distension is usually due to retention of air or ileus. A thorough and serial clinical evaluation must be performed. Severe pain, worsening symptoms and signs should prompt a judicious use of imaging to evaluate for perforation. Use of carbon dioxide can decrease post procedure abdominal pain due to distension.
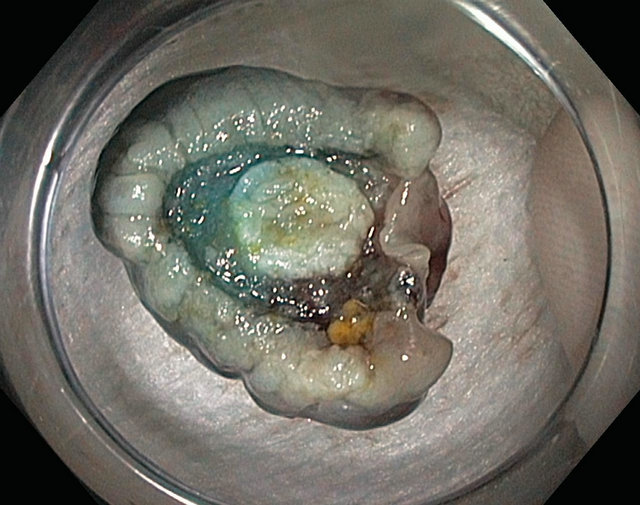
Figure 10. Target sign.
1.2.2. Delayed Complications
Bleeding from EMR site can occur between days to weeks after procedure. Depending on the clinical scenario, observation, transfusion, resuscitation with fluids may be appropriate. Colonoscopy may be indicated if the lesion continues to bleed despite initial conservative therapy. Rarely emergent angiography may be indicated in cases of massive bleeding and hemodynamic instability. Delayed perforation usually requires surgical intervenetion. Endoscopic closure of perforation may not be feasible as the patient cannot be prepped and endoscopy is generally precluded. Post-polypectomy syndrome is characterized by pain, fever, elevated white count with localized abdominal signs without perforation. It is related to deep cautery effect causing localized inflammation and peritonitis. The syndrome responds well to conservative management with bowel rest, IV antibiotics and hydration. Polyp recurrence: In a retrospective study done at our center, a polyp recurrence rate of 12% was found [33]. The only significant predictor of recurrence was piecemeal removal during index EMR. For this reason, close surveillance intervals are usually recommended such as repeat colonoscopy in 2 - 6 months to ensure complete eradication.
2. Discussion
Outcomes: A significant amount of research has been done to examine the factors that impact clinical outcome but most studies are retrospective [34]. A large prospective study by Moss et al. showed that a prior attempt at EMR, ileocecal valve involvement increased the risk of ineffective EMR and size greater than 4 cms and use of salvage APC was associated with polyp recurrence [35]. Our retrospective analysis showed that piecemeal removal of polyps was the only risk factor for polyp recurrence [33]. Both studies showed an overall failure rate of about 10% - 11%. In a study that specifically evaluated early colon cancer treated by EMR, Kim et al. found that 64/129 subjects with intramucosal cancer were completely and successfully treated with EMR and of the 64/129 subjects with submucosal cancer, 7 had progresssion to colon cancer [36]. Mannath et al. showed that piecemeal recurrence was the main risk factor for polyp recurrence and also that APC use did not impact recurrence [37].
Surveillance: In all studies close follow up evaluation is recommended however there are no reliable prospective data regarding the appropriate colonoscopy intervals. The most common practice, also followed at our center, is surveillance colonoscopy at 3, 12 and 36 months [33, 35].
Pathology: Acceptable complete resection of early colon cancer is generally defined as negative deep and lateral margins with no lymphovascular invasion, a differentiated tumor, and <1000 microns of submucosal invasion [38]. Studies have shown that the large majority of T1a lesions are cured by EMR/ESD [35,36].
3. Conclusion
Endoscopic detection and removal of advanced polyps and early invasive cancers is now widely recognized as an effective alternative to surgery. The methods require advanced endoscopic skills and training and are associated with increased risk compared with small polyps, but likely lower risk compared to surgical resection. The vast majority of non-invasive polyps, even giant polyps greater than 4-6 cm can typically be removed safely and effectively in expert hands. The take home points for EMR of early colon cancer include appropriate documentation and photography of large polyp, limited cold forceps biopsy, avoiding cautery methods (hot snare, hot biopsy) for sampling, tattooing at a location slightly away from polyp but not underneath it, discussion of therapeutic options based on pathology, and referral to advanced endoscopist who practices EMR [34].
REFERENCES
- A. C. Society, Cancer Facts and Figures 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
- S. J. Winawer, et al., “Prevention of Colorectal Cancer by Colonoscopic Polypectomy. The National Polyp Study Workgroup,” The New England Journal of Medicine, Vol. 329, No. 27, 1993, pp. 1977-1981. doi:10.1056/NEJM199312303292701
- A. G. Zauber, et al., “Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths,” The New England Journal of Medicine, Vol. 366, No. 8, 2012, pp. 687-696. doi:10.1056/NEJMoa1100370
- V. M. Ussui, and M. B. Wallace, “Advances in Colonoscopy,” Discovery Medicine, Vol. 13, No. 71, 2012, pp. 313-321.
- S. V. Kantsevoy, et al., “Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection,” Gastrointestinal Endoscopy, Vol. 68, No. 1, 2008, pp. 11-18. doi:10.1016/j.gie.2008.01.037
- S. C. Ng, et al., “The Efficacy of Cap-Assisted Colonoscopy in Polyp Detection and Cecal Intubation: A MetaAnalysis of Randomized Controlled Trials,” The American Journal of Gastroenterology, Vol. 107, No. 8, 2012, pp. 1165-1173. doi:10.1038/ajg.2012.135
- Y. T. Lee, et al., “Efficacy of Cap-Assisted Colonoscopy in Comparison with Regular Colonoscopy: A Randomized Controlled Trial,” The American Journal of Gastroenterology, Vol. 104, No. 1, 2009, pp. 41-46. doi:10.1038/ajg.2008.56
- H. P. Tee, et al., “Prospective Randomized Controlled Trial Evaluating Cap-Assisted Colonoscopy vs. Standard Colonoscopy,” World Journal of Gastroenterology, Vol. 16, No. 31, 2010, pp. 3905-3910. doi:10.3748/wjg.v16.i31.3905
- D. G. Hewett and D. K. Rex, “Cap-Fitted Colonoscopy: A Randomized, Tandem Colonoscopy Study of Adenoma Miss Rates,” Gastrointestinal Endoscopy, Vol. 72, No. 4, 2010, pp. 775-781. doi:10.1016/j.gie.2010.04.030
- L. Wu, et al., “The Diagnostic Accuracy of Chromoendoscopy for Dysplasia in Ulcerative Colitis: Meta-Analysis of Six Randomized Controlled Trials,” Colorectal Disease, Vol. 14, No. 4, 2012, pp. 416-420. doi:10.1111/j.1463-1318.2010.02505.x
- F. A. Farraye, et al., “AGA Medical Position Statement on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease,” Gastroenterology, Vol. 138, No. 2, 2010, pp. 738-745. doi:10.1053/j.gastro.2009.12.037
- C. J. Kahi, et al., “High-Definition Chromocolonoscopy vs. High-Definition White Light Colonoscopy for Average-Risk Colorectal Cancer Screening,” The American Journal of Gastroenterology, Vol. 105, No. 6, 2010, pp. 1301-1307. doi:10.1038/ajg.2010.51
- J. Pohl, et al., “Pancolonic Chromoendoscopy with Indigo Carmine versus Standard Colonoscopy for Detection of Neoplastic Lesions: A Randomised Two-Centre Trial,” Gut, Vol. 60, No. 4, 2011, pp. 485-490. doi:10.1136/gut.2010.229534
- V. Subramanian, et al., “High Definition Colonoscopy vs. Standard Video Endoscopy for the Detection of Colonic Polyps: A Meta-Analysis,” Endoscopy, Vol. 43, No. 6, 2011, pp. 499-505. doi:10.1055/s-0030-1256207
- G. Tribonias, et al., “Comparison of Standard vs. HighDefinition, Wide-Angle Colonoscopy for Polyp Detection: A Randomized Controlled Trial,” Colorectal Disease, Vol. 12, No. 10, 2010, pp. e260-e266. doi:10.1111/j.1463-1318.2009.02145.x
- A. Rastogi, et al., “Randomized, Controlled Trial of Standard-Definition White-Light, High-Definition WhiteLight, and Narrow-Band Imaging Colonoscopy for the Detection of Colon Polyps and Prediction of Polyp Histology,” Gastrointestinal Endoscopy, Vol. 74, No. 3, 2011, pp. 593-602. doi:10.1016/j.gie.2011.04.050
- S. A. Gross, et al., “A Comparison of High DefinitionImage Enhanced Colonoscopy and Standard White-Light Colonoscopy for Colorectal Polyp Detection,” Endoscopy, Vol. 43, No. 12, 2011, pp. 1045-1051. doi:10.1055/s-0030-1256894
- A. M. Buchner, et al., “High-Definition Colonoscopy Detects Colorectal Polyps at a Higher Rate than Standard White-Light Colonoscopy,” Clinical Gastroenterology and Hepatology, Vol. 8, No. 4, 2010, pp. 364-370. doi:10.1016/j.cgh.2009.11.009
- Y. K. Sano and M. Y. Hamamoto, “New Diagnostic Method Based on Color Imaging Using Narrow Band Imaging (NBI) System for Gastrointestinal Tract,” Gastrointestinal Endoscopy, Vol. 53, No. 5, 2001, p. AB125. doi:10.1016/S0016-5107(01)80239-X
- A. Adler, et al., “A Prospective Randomised Study on Narrow-Band Imaging versus Conventional Colonoscopy for Adenoma Detection: Does Narrow-Band Imaging Induce a Learning Effect?” Gut, Vol. 57, No. 1, 2008, pp. 59-64. doi:10.1136/gut.2007.123539
- X. F. Jin, et al., “Meta-Analysis for Evaluating the Accuracy of Endoscopy with Narrow Band Imaging in detecting colorectal adenomas,” Journal of Gastroenterology and Hepatology, Vol. 27, No. 5, 2012, pp. 882-887. doi:10.1111/j.1440-1746.2011.06987.x
- A. Nagorni, G. Bjelakovic and B. Petrovic, “Narrow Band Imaging versus Conventional White Light Colonoscopy for the Detection of Colorectal Polyps,” Cochrane Database of Systematic Reviews, Vol. 1, 2012, p. CD008361.
- S. F. Pasha, et al., “Comparison of the Yield and Miss Rate of Narrow Band Imaging and White Light Endoscopy in Patients Undergoing Screening or Surveillance Colonoscopy: A Meta-Analysis,” The American Journal of Gastroenterology, Vol. 107, No. 3, 2012, pp. 363-370. doi:10.1038/ajg.2011.436
- M. Classen, M. D. Guido, N. J. Tytgat and C. J. Lightdale, “The Paris Endoscopic Classification of Superficial Neoplastic Lesions: Esophagus, Stomach, and Colon: November 30 to December 1, 2002,” Gastrointestinal Endoscopy, Vol. 58, No. 6, 2003, pp. S3-43.
- Endoscopic Classification Review Group, “Update on the Paris Classification of Superficial Neoplastic Lesions in the Digestive Tract,” Endoscopy, Vol. 37, No. 6, 2005, pp. 570-578. doi:10.1055/s-2005-861352
- S. Kudo, et al., “Colorectal Tumours and Pit Pattern,” Journal of Clinical Pathology, Vol. 47, No. 10, 1994, pp. 880-885. doi:10.1136/jcp.47.10.880
- T. Uraoka, et al., “Sano’s Capillary Pattern Classification for Narrow-Band Imaging of Early Colorectal Lesions,” Journal of Digestive Endoscopy, Vol. 23, Suppl. 1, 2011, pp. 112-115. doi:10.1111/j.1443-1661.2011.01118.x
- D. G. Hewett, et al., “Validation of a Simple Classification System for Endoscopic Diagnosis of Small Colorectal Polyps Using Narrow-Band Imaging,” Gastroenterology, Vol. 143, No. 3, 2012, pp. 599-607.
- V. M. Ussui and M. B. Wallace, “Imaged-Enhanced Technologies for Colorectal Polyp Detection and Classification,” The American Journal of Gastroenterology, Vol. 107, No. 4, 2012, pp. 551-553. doi:10.1038/ajg.2012.15
- A. M. Buchner, et al., “Comparison of Probe-Based Confocal Laser Endomicroscopy with Virtual Chromoendoscopy for Classification of Colon Polyps,” Gastroenterology, Vol. 138, No. 3, 2010, pp. 834-842. doi:10.1053/j.gastro.2009.10.053
- M. W. Shahid, et al., “Diagnostic Accuracy of ProbeBased Confocal Laser Endomicroscopy and Narrow Band Imaging for Small Colorectal Polyps: A Feasibility Study,” The American Journal of Gastroenterology, Vol. 107, No. 2, 2012, pp. 231-239. doi:10.1038/ajg.2011.376
- J. C. Brooker, et al., “Treatment with Argon Plasma Coagulation Reduces Recurrence after Piecemeal Resection of Large Sessile Colonic Polyps: A Randomized Trial and Recommendations,” Gastrointestinal Endoscopy, Vol. 55, No. 3, 2002, pp. 371-375. doi:10.1067/mge.2002.121597
- T. A. Woodward, et al., “Predictors of Complete Endoscopic Mucosal Resection of Flat and Depressed Gastrointestinal Neoplasia of the Colon,” The American Journal of Gastroenterology, Vol. 107, No. 5, 2012, pp. 650-654. doi:10.1038/ajg.2011.473
- V. Chandrasekhara, and G. G. Ginsberg, “Endoscopic Management of Large Sessile Colonic Polyps: Getting the Low down from down under,” Gastroenterology, Vol. 140, No. 7, 2011, pp. 1867-1871. doi:10.1053/j.gastro.2011.04.024
- A. Moss, et al., “Endoscopic Mucosal Resection Outcomes and Prediction of Submucosal Cancer from Advanced Colonic Mucosal Neoplasia,” Gastroenterology, Vol. 140, No. 7, 2011, pp. 1909-1918. doi:10.1053/j.gastro.2011.02.062
- M. N. Kim, et al., “Clinical Features and Prognosis of early Colorectal Cancer Treated by Endoscopic Mucosal Resection,” Journal of Gastroenterology and Hepatology, Vol. 26, No. 11, 2011, pp. 1619-1625. doi:10.1111/j.1440-1746.2011.06749.x
- J. Mannath, et al., “Polyp Recurrence after Endoscopic Mucosal Resection of Sessile and Flat Colonic Adenomas,” Digestive Diseases and Sciences, Vol. 56, No. 8, 2011, pp. 2389-2395. doi:10.1007/s10620-011-1609-y
- S. B. Edge and C. C. Compton, “The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM,” Annals of Surgical Oncology, Vol. 17, No. 6, 2010, pp. 1471-1474.
- Y. Wada, et al., “Vascular Pattern Classification of Colorectal Lesions with Narrow Band Imaging Magnifying Endoscopy,” Journal of Digestive Endoscopy, Vol. 23, Suppl. 1, 2011, pp. 106-111. doi:10.1111/j.1443-1661.2011.01109.x
NOTES
*Corresponding author.

