American Journal of Plant Sciences
Vol.5 No.3(2014), Article ID:42762,6 pages DOI:10.4236/ajps.2014.53053
Influence of Bavistin, Cefotoxime, Kanamycin and Silver Thiosulphate on Plant Regeneration of Solanum viarum (Dunal)—An Important Anticancer Medicinal Plant
Department of Biotechnology, Dravidian University, Kuppam, India.
Email: *challagundlav@yahoo.co.in
Copyright © 2014 M. D. Mahadev et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property M. D. Mahadev et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received November 29th, 2013; revised January 18th, 2014; accepted January 29th, 2014
KEYWORDS
Solanum viarum; Bavistin; Cefotoxime; Kanamycin; Silver Thiosulphate; Plant Growth Regulators
ABSTRACT
The effects of bavistin, cefotoxime and kanamycin along with the ethylene inhibitor silver thiosulphate on in vitro regeneration using the axillary buds of Solanum viarum (D.) have been investigated. Axillary bud explants when cultured on MS medium supplemented with bavistin (100 mg/l) maximum number of shoots (4.21 ± 0.83) were observed. In a separate experiment, bavistin along with growth regulators such as BAP and TDZ was added to MS medium containing sucrose (3%). Maximum number of shoots was obtained (5.55 ± 0.28) in MS medium supplemented with bavistin (100 mg/l) and BAP (2.0 mg/l). Cefotoxime and kanamycin (10 - 150 μM/L) were added to MS medium supplemented with BAP (2.0 mg/l) with sucrose (3%) having shown the maximum shoot regeneration (4.38 ± 0.45 and 3.4 ± 0.48) at 80 μM/L and 50 μM/L respectively. Similarly the regeneration medium was supplemented with (5.0 - 100 μM/L) of STS. Maximum number of shoots (4.75 ± 0.43) was seen with 40 μM/L. These plantlets were further maintained for root emergence on a rooting medium supplemented with growth regulators such as (0.5 - 2.0 mg/l) IAA, IBA and NAA. In MS medium supplemented with IBA (1.0 mg/l), maximum number of roots (24.3 ± 0.31) was seen with 100% regeneration. The rooted plants were acclimatized and transferred to field plots with (95%) of plant surviving in the field.
1. Introduction
Solanum viarum (Dunal) commonly referred as Tropical Soda apple is one of the multipurpose medicinal plant. It is the spiny, perennial plant of the angiospermic family Solanaceae. The Tropical soda apple plant is native to Brazil, Paraguay, Uruguay and Argentina. Thereafter it was introduced in the South-eastern United States, Africa, India, Nepal and other parts of the tropical Asia [1]. Because of its alkaloid contents, the solution of the boiledleaf of this weed is used as a drug in Senegal by young teenagers. In another part in West Africa, Chad, a solution of S. viarum roots is locally used as a tooth medicine. In India, it is also regarded as a medicinal crop [2]. Solasodine present in the fruits is used as a precursor to produce complex steroidal compounds and contraceptive pills [3]. Steroids produced by the plant have been used for the treatment of cancer, rheumatic arthritis, Addison’s disease, chronic asthma, leukemia, obesity and other skin diseases [4]. Antimicrobial agents and fungicides are generally used in plant tissue culture media to eliminate microorganisms that are present in the explants or arise as laboratory contaminants. Several such agents are reported to effect in vitro cell culture and plant regeneration. The influences of bavistin and silver thiosulphate have been well documented in many medicinal plants such as Mentha piperita [5], Stevia rebaudiana [6], Solanum nigrum [7] and the effect of bavistin with adenine thiosulphate (ATS) has been studied in Picrorhiza scrophulariiflora [8]. Since studies on in vitro plant regeneration of this species using different antimicrobial agents and ethylene inhibitor STS are almost limited, hence in the present study, we report a protocol on the effect of different concentrations of bavistin; cefotoxime and kanamycin along with STS were examined.
2. Materials and Methods
2.1. Plant Material
Axillary buds were excised from the in vitro seed germinated of four week old S. viarum. The seeds were inoculated on MS medium [9] containing 0.8% (w/v) agar supplemented with various concentrations of cytokinins (BAP and Kinetin) and auxins (IAA, NAA and IBA).
2.2. Culture Medium and Culture Conditions
Axillary bud explants (1.0 - 2.0 cm) from in vitro seed germinated of four week old S. viarum were inoculated on MS medium containing different concentrations (10 - 120 mg/l) of bavistin singly and in combination of BAP and TDZ to induce multiple shoots. In the same way, axillary bud explants were also inoculated on MS medium containing different concentrations of cefotoxime and kanamycin (10 - 150 µM/L) and silver thosulphate (5.0 - 100 µM/L). Both proliferation and rooting media containing (3%) sucrose gelled with (0.8%) agar (Himedia, India). The PH of the medium was adjusted to 5.8 using 0.1 N NaOH and 0.1 N HCl and gelled with addition of agar and autoclaved at 121˚C, 15 lbs. pressure for 15 minutes. The culture room conditions maintained for in vitro cultures were 26˚C ± 2˚C and 60% to 70% relative humidity. Light intensity was 3000 lux with a photoperiod of 18 hours day light and 6 hours in dark.
2.3. Sub Culturing
Sub culturing was carried out at regular intervals of thirty days visual observations of the cultures were taken for every transfer and the effects of different treatments were quantified on the basis of percentage of cultures showing response on shoot proliferation, shoot development, number of shoots per explant, length of the regenerated shoots, number of roots per shoot and root length.
2.4. Data Analysis
The variables such as shoot regeneration frequency, number of shoots per explant, shoot length, number of roots per shoot and root length were recorded.
2.5. Statistical Analysis
All experiments were conducted with a minimum of 20 explants. All assays were repeated at least three times. The experimental data were statistically analyzed by one-way ANOVA using the DMRT (Duncan’s Multiple Range Test) (P < 0.05) and were presented as the average ± standard error (SE).
3. Results and Discussion
3.1. Effect of Bavistin on the in vitro Plant Regeneration from Axillary Bud Explants
In the present investigation, axillary bud explants responded well with bavistin. Maximum frequency of regeneration (85%), maximum mean shoot number (4.21 ± 0.83) and shoot length (4.9 ± 0.80 cm) was obtained in bavistin (100 mg/L). Bavistin in combination with BAP (2.0 mg/L) showed increased regeneration frequency (90%), maximum mean shoot number (5.55 ± 0.28) with decreased shoot length (4.5 ± 0.38 cm) when compared with bavistin alone supplemented MS medium. Table 1 (Figure 1(A)). Bavistin with TDZ also showed less regeneration frequency (83%), mean shoot number (4.13 ± 0.44) and shoot length (3.9 ± 0.29 cm) compared with the cultures having only bavistin. Bavistin is a systemic fungicide that belongs to benzimidazole family. Benzimidazole is group of organic fungicides with systemic action that are extensively used in agriculture [10]. It has been reported that the molecular structure of methyl benzimidazole carbonate has some semblance to cytokinins based on adenine [11]. One of the most effective and extensively used benzimidazole has a cytokinin activity in soy and radish [12,13]. Bavistin was found to be the least toxic to plant cells and has a broad-spectrum fungicidal activity [14]. It has also been demonstrated that these compounds can have beneficial effects on the physiology of the plant [15]. In present study it has been known that the usage of bavistin to control the fungal contamination does not show any negative effect on S. viarum cultures and further promotes the shoot regeneration.
3.2. Effect of Cefotoxime on the in vitro Plant Regeneration from Axillary Bud Explants
The obtained results reveal the influence of cefotoxime on the regeneration of axillary bud cultures of S. viarum. Maximum number of shoot formation (4.38 ± 0.45) with a maximum shoot length (4.2 ± 1.35 cm) and high frequency of regeneration (88%) at a concentration of (80 µM/L). At highest concentration, cefotoxime (150 µM/L) showed decreased frequency of regeneration (50%), shoot number (1.5 ± 0.64) and shoot length (2.5 ± 0.37 cm). A concentration of (80 µM/L) of cefotoxime was chosen as the optimum concentration for S. viarum cultures Table 2 (Figure 1(B)). Similar results reported as
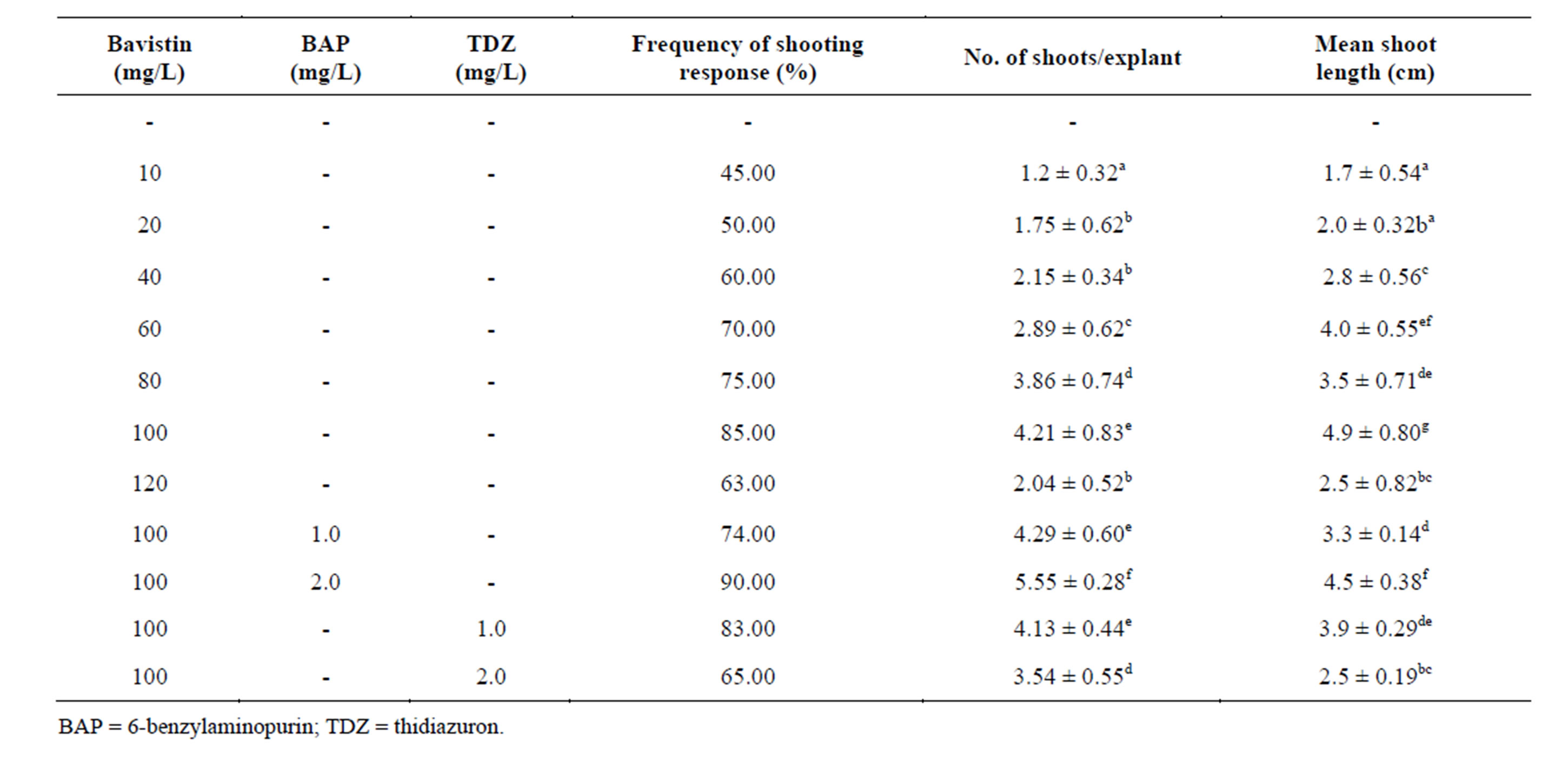
Table 1. Effect of Bavistin alone and in combination with cytokinins on shoot organogenesis from axillary bud explants of in vitro grown plants of S. viarum. Data represent treatment means ± SE fallowed by different letter(s) within column indicate significant differences according to ANOVA and DMRT test (P < 0.05).
the cefotoxime alone stimulated the shoot organogenesis in the axillary bud explants of Mentha piperita whereas in case of apricot cefotoxime alone did not induce the shoot organogenesis but in combination with the other antibiotics such as timentin or vancomycin, stimulated the shoot organogenesis [16]. The activity of cefotoxime in culture is that the molecule mimics a plant growth regulator. The chance that cell metabolism converts cefotoxime to a compound with growth regulator activity has been reported in embryogenesis and regeneration in Triticum aestivum [17]. The addition of cefotoxime in the culture medium also boosted up the photosynthetic machinery of sugarcane plants [18]. Several reports concluded that cefotoxime might be killing endophytic bacteria that finally result in disease-free and vigorously growing plant cultures As a matter of fact; cefotoxime is a β-lactam antibiotic that inhibits cell wall synthesis in dividing bacterial cells and results in cell lysis [19].
3.3. Effect of Kanamycin on the in vitro Plant Regeneration from Axillary Bud Explants
The influence of kanamycin on regeneration of S. viarum was evaluated at different concentrations. Shoot initiation was observed after one week of inoculation. MS medium supplemented with 50 µM/L kanamycin induced maximum number of shoots (3.4 ± 0.48) with maximum shoot length (5.2 ± 0.12 cm) and highest mean shoot regeneration frequency (95%). The regeneration frequency gradually decreased with the increase in concentration up to (150 µM/L). Higher concentrations above (150 µM/L) tested completely inhibited regeneration Table 3 (Figure 1(C)). Kanamycin above (50 μM/L) concentration, the regeneration frequencies gradually decreased with increase in concentrations up to (150 μM/L). Higher concentrations above (150 μM/L) tested completely inhibited regeneration. Similar results were also found with quite tolerant species such as pear [20], olive [21]. The use of kanamycin along with the nutrient media is also useful to inhibit the growth of a wide range of unwanted bacteria in tissue culture vessels. The possible mechanism of stimulatory effect of antibiotics on regeneration may involve generation of stress that makes cells competent for regeneration. Alternatively, the antibiotics may mimic plant growth regulators [22]. Earlier reports demonstrated that certain antibiotics like chloramphenicol exhibited stimulatory effect on shoot organogenesis in callus cultures of Hyoscyamus muticus [23].
3.4. Effect of Ethylene Inhibitor Silver Thiosulphate on Axillary Bud Explants
In in vitro conditions the axillary bud explants bud break was observed one week after inoculation on MS medium supplemented with different concentrations of silver thosulphate. At any concentration tested with silver thiosulphate had a positive effect and showed shoot formation. Highest regeneration frequency (90%) was observed at (40 µM/L) STS and maximum number of shoots (4.75 ± 0.43) and highest shoot length (5.5 ± 0.35 cm). Lower
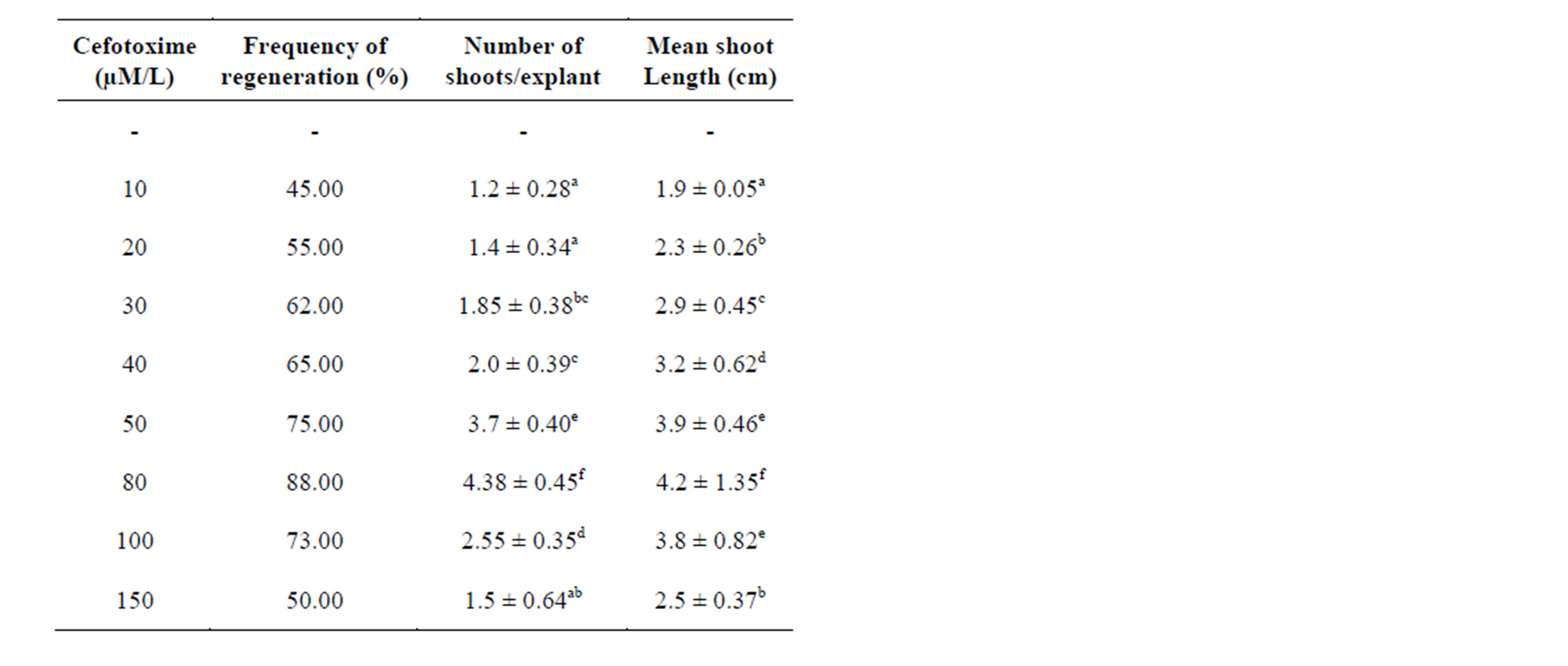
Table 2. Influence of cefotoxime added to MS medium supplemented with BAP (2.0 mg/l) on regeneration of plantlets from axillary bud explants of Solanum viarum. Data represent treatment means ± SE fallowed by different letter(s) within column indicate significant differences according to ANOVA and DMRT test (P < 0.05).
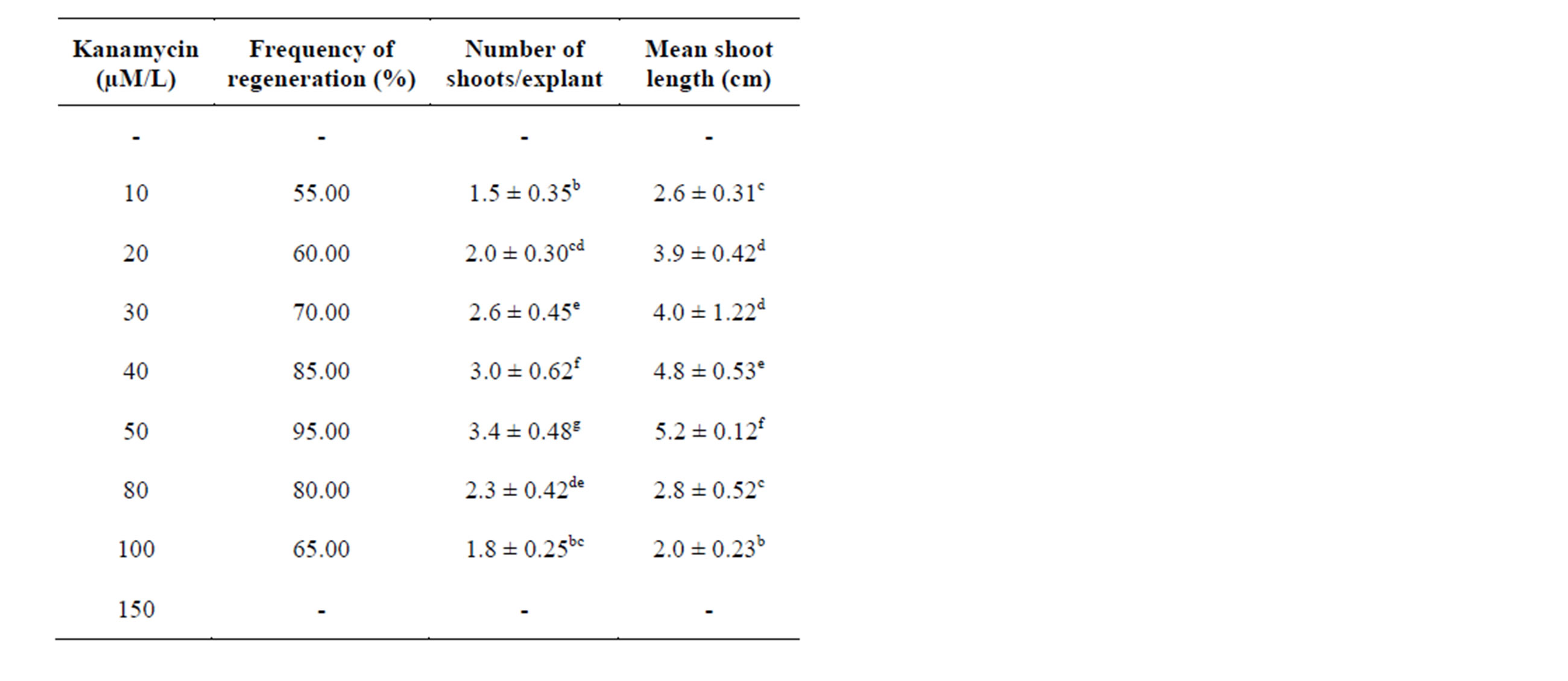
Table 3. Influence of Kanamycin added to MS medium supplemented with BAP (2.0 mg/l) on regeneration of plantlets from axillary bud explants of Solanum viarum. Data represent treatment means ± SE fallowed by different letter(s) within column indicate significant differences according to ANOVA and DMRT test (P < 0.05).
concentrations of STS favoured the high frequency of generation and highest number of shoots. The most favorable range of STS concentrations recorded was 10 - 40 µM/L Table 4 (Figure 1(D)). The addition of STS to the regeneration media increased organogenesis in higher plants [24] were Ag+ ions inhibit ethylene action in a wide variety of ethylene induced responses in plants by reducing the receptor capacity to bind ethylene. Thus, silver thiosulphate might be useful as a media supplement to develop efficient protocols for in vitro propagation of S. viarum as it favors the shoot formation. The beneficial effects of the ethylene inhibitor STS on organogenesis have been widely reported regeneration in organogenesis of Asclepias curassavica [25]. The silver ions inhibit ethylene action in a wide variety of ethylene induced responses in plants. The ethylene inhibiting effect of silver is believed to be due to an interference with ethylene binding [26]. The positive effective of silver in shoot organogenesis suggests that ethylene produced by cultured explants inhibit shoot organogenesis of those explants.
3.5. In vitro Rooting
After regeneration and sufficient elongation of the micro shoots length (3.0 - 5.0 cm) were carefully excised and transferred on MS medium supplemented with different concentrations of auxins such as IBA, IAA and NAA (0.5 - 2.0 mg/l) separately. IBA (1.0 mg/l) produced the maximum number of roots (24.3 ± 0.31) with root length (5.7 ± 0.62 cm) and highest regeneration frequency (100%) whereas the least number of roots (7.4 ± 0.21) with root length (1.8 ± 0.23) were seen in IAA (2.0 mg/l). NAA (1.0 mg/l) gave the second highest number of roots (18.3 ± 0.31) with root length (4.7 ± 0.21 cm) was observed Table 5 (Figures 1(E) and (F)).
3.6. Acclimatization and Hardening
Well rooted plantlets were separated from the culture
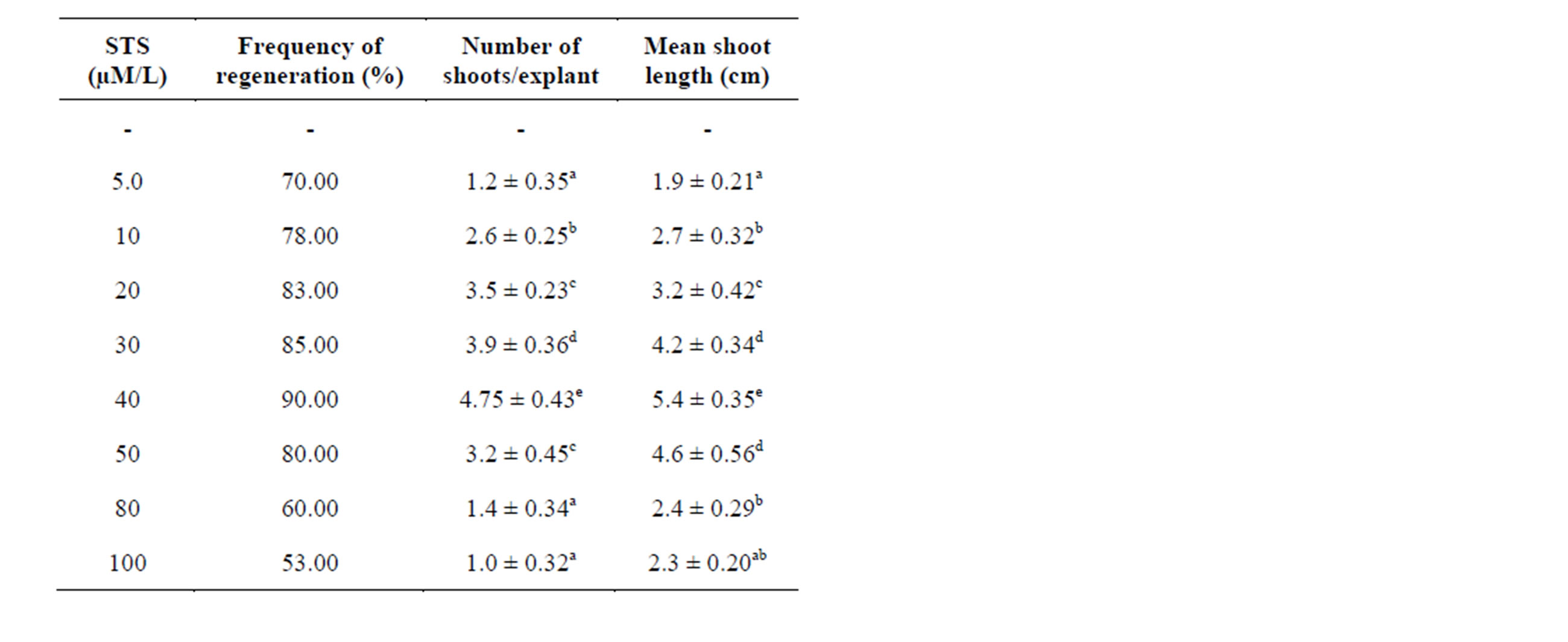
Table 4. Effect of different concentrations of silver thiosulphate (STS) supplemented in MS medium containing BAP (2.0 mg/l) on regeneration of plantlets from axillary bud explants of Solanum viarum. Data represent treatment means ± SE fallowed by different letter(s) within column indicate significant differences according to ANOVA and DMRT test (P < 0.05).

Figure 1. Axillary bud explants of Solanum viarum cultured on MS medium supplemented with various concentrations of bavistin, cefotoxime, kanamycine and silver thiosulphate. A. Multiple shoots regenerated from axillary bud explant with MS + bavistin (100 mg/L) + BAP (2.0 mg /L); B. Shoot regeneration from axillary bud explant on MS + cefotaxime (80 μM/L); C. Maximum shoots formed on MS + Kanamycin (50 μM/L); D. MS + sliver thiosulphate (40 μM/L); E. Rooting from regenerated shoots on MS medium (IBA 1.0 mg/L); F. Well rooted plants ready for hardening; G & H. Hardened plants in polycups and pots containing sterile soil and vermiculate (1:1 ratio).
tubes, washed and transferred to polycups containing soil and vermiculate in (1:1) ratio for hardening. Lastly the hardened plantlets were transferred to field conditions. (Figures 1(G) and (H)). Rooted shoots showed the maximum percentage of survival.
4. Conclusion
In the present investigation, it is noticeable that the antimicrobial agents eliminate the contaminating microorganisms and do not show any negative effect on S. viarum cultures and further it promotes shoot regeneration. Hence the shoot promoting activities of these antimicrobial agents are very useful in micropropagation and conversion of this very important medicinal plant.

Table 5. Root organogenesis of in vitro derived shoot lets of Solanum viarum supplemented with various concentrations of IAA, NAA and IBA using MS medium. Data represent treatment means ± SE fallowed by different letter(s) within column indicate significant differences according to ANOVA and DMRT test (P < 0.05).
Acknowledgements
The authors are grateful to the UGC BSR (Non-SAP) New Delhi, India for providing financial assistance in the form of fellowship.
REFERENCES
- M. Nee, “Synopsis of Solanum Section Acanthophora: A Group of Interest for Glycoalkaloids,” In: J. G. Hawkes, R. N. Lester, M. Nee and N. Estrada, Eds., Solanaceae III: Taxonomy, Chemistry, Evolution, Kew and Linnean Society of London, London, 1991, pp. 257-266.
- N. S. Talekar, T. B. H. Hau and W. C. Chang, “Solanum viarum, a Trap Crop for Helicoverpa armigera,” Insect Environment, Vol. 5, No. 3, 1999, p. 142.
- C. R. Babu and F. N. Hepper, “Taxonomy and Nomenclature of Solanum khasianum and Some of Its Relatives,” Kew Bulletin, Vol. 34 No. 2, 1979, pp. 407-411. http://dx.doi.org/10.2307/4110004
- A. R. Pingle and V. R. Dhyansagar, “Solanum viarum as a Source of Solasodin,” Indian Drugs, Vol. 17, No. 36, 1980, pp. 6-370.
- P. Sujana and C. V. Naidu, “Influence of Bavistin, Cefotoxime, Kanamycin and Silver Thiosulphate on Plant Regeneration of Mentha piperita (L.)—An Important Multipurpose Medicinal Plant,” Journal of Phytology, Vol. 3, No. 5, 2011, pp. 36-40.
- D. Preethi, T. M. Sridhar and C. V. Naidu, “Effect of Bavistin and Silver Thiosulphate on in Vitro Plant Regeneration of Stevia rebaudiana,” Journal of Phytology, Vol. 3, No. 5, 2011, pp. 74-77.
- T. M. Sridhar and C. V. Naidu, “Antimicrobial Agents Alter the in Vitro Plant Regeneration in Solanum nigrum (L.),” Journal of Phytology, Vol. 3, No. 5, 2011, pp. 65-68.
- P. Bantawa, O. S. Roy, P. Ghosh and T. K. Mondal, “Effect of Bavistin and Adenine Sulphate on in Vitro Shoot Multiplication of Picrorhiza scrophulariiflora Pannell: An Endangered Medicinal Plant of Indo-China Himalayan regions,” Plant Tissue Culture and Biotechnology, Vol. 19, No. 2, 2009, pp. 237-245.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Plant Physiology, Vol. 15, No. 3, 1962, pp. 473- 479. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
- C. J. Delp, “Benzimidazole and Related Fungicides,” In: H. Lyr, Ed., Modern Selective Fungicides, 2nd Edition, Gustav Fisher Verlag, Jena, 1987, pp. 291-303.
- R. K. Tripathi and S. Ram, “Induction of Growth and Differentiation of Carrot Callus Cultures by Carbendazim and Benzimidazole,” Indian Journal of Experimental Biology, Vol. 20, No. 9, 1982, pp. 674-677.
- K. G. M. Skene, “Cytokinin-Like Properties of the Systemic Fungicide Benomyl,” Journal of Horticultural Science, Vol. 47, 1972, pp. 179-182.
- T. H. Thomas, “Investigations into the Cytokinin-Like Properties of Benzimidazole Derived Fungicides,” Annals of Applied Biology, Vol. 76, No. 2, 1974, pp. 237-241. http://dx.doi.org/10.1111/j.1744-7348.1974.tb07977.x
- R. Shields, S. J. Robinson and P. A. Anslow, “Use of Fungicides in Plant Tissue Culture,” Plant Cell Reports, Vol. 3, No. 1, 1984, pp. 33-36.
- P. C. Garcia, R. M. Rivero, J. M. Ruiz and L. Romero, “The Role of Fungicides in the Physiology of Higher Plants: Implications for Defense Responses,” Botanical Review, Vol. 69, No. 2, 2003, pp. 162-172.
- L. Burgos and N. Alburquerque, “Ethylene Inhibitors and Low Kanamycin Concentrations Improve Adventitious Regeneration from Apricot Leaves,” Plant Cell Reports, Vol. 21, No. 12, 2003, pp. 1167-1174. http://dx.doi.org/10.1007/s00299-003-0625-6
- R. J. Mathias and L. A. Boyd, “Cefotoxime Stimulates Callus Growth, Embryogenesis and Regeneration in Haploid Breed Wheat (Triticum aestivum L.),” The Plant Science, Vol. 46, No. 3, 1986, pp. 217-223. http://dx.doi.org/10.1016/0168-9452(86)90195-0
- O. M. F. Zaghmout and W. A. Torello, “Plant Regeneration from Callus and Protoplasts of Perennial Rye Grass (Lolium perenne L.),” Journal of Plant Physiology, Vol. 140, No. 1, 1992, pp. 101-105. http://dx.doi.org/10.1016/S0176-1617(11)81065-5
- S. Selwyn, “The Beta-Lactam Antibiotics,” Hodder and Stoughton, London, 1980, pp. 56-90.
- E. Chevreau F. Mourgues M. Neveu and M. Chevalier, “Effect of Gelling Agents and Antibiotics on Adventitious Bud Regeneration from in Vitro Leaves of Pear,” In Vitro Cellular and Developmental Biology-Plant, Vol. 33, No. 3, 1997, pp. 173-179. http://dx.doi.org/10.1007/s11627-997-0017-7
- M. Mencuccini, M. Micheli, A. Angiolillo, L. Baldoni, G. Frugis and D. Mariotti, “Agrobacterium Mediated DNA Transfer in Olive Callus (Olea europaea L.),” Advances in Horticultural Science, Vol. 13, 1999, pp. 25-28.
- T. Holford and H. J. Newbury, “The Effects of Antibiotics and Their Breakdown Products on the in Vitro Growth of Antirrhinum majus,” Plant Cell Reports, Vol. 11, No. 2, 1992, pp. 93-96. http://dx.doi.org/10.1007/BF00235261
- K. Kukreja, A. K. Mathur and P. S. Ahuja, “Effect of Antibiotics on Morphogenesis and Alkaloid Production in Callus of Hyoscymaus muticus,” In: G. M. Reddy, Ed., Proceedings of the Symposium on Plant Cell and Tissue Culture of Economically Important Plants, Hyderabad, 1985, pp. 399-404.
- S. F. Yang and N. E. Hoffman, “Ethylene Biosynthesis and Its Regulation in Higher Plants,” Annual Review, Plant Physiology, Vol. 35, 1984, pp. 155-189. http://dx.doi.org/10.1146/annurev.arplant.35.1.155
- S. H. Reddy, M. Chakravarthi, K. N. Chandrashekara and C. V. Naidu, “Influence of Bavistin and Silver Thiosulphate on in Vitro Regeneration of Asclepias curassavica (L). Using Nodal Explants,” American Journal of Plant Sciences, Vol. 3, No. 7, 2012, pp. 941-946.
- E. M. Bayer, “Effect of Silver Ion, Carbon Dioxide and Oxygen on Ethylene action and Metabolism,” Plant Physiololgy, Vol. 63, No. 1, 1979, pp. 169-173.
NOTES
*Corresponding author.

