Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 10, No.5, pp.397-407, 2011 jmmce.org Printed in the USA. All rights reserved 397 Influence of Processing Parameters on the Magnetic Properties of Mn-Zn Ferrites S. A. El-Badry ME Lab., Phys. Dept., Faculty of Science, Al-Azhar Univ., Nasr City, Cairo, Egypt. ABSTRACT Pure MnO 2 , ZnO and Fe 2 O 3 were used to prepare a Mn-Zn Ferrite sample of the nominal composition Mn 0.64 Zn 0.29 Fe 2 . 07 O 4 . These oxides were mixed firstly for 1hr, and then were milled for 20 and for 40 hrs. The as-mixed and the milled powders were examined by XRD and ME spectroscopy. The investigated samples were further mixed with PVA, granulated, cold pressed and sintered at different temperatures (1000, 1300 and 1400 º C) for 2 hrs and were then reinvestigated again. The magnetic properties of all samples before and after sintering were characterized using VSM at a field of 15 k Oe. When the powder oxides were milled for 20 hrs, detectable diffusion reaction was observed where the centers of all XRD peaks (due to Fe 2 O 3 and MnO 2 ) shifted to higher 2θ angles, suggesting that Zn 2+ cations had diffused through Fe 3+ and/or Mn 4+ lattices. The observed increase in the width of the XRD peaks can be attributed to the refinement of the powders by milling. Milling of the powder for 40 hrs resulted in the formation of spinel phase of (Zn, Fe), but MnO 2 was disappeared probably due to the formation of amorphous structure. Sintering at 1000, 1300, and 1400 º C resulted in the formation of different spinel (Mn-Zn) ferrites. The ME measurements followed the gradual formation the manganese zinc ferrite until complete formation which observed in the sample that milled for 40 hrs followed by sintering at 1300 º C for two hrs. However, it can be concluded that, the processing conditions of such sample represent are the best conditions for obtaining a soft manganese zinc ferrite (single phase). Key words: Mn-Zn ferrite, Ferrites, Soft magnets, XRD, ME spectroscopy and Magnetic properties. 1. INTRODUCTION Soft magnets are presently the basic elements of many electronic, electrotechnics and mechanical devices. They are used now from the base appliances up to different techniques in the space shuttle. Mn-Zn ferrites represent a group of the most widely used soft magnets in  398 S. A. El-Badry Vol.10, No.5 many electronic applications, eg. Choke coils, loud speaker, noise filters, recording heads, broad band impulse transformers, etc [1-3]. These materials exhibit excellent properties such as high permeability, high saturation magnetization, high resistivity and low power loss [4]. But in all cases the preparation technique is a matter of interest. The usual solid state reaction (ceramic) method appeared now of low interset since it yields ferrite with comparatively large grain size. Nowadays, powder milling method has been wildly used to prepare small particle size spinel ferrites [5-11]. It was found that, the magnetic properties of Mn-Zn ferrite are very sensitive to the processing parameters such as composition, additives, raw materials, attrition milling, and sintering conditions. The change in one or more of these parameters affects widely the magnetic properties of such materials [11-15]. On the other hand, 57 Fe ME spectroscopy has been succesflly applied to investigate the structure of iron compounds including variety of spinel ferrite systems as well as to get interesting information about their hyperfine structure, cation distribution and their internal magnetic properties [16]. Accordingly, this article was devoted to investigate the effect of the milling time as well as the sintering temperature on the magnetic properties of Mn-Zn ferrite applying XRD, ME spectroscopy and VSM techniques. 2. EXPERIMENTAL Pure manganese dioxide, zinc oxide and ferric oxide were used to prepare a Mn-Zn ferrite sample of the nominal composition Zn 0.29 Mn 0.64 Fe 2.07 O 4 . The mean particle size of the used oxides was between 63~20 µm. These oxides were mixed together using a double cone mixer until almost complete mixing was obtained (after 1 hr). The mixed powder was firstly examined by XRD and ME spectral analysis, and was then divided into two parts. The first part was further mixed with 6 % PVA, granulated and then cold pressed at 40 Mpa, in a floating die pressing, several compacts of rectangular shape. Some of these compacts were sintered at 1000 º C, some others at 1300 º C, and the rest of the compacts were sintered at 1400 º C for two hrs. The second part was subjected to mechanical milling using three dimensional hardened steel balls vial containing high chromium steel balls of 6 mm-diameter (spex 8000). The weight ratio between the balls and the charged powder was 10:1. The milling operation was continued for 40 hrs, but some of the milled powders for 20 and 40 hrs were mixed with 6 % PVA, granulated, and were cold compacted at 40 Mpa. These compacts were further sintered at one of the following temperatures: 1000, 1300 or 1400 º C for two hrs. Extensive investigation by XRD for different processing condition was performed using Phillips P.W. 1390 diffractometer using Co-K α radiation and Mn single-crystal monchromater. Density measurements were also measured using Archimedes principle in toluene liquid.  Vol.10, No.5 Influence of Processing Parameters on the Magnetic Properties 399 A conventional constant acceleration ME spectrometer outfitted with 25 m Ci 57 Co radioactive source was used to obtain the Mossbauer spectra of the studied samples at room temperature. The magnetic properties of the powder samples before and after sintering were characterized using VSM up to a field of 15 k Oe. 3. RESULTS AND DISCUSSION 3.1. X-Ray Analysis The XRD technique was firstly employed here to investigate the structure of the selected samples (as-mixed powder, after milling for 20 hrs, after milling for 40hrs as well as after sintering all samples at 1000, 1300 and 1400 º C for 2 hrs). The obtained XRD pattern of the as-mixed powder oxides is shown in (Fig.1 a). The analysis of this pattern indicated that the XRD peaks of MnO 2 , ZnO and Fe 2 O 3 are all present where they are the basic constituting oxides. When these mixed powder oxides were subjected to milling for 20 hrs, it appeared that the centers of all peaks due to Fe 2 O 3 and MnO 2 shifted to higher 2θ angles, but the centers of all peaks due to ZnO shifted to lower angles (Fig.1 b). However, it can be supposed that a detectable diffusive reaction occurred. It was suggested also that Zn 2+ cations had diffused through the Fe 3+ and/or Mn 4+ lattices. Also, it was noticed that the line width of the XRD peaks increased when the as mixed powder oxides were milled and their intensity decreased. This broadening may be due mainly to the refinement of the crystallite size and the accumulation of the internal strain in the milled powders [17]. With further milling up to 40 hrs (Fig.1C), the diffusive reaction continued and the centers of Fe 2 O 3 shifted to higher angles and the broadening of the peaks is also increased and their intensity show great decrease. This was associated with the formation of (Zn,Fe) spinel phases of the composition (Zn 0.664 Fe 0.336 ) (Fe 1.934 Zn 0.66 )O 4 , and the MnO 2 was completely disappeared. The formation of (Zn,Fe) spinel is due to the continuation of the diffusive reaction of Zn 2+ into Fe 3+ . The disappearance of the MnO 2 could be attributed to the formation of amorphous structure as a result of the accumulation of internal strain. The as mixed powder oxides as well as those milled for 20 hrs and for 40 hrs were all compacted and sintered for 2 hrs. They were then subjected to extensive XRD investigation (as exhibited in the experimental part). The analysis of the obtained XRD patterns of all these compacts that sintered at 1000 º C for 2 hrs indicated that: 1- The sintered compacts made from the as mixed powder oxides only (before milling) showed the formation of a manganese ferrite phase of the composition [(FeO) 1.099 (MnO) 0.011 ] and a (Zn,Fe) spinel phase of the composition [Zn 0.664 Fe 0.336 ] and a (Zn,Fe) spinel phase of the composition [Zn 0.664 Fe 0.336 ) (Fe 1.934 Zn 0.066 ]O 4 . 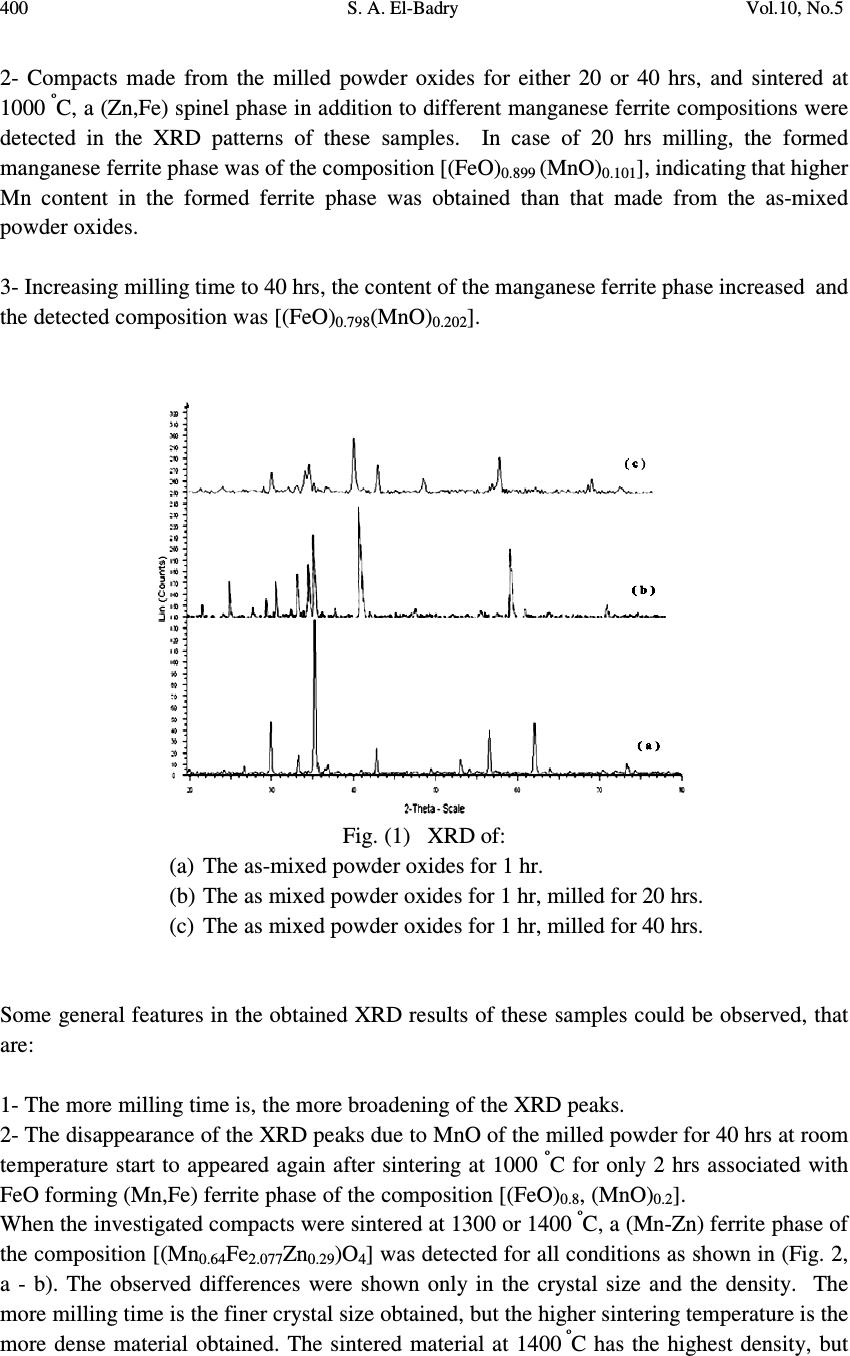 400 S. A. El-Badry Vol.10, No.5 2- Compacts made from the milled powder oxides for either 20 or 40 hrs, and sintered at 1000 º C, a (Zn,Fe) spinel phase in addition to different manganese ferrite compositions were detected in the XRD patterns of these samples. In case of 20 hrs milling, the formed manganese ferrite phase was of the composition [(FeO) 0.899 (MnO) 0.101 ], indicating that higher Mn content in the formed ferrite phase was obtained than that made from the as-mixed powder oxides. 3- Increasing milling time to 40 hrs, the content of the manganese ferrite phase increased and the detected composition was [(FeO) 0.798 (MnO) 0.202 ]. Fig. (1) XRD of: (a) The as-mixed powder oxides for 1 hr. (b) The as mixed powder oxides for 1 hr, milled for 20 hrs. (c) The as mixed powder oxides for 1 hr, milled for 40 hrs. Some general features in the obtained XRD results of these samples could be observed, that are: 1- The more milling time is, the more broadening of the XRD peaks. 2- The disappearance of the XRD peaks due to MnO of the milled powder for 40 hrs at room temperature start to appeared again after sintering at 1000 º C for only 2 hrs associated with FeO forming (Mn,Fe) ferrite phase of the composition [(FeO) 0.8 , (MnO) 0.2 ]. When the investigated compacts were sintered at 1300 or 1400 º C, a (Mn-Zn) ferrite phase of the composition [(Mn 0.64 Fe 2.077 Zn 0.29 )O 4 ] was detected for all conditions as shown in (Fig. 2, a - b). The observed differences were shown only in the crystal size and the density. The more milling time is the finer crystal size obtained, but the higher sintering temperature is the more dense material obtained. The sintered material at 1400 º C has the highest density, but  Vol.10, No.5 Influence of Processing Parameters on the Magnetic Properties 401 with coarser grain size than the other sintered compacts at relatively lower temperatures (1300 º C). The crystal sizes of the studied ferrite sample in different stages of milling time and sintering temperature, was calculated from the XRD and density measurements. All these results are presented in Table (1). Fig. (2) XRD patterns of the milled compacts for 40 hrs and sintered for 2 hrs at: (a) 1300 º C , (b) 1400 º C. Table (1). Effect of milling time and sintering temperatures on the crystal size and density measurements of the studied Mn-Zn-ferrites. Milling time , hr Sintering temp. ºC Crystal size nm Density g/c 3 As mixed compacts RT 202 1.65 1000 215 3.2 1300 225 3.83 1400 231 4.65 20hrs milled compact RT 96 1.03 1000 101 3.15 1300 160 4.3 1400 164 4.75 40hrs milled compact RT 45 0.83 1000 90 3.11 1300 106 4.2 1400 120 4.75  402 S. A. El-Badry Vol.10, No.5 From Table (1), it is clear that at RT, with increasing milling time the crystal sizes and density decreases as a result of decreasing particle sizes of the used powders. While for mixed compacts, 20 hr milled compacts and 40 hrs compacts with increasing milling time the crystal size decreases and density increases. The increases in the density as the milling time and sintering temperature can be attributed to the decrease of starting milled powder and so increase in the diffusion rate during sintering. 3.2. Mossbauer Spectroscopy It is interesting to apply Mossbauer Effect (ME) spectroscopic analysis to follow the changes due to the processing parameters (milling time and sintering temperature). It can be also applied to study the hyperfine structure of the studied samples as well as to follow Zinc ferrite formation. However, the obtained RT Mossbauer spectra of the studied samples are presented in both Fig. (3) and Fig. (4). -10 -50510 Fe 2 O 3 Transsmation % Velocity (mm/s) Fig. (3). ME spectra of: The as mixed power oxides for 1 hr, and ferric oxide (Fe 2 O 3 ) Fig.(3) exhibits the ME spectrum of the as mixed powder oxides - only after mixing just for one hour by the double-cone mixer together with the obtained ME spectrum due to pure α- Fe 2 O 3 for comparison. The only observable difference between the two spectra is the appearance of a slight doublet in the spectrum due to the mixed powder oxides. Except this small doublet, both spectra in this figure show typical coincident. In addition, the calculated parameters of the magnetic phase in the spectrum of the as mixed powder oxides and that due to α-Fe 2 O 3 appeared the same. This can be obviously seen in Table (2). On the other hand, the calculated parameters of the small doublet that appeared in the spectrum of the as mixed powder oxides, are also presented in Table (2). It was found that, for that doublet, the quadruple splitting (QS) energy was 0.318 (± 0.01) mm/s, while the isomer shift (IS) energy was 0.31 (±0.017) mm/s. Also it showed a line width value of 0.338 (± 0.04) mm/s. This  Vol.10, No.5 Influence of Processing Parameters on the Magnetic Properties 403 indicated that the ferrite formation starts in very little proportions with just mixing for one hour in the double cone-mixer. Table (2). RT Mossbauer Effect parameters for the measured Mn-Zn ferrite (Some representative samples) Sample Phase Qs Is Lw Hf A% As mixed powder 1 2 0.201 0.318 0.367 0.307 0.5 0.34 514 - 93 7 Milled for 20 hrs 1 2 3 0.041 0.0185 1.79 0.279 0.516 0.526 1.085 1.63 0.658 451 383 - 25.1 63.3 11.6 Milled for 40 hrs 1 2 3 4 0.241 0.799 1.36 0.806 0.371 0.428 0.512 1.01 0.613 1.73 1.1 0.557 514 376 - - 20.1 46.6 13.9 19.4 Milled for 40 hr and sintered at 1300 º C 1 2 0.267 0.681 0.405 0.363 0.471 0.733 508 - 18.8 81.2 Fig. (4). ME spectra of milled oxides, (1) mixed for 1 hr, (2) milling for 20 hrs, (3) milling for 40 hrs , and (4) after milling for 40 hrs and then calcinations for 1 hr at 1000 º C and sintering for 2 hrs at 1300 º C. Fig. (4) exhibits five representative ME spectra, where these spectra are due to:- 1- The ME spectrum of the as mixed powder oxides after mixing by the double-cone mixer for only one hour. 2- The ME spectrum of the mixed powder oxides after milling for 20 hrs. 3- The ME spectrum of the mixed powder oxides after milling for 40 hrs. -10 -50510 (4) Transsmation % Velocity (mm/s) (3) (2) (1)  404 S. A. El-Badry Vol.10, No.5 4- The ME spectrum of the mixed powder oxides after calcinations at 1000 º C for one hr and sintering at 1300 º C for 2 hrs. From these spectra, it is easily to observe the successive decrease of the sub-spectrum due to the ferromagnetic α-Fe 2 O 3 together with the gradual increase of the paramagnetic (soft- magnetic) ferrite material. The computer analysis and fitting of the as mixed powder oxides milled for 20 hrs indicated that, two magnetic iron phases (two sestets) are present. These two sextets may be due to both A and B sites. On going from spectrum (1) to spectrum (5) the ferromagnetic phases decreased gradually until complete disappearance in spectrum (5), while the central paramagnetic doublet increases also gradually, which means the complete formation of a soft magnetic (Mn,Zn) spinal ferrite. The Obtained ME parameters for all the measured samples are also presented in Table (2). Inspecting the ME spectra 2 and 3, it can be supposed that little relaxation effect appear which may be due to a super-paramagnetic relaxation. This may be in turn due to the effect of milling and the transformation of the particles to the nano-structural size, as concluded from XRD. It can be supposed also that the change of the ferromagnetic to paramagnetic material may be due to the small grain size as well as the disorder introduced by the milling process [18, 19]. Also, The spectra 1, 2, and 3 show in addition to the central doublets, a complex spectra in which two supper-imposed sextets appeared by fitting corresponding to the tetrahedral A and the octahedral B sites of iron cations. 3.3. Magnetic Properties Fig. (5) shows the magnetization curves for the as mixed powder oxides as well as those milled for 20 and for 40 hrs, and all these samples were sintered at 1300 º C, Fig. (5) Some representative hysteresis loops of: (1) The as mixed powder oxides, (2) Milled for 20 hr, and (3) Milled for 40 hrs.  Vol.10, No.5 Influence of Processing Parameters on the Magnetic Properties 405 Table (3) summarizes the effect of milling time and sintering temperature on the magnetic properties of the investigated materials. From this Table, it could be seen that when the milling time of the as mixed powder oxides was gradually increased (before sintering), the coercive force (Hc) decreased gradually from 234.3 to 75.81 Oe. It was observed also that the reminence magnetization (Br) and the saturation magnetization (Bs) increased by about 5 and 8 orders when the compacts were milled for 20 or 40 hrs respectively (see Fig. 6). As the sintering temperature was gradually increased from 1000 up to 1400 º C, a critical drop of the coercive force was obviously seen, while the reminence and the saturation magnetization showed sharp increase. It is worth to note that, all the values of the measured parameters (Hc, Br and Bs) of the sintered compacts at 1300 or 1400 º C showed approximately similar magnetic behavior. The observed changes in the magnetic properties of the studied materials could be attributed to both the reduction in the grain size and the formation of spinel (Zn-Fe) ferrite. It was concluded that the measured parameters of the compact sintered at 1400 º C exhibited superior magnetic properties than all other sintered compacts which may be due to its highest density value (due to the highest sintering temperature). But, it was easy to observe that the compacts sintered at 1300 º C have almost the same magnetic properties of that sintered at 1400 º C, but the former have finer grain sizes than the later compact that have coarser grain size. In addition to this the difference between the density values of both these compacts was small and they appeared to be close to each other. However, it could be concluded that the best sample is that milled for 40 hrs and sintered at 1300 º C for 2 hrs. Such processing conditions could form the same ferrite phase with finer average grain size and could produce approximately similar magnetic properties to that sintered at 1400 º C. In addition much thermal energy could be consumed when compared with the compact sintered at 1400 º C. Table (3) Effect of milling time and sintering temperatures on the magnetic properties of the studied Mn-Zn-ferrites during different stage of processing. Milling time , hr Sintering temp. º C Hc, Oe Br, emu/g Bs, emu/g As mixed compacts 20hr milled compact 40hr milled compact R.T. 1000 1300 1400 R.T. 1000 1300 1400 R.T. 1000 1300 1400 234.4 70.04 11.33 9.76 108.8 55.84 9.14 8.40 75.81 51.09 6.035 5.033 0.030 0.191 0.203 0.246 0.142 0.275 0.344 0.365 0.186 0.333 0.434 0.479 0.24 6.23 18.6 19.3 1.98 8.188 21.72 22.46 3.531 12.641 23.7 24.61  406 S. A. El-Badry Vol.10, No.5 010 20 30 40 0 20 40 60 80 100 120 140 160 180 200 220 240 Corecivity, HC Milling Time, hrs RT 1000C 1300C 1400C ( a ) 010 20 30 40 0.0 0.1 0.2 0.3 0.4 0.5 Reminance, Br Milling Time, hrs RT 1000C 1300C 1400C ( b ) Fig. (6) Effect of milling time and sintering temperature on the magnetic properties of the studied Mn-Zn-ferrites during different stages of processing. 4. CONCLUSION The obtained results demonstrate that the process consisting of both mechanical milling before sintering and then sintering at high temperature is useful for obtaining Mn-Zn ferrite of homogenous structure with fine size distribution which exhibit superior magnetic properties Inspecting the obtained results, it could be concluded that mixing the starting oxides for only one hour was enough to start the ferrite phase formation, as detected by ME spectroscopy. When the milling time was increased, this acted to decrease the particle size, resulting in a nano-particles structure. As the sintering temperature was increased, the density of the studied sample increased and the particle size became coarser, as well as the measured magnetic parameters became also better. Also, it could be seen that the magnetic parameters of the samples milled for 40 hrs and sintered at 1300 º C and 1400 º C respectively appeared to be close to each other. Therefore it could be concluded that the best processing conditions were the milling for 40 hrs followed by the sintering at 1300 º C for 2 hrs. REFERENCES 1. D. J. Fatemi, V. G. Harris, M. X. Chen, S. K. Malik, W. B. Yelon, . J. Long and A. Mohan, J. Appl. Phys., P. 5172, (1999). 2. J. S. Jiang, L. Gao, X. L. Yang, J. K. Guo and H. L. Shen, J. Mater. Sci. Lett. 18, P. 6867 (1999) 010 20 30 40 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Magnetization, Bs Milling Time, hrs RT 1000C 1300C 1400C ( C )  Vol.10, No.5 Influence of Processing Parameters on the Magnetic Properties 407 3. D. J. Fatemi, V. G. Harris,V. M. Browining and J. P. Kirkland, J. Appl. Phys. 83, p. 6867, (1998) 4. S. J. Shukla, K. M. Jadhav. And G.K. Bichile, J. Pure Phys. 39, P. 226 (2001). 5. A. Thakur and M. Singh, Ceram. Int. 29, P. 505 (2003). 6. A. Verma, T. C. Goel, R. G. Mendiratta, in : Second International Conference on processing Materials for properties, The Mineral, Metal & material Society, P. 493 (2000). 7. A. Thakur, P. Mathur and M. Singh, J. of Phy. and Chem. Of Solids, (2006). 8. A. L. Greer, Phil. Mag. B 61, P. 525 (1990). 9. G. Jain, B. Das, and S.Kumari, IEEE Magn., 16(6), P.1428 (1980). 10. T. Otsuka, E. Otsuki, and T. Sato, ICF6 (6 th Inter. Ceramic Conf.), P.317 (1992). 11. J. Fan, and F. Sale, IEEE Trans. Magn., 32(5), P. 4854 (1996). 12. H. J. Fecht, Nanostruct Mater. 1, P. 125 (1992). 13. H. Gleiter, Nanostruct Mater. 6, P. 3 (1995). 14. C. Suryanarayana, Prog., Mater. Sci. 46, P.184, (2001). 15. I. Lin, R. Mishra, and G. Thoma, IEEE Trans. Magn. 22(3), P. 175 (1986). 16. S. M. Attia, Egypt. J. Solids, 29, No.2, P. 329 (2006). 17. C. Linu, J. Wu, C. Chen, and M. Tung, J. magn. Magn. Mater., 133, P.478(1994). 18. J. Xu, J. He and E. Ma, Metall and Mater. Trans., 28A, P, 1969 (1997). 19. R. A. Dunlap, A. Alghamdi, J. W. O‘Brien and S. J. Penney, J. Alloys and Compounds, Vol. 365, Issues 1-2, P.84 (2004). |

