 Vol.2, No.6, 551-556 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.26069 Copyright © 2010 SciRes. OPEN ACCESS Effect of prolonged intake of iron enriched diet on testicular functions of experimental rats Mohamed M. El-Seweidy*, Mervat E. Asker, Sousou I. Ali, Hebatallah H. Atteia Biochemistry Department, Faculty of Pharmacy, Zagazig University; *Corresponding Author: mmelseweidy@yahoo.com Received 28 December 2009; revised 8 January 2010; accepted 23 March 2010. ABSTRACT Iron deficiency anemia represents a common nutritional problem which affects many socie- ties allover the world and iron fortified diet has been suggested as one of possible tools to combat and solve such problem. Present study was designed to illustrate the effect of dietary iron intake on certain biochemical markers dealing with oxidative stress, inflammatory re- sponse and cellular alterations of testicular tissues. Adult male rats which were fed on bis- cuits fortified with iron (0.3% ferrous sulfate) daily for 10 weeks (iron group) showed increa- sed serum iron, ferritin, tumor necrosis factor- alpha (TNF-α), nitric oxide (NO) and decreased Testosterone level (p < 0.05). Testicular tissues content of Malondialdehyde (MDA), hydroxypro- line (Hyp), iron showed significant increase (p < 0.05) and decreased glutathione (GSH) as com- pared to control group. Testicular tissues dem- onstrated massive iron distribution in sertoli interstial tissues and degeneration of germinal epithelial cells. Apparent reduction in number of sperms and spermatogenic cells were also obs- erved. These symptoms may demonstrate that prolonged intake of Biscuit fortified with iron causes certain testicular damage through cert- ain mechanism. Keywords: Iron Overload; Oxidative Stress; Inflammatory Response of Rat Testis 1. INTRODUCTION Iron is an essential element for many cellular activities like oxygen transport, electron transfer [1] and gene reg- ulation. However, an increase in cellular iron content may be toxic due to generation of reactive oxygen spe- cies (ROS) that damage proteins, lipids and DNA [2,3]. Iron deficiency anemia represents a common nutri- tional problem affecting large sector of individuals all- over the world. Accordingly iron fortified diet was con- sidered by some nutritionists as an effective tool and long term strategy to combat such kind of anemia. This program was adopted and applied during last decades in different countries using different bakeries and breads fortified with Iron. Objectors to these idea stated that the possibility of toxicity from excess iron absorption of fortified diet was too great and thus such a measure should not be sponsored.Ten years ago Egyp- tian ministry of education, through its distributed insti- tutes had adopted iron fortification program for different sectors of students especially young ages. This was achieved through daily supplementation to students of iron fortified biscuits (0.3% w/w). Till now this program was not evaluated properly, taken in consideration that additive iron here is greatly high. Healthy individuals may however face iron storage state even with moderate fortification of diet with iron, inducing inturn many va- rieties of chronic diseases [4]. Continuous iron intake may contribute to generation of free radicals and oxida- tion of cell components, an example is hepatocellular damage [5]. Testicular functions like liver cells are particularly vulnerable to such kind of injury and mostly mediated by reactive oxygen species (ROS) in consequence to iron overload. Oxidative damage here can either affect sperm cells or influence spermatogenic process which could change sperm functions. ROS can also activate transc- ription factors as nuclear factor-kappa B (NF-kB) which upregulates the transcription of adhesion molecules, cy- tokines and Enzymes as collective factors, mostly cont- ributing to inflammatory response [6]. Chronic iron overload (CIO) can enhance proliferation and increase TNF-α. The latter can also induce nitric oxide (NO) production from the activated polmorphonuclear leuko- cytes in response to tissues inflammation [7,8]. NO is an inorganic reactive nitrogen species synthesized in liver by iNos located in hepatocytes, kupffer, endothelial cells [9]. It represents a hepatoprotective mechanism against  M. M. El-Seweidy et al. / Natural Science 2 (2010) 551-556 Copyright © 2010 SciRes. OPEN ACCESS 552 CIO toxicity [10]. Decreased level of Testosterone in male suffering from idiopathic hemochromatosis was reported before [11]. Androgenic deficiency as mention- ned there not only produced by pituitary failure but also by testicular dysfunction due to hemosiderin deposition in testes. Oxidative stress may suppress also steroido- genesis. This is through substantial decrease in mRNA of steroid Acute regulatory protein (Star) as well as ac- tivities of testicular ∆5-3ß and 17-ß hydroxysteroid de- hydrogenases [12]. Therefore present study aimed mainly to illustrate the effect of prolonged intake of iron enriched biscuits on oxidative stress, inflammation, tes- ticular injuries and its degree in experimental rats. This may add great focus on such program having higher for- tification degree with iron. 2. MATERIALS AND METHODS A total of 30 wistar male albino rats (180 ± 20 g, 3 mon- ths old) were supplied by the Egyptian Organization for Biological Products and Vaccines (Helwan farm, Cairo). Rats were housed in stainless steel rodent cages and kept under environmentally controlled conditions and allow- ed one week for acclimatization at room temperature with a 12 h dark/light cycle before starting the experim- ental work. During such time animals received normal rat chow (El-Nasr Pharmaceuticals Chemicals, Egypt), and allowed free access of drinking water. 3. WORK DESIGN The rats were then divided into 2 groups, first one re- ceived in addition to normal chow, iron enriched biscuits (0.3% ferrous sulphate w/w) [13] daily for ten weeks (Fe group). Second group received iron free biscuits and served as control. This protocol was approved by the animal care and use committee of the Biochemistry De- partment, Faculty of Pharmacy, Zagazig University. Both groups, approximately consumed equal quantities of biscuits, its main components were: wheat flour, eggs, Butter, cane sugar and vanillin as flavor. Ten weeks after feeding, rats were fasted overnight and blood was col- lected via retro-orbital bleeding. Serum was prepared, and aliquots were stored at –20℃ for later determina- tions of: iron, ferritin, TNF-α, testosterone and nitric oxide (NO). Rats were killed by dissection. Testicular tissues were removed, rinsed with cold normal saline, divided into parts and dried with filter paper. First part was quickly frozen in liquid nitrogen (–170℃) then stored at –20℃ for determination of biochemical parameters: malondial- dehyde (MDA), Glutathione (GSH) Hydroxyproline (Hyp) and iron contents. The other part was kept in 10% formalin-saline at 4ºC for 1 week, subsequently dehydrated with a series of ethanol solution from 75 to 100% before embedding in paraffin. Cross sections (5 µm thick) were stained with hematoxylin and eosin (H & E) for routine light micros- copy assessments, Perl’s Prussian blue stain to localize deposited iron and Mallory trichrome stain to illustrate collagen fibers. 4. ANALYTICAL PROCEDURES I: Serum iron was determined Colorimetrically by using The kits supplied by spinreact, (S.A., Spain) [14], NO as nitrite [15]. TNF-α was measured by using ELISA kits supplied by Biosource Int. (California, USA) [16]. Se- rum testosterone and ferritin were measured by electro- chemiluminscence immunoassay “ECLIA” using com- mercial kits (Roche Diagnostics, USA) and Roche Elec- sys 2010 immunoassay analyzers [17,18]. II: Testicular tissues: MDA was determined spectro- photometrically by using thiobarbituric acid, TBA [19]. 0.5 G tissue was homogenized in 5 ml phosphate buffer (PH = 7.2), centrifuged at 2000 g for 10 min, supernatant fraction was used for MDA determination. GSH content was determined spectrophotometrically using Ellman’s reagent according to modified method [20]. 0.1 G of tissues was homogenized in 1 ml phosphate buffer (PH = 8) at 4℃. 0.5 ml of homogenate was mixed with 0.5 ml of 10 percent TCA in 5 mM EDTA sodium, mixed well and centrifuged at 2000 g for 5 min. Supernatant was used for determination of reduced GSH. Iron content was determined by flame atomic absorption Spectro- photometer [21]. About 0.1 G tissues was digested in 2ml conc Nitric acid and 2 ml perchloric acid at room temperature for 24 hours, the samples were filtered, di- luted and absorption was measured at 248 nm. Hyp was determined spectrophotometrically by Ehrlich reagent [22]. 0.1 G tissues was pulverized ground, 500 ul of 6 N Hcl were added and incubated overnight at 120 c. 5 ul of acid hydrolysate were mixed with 5 ul of citrate acetate buffer and 100 ul chloramines T in ELISA plate and incubated for 20 minutes at room temperature before addition of Ehrlich solution. 5. STATISTICAL ANALYSIS Analysis was carried out by using SPSS program for windows version 10 (SPSS, Chicago, USA). All results are presented as “Mean ± SD”, student “t” test was used for the comparison between groups. Statistical signifi- cance was defined at P < 0.05. 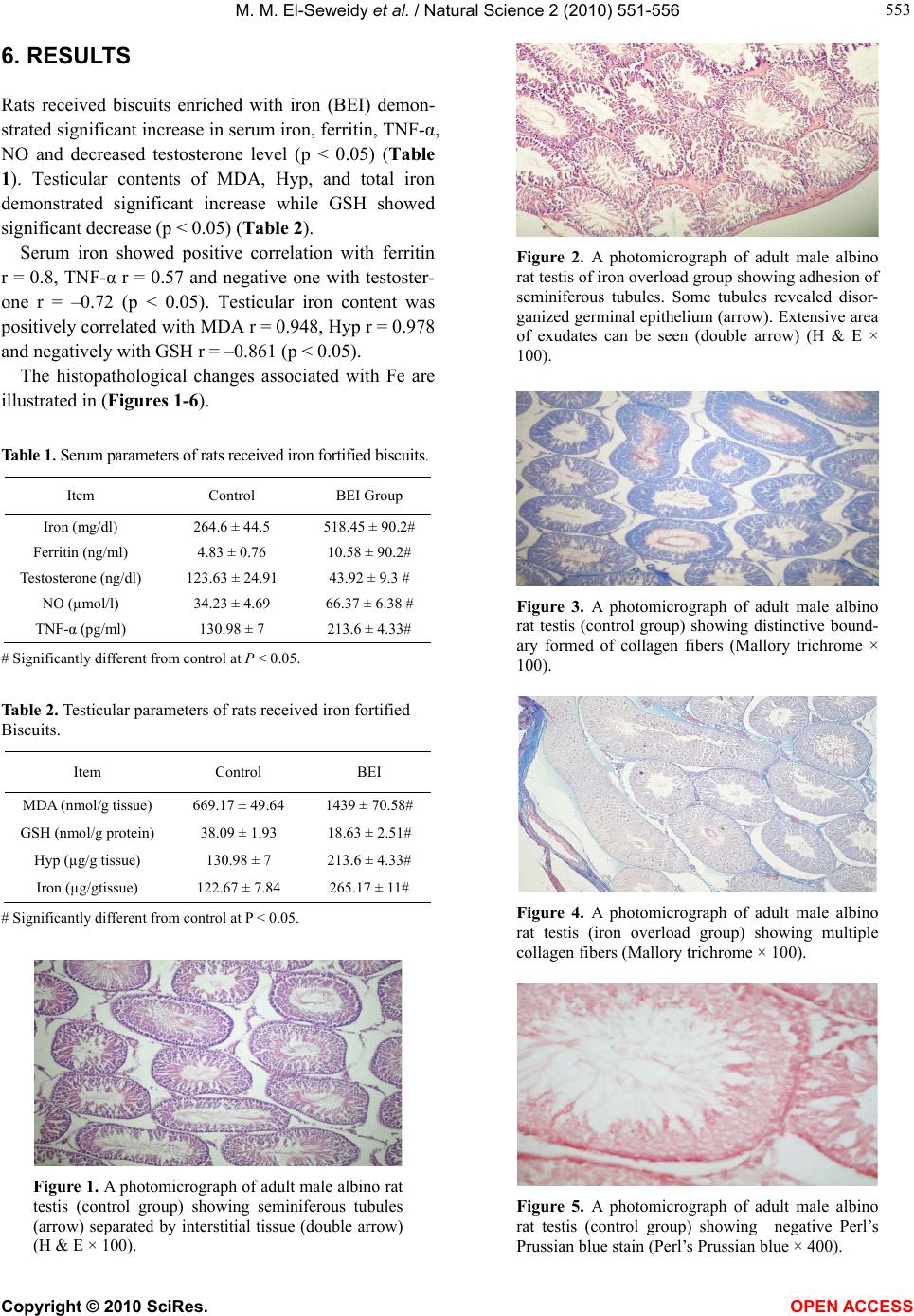 M. M. El-Seweidy et al. / Natural Science 2 (2010) 551-556 Copyright © 2010 SciRes. OPEN ACCESS 553 553 6. RESULTS Rats received biscuits enriched with iron (BEI) demon- strated significant increase in serum iron, ferritin, TNF-α, NO and decreased testosterone level (p < 0.05) (Table 1). Testicular contents of MDA, Hyp, and total iron demonstrated significant increase while GSH showed significant decrease (p < 0.05) (Table 2). Serum iron showed positive correlation with ferritin r = 0.8, TNF-α r = 0.57 and negative one with testoster- one r = –0.72 (p < 0.05). Testicular iron content was positively correlated with MDA r = 0.948, Hyp r = 0.978 and negatively with GSH r = –0.861 (p < 0.05). The histopathological changes associated with Fe are illustrated in (Figures 1-6). Table 1. Serum parameters of rats received iron fortified biscuits. Item Control BEI Group Iron (mg/dl) 264.6 ± 44.5 518.45 ± 90.2# Ferritin (ng/ml) 4.83 ± 0.76 10.58 ± 90.2# Testosterone (ng/dl) 123.63 ± 24.91 43.92 ± 9.3 # NO (µmol/l) 34.23 ± 4.69 66.37 ± 6.38 # TNF-α (pg/ml) 130.98 ± 7 213.6 ± 4.33# # Significantly different from control at P < 0.05. Table 2. Testicular parameters of rats received iron fortified Biscuits. Item Control BEI MDA (nmol/g tissue) 669.17 ± 49.64 1439 ± 70.58# GSH (nmol/g protein) 38.09 ± 1.93 18.63 ± 2.51# Hyp (µg/g tissue) 130.98 ± 7 213.6 ± 4.33# Iron (µg/gtissue) 122.67 ± 7.84 265.17 ± 11# # Significantly different from control at P < 0.05. Figure 1. A photomicrograph of adult male albino rat testis (control group) showing seminiferous tubules (arrow) separated by interstitial tissue (double arrow) (H & E × 100). Figure 2. A photomicrograph of adult male albino rat testis of iron overload group showing adhesion of seminiferous tubules. Some tubules revealed disor- ganized germinal epithelium (arrow). Extensive area of exudates can be seen (double arrow) (H & E × 100). Figure 3. A photomicrograph of adult male albino rat testis (control group) showing distinctive bound- ary formed of collagen fibers (Mallory trichrome × 100). Figure 4. A photomicrograph of adult male albino rat testis (iron overload group) showing multiple collagen fibers (Mallory trichrome × 100). Figure 5. A photomicrograph of adult male albino rat testis (control group) showing negative Perl’s Prussian blue stain (Perl’s Prussian blue × 400).  M. M. El-Seweidy et al. / Natural Science 2 (2010) 551-556 Copyright © 2010 SciRes. OPEN ACCESS 554 Figure 6. A photomicrograph of adult male albino rat testis (iron overload group) showing positive Perl’s prussian blue stain (arrow) in interstitial tissue (Perl’s Prussian blue × 400). 7. DISCUSSION As mentioned above prolonged intake of iron enriched diet induced significant increase in serum iron and fer- ritin levels, in agreement with reported studies [23,24]. Previous report suggested that iron overload in rats can specifically activate target genes in the liver (i.e., L fer- ritin and procollagen) [25]. Similar study supported this suggestion, illustrated an increased expression of the heavy-chain isoform of ferritin mRNA in liver of iron overloaded rats [26]. Others suggested that the elevated non-transferrin bound iron (NTB iron); observed in iron- overloaded diseases may reflect two factors. First one,by increasing saturation degrees of transferrin it will reduce the number of plasma binding sites for NTB iron .This may increase the total plasma iron fraction in the NTB iron pool. The second factor may be liver damage [27]. Testicular iron content demonstrated significant in- crease (Table 2), in agreement with previous studies [28,29] which considerd testicular tissues as secondary target for accumulated iron. This observation also im- plicates a regulation of iron transport which greatly dif- fers from liver. It considers also that testis produce their own transferrin, and participates in iron shuttle system which fulfill the requirements of this metal for sper- matogenesis [30]. Pro-oxidant effects of iron in testicular tissue are rea- sonable factor which mediates MDA increase and GSH decrease [31]. Other studies demonstrated GSH deple- tion, reduced activities of antioxidant enzymes like glu- tathione peroxidase, catalase, and superoxide dismutase [32]. In vitro study reported also similar findings [33]. Testicular content of Hyp demonstrated also signific- ant increase in consistent with. Previous study [34] wh- ich may be due to increased activity of prolyl hydroxy- lase [35]. It was demonstrated before that iron overload induced moderate fibrosis in testes interstitium, intersti- tial macrophages, fibroblast and Leydig cells [36] mean- while present study configurated similar findings (Fig- ure 4). Last years many studies has been oriented to illustrate iron-induced oxidative stress, its effect on activation of the transcription factor nuclear factor-kappa B (NF-kB) [37]. NF-kB exists in an inactive state in the cytosol, usually as two subunits, p50and p65, complexed with inhibitory proteins (IkB). Degradation of inhibitory pro- teins can translocate NF-kB to the nucleus where it alters transcription of a number of genes involved in cell growth, differentiation, inflammation, and immune func- tion. Such study suggested that NF-kB activation can be modulated by the redox status of the cell [38]. ROS can increase NF-kB activation while, antioxidant compounds including iron chelators can inhibit it [39]. NF-kB activation also plays a key role in the inflam- matory process and upregulates the transcription of ad- hesion molecules, cytokines and enzymes involved in the inflammatory responses [40]. Present study demon- strated significant increase in TNF-α of iron group and, in agreement with recent study [13]. TNF-α may also activate NO production from the ac- tivated polymorphnuclear leukocytes in response to tis- sue inflammation [6]. The presence of nitrogen reactive species can explain the iron sequestration pattern which characterizes macrophages under inflammatory condi- tions.It may refer also to a complex relationship between iron and NO so that iron overload can also increase iron uptake by the liver Kupffer cells while No increase is mostly attributed to activated nitric oxide synthase [41]. NO expression is controlled also by the redox-sensit- ive transcription factor NF-KB [10], acting in iron over- load state as hepatoprotective agent against iron toxicity. This may be associated with increased NF-kB DNA binding to the iNOS gene promoter and higher expres- sion and activity of iNOS mRNA [42]. Decreased serum testosterone in Fe group may be at- tributed to haemosiderin deposition in the pituitary gland [43] or cytotoxic effects of iron on the gonadotrophs [44]. Reported histopathological study indicated that exces- sive iron deposition caused degranulation of the adeno- hypophysiocytes and decreased hormone storage within such cells [45], additionally iron deposition in the ante- rior lobe of the pituitary gland can decrease its response to gonadotropin-releasing hormone [12]. Involvement of oxidative stress in the suppression of steroidogenesis was also reported before. This may be due to substantial reduction in the mRNA of steroid acute regulatory pro- tein as well as activities of testicular Δ5-3β and 17-β hy- droxysteroid dehydrogenases (HSD) through strong af- finity of divalent heavy metal to the thiol groups of these proteins and enzymes [46]. Other reported studies indicated that TNF-α induced  M. M. El-Seweidy et al. / Natural Science 2 (2010) 551-556 Copyright © 2010 SciRes. OPEN ACCESS 555 555 an early and transient increase in serum luteinizing hor- mone (LH) , followed by a transient decrease in serum testosterone levels, while FSH demonstrated no change [47]. 8. CONCLUSIONS Taken together, the present study indicates that iron ove- rload causes impairment in testicular activity and affects the androgenicity of male rats .This may be a reflection of iron deposition in testicular tissues, a matter which deserve great focus and attentions regarding such dietary program which have higher fortification degree with iron. 9. ACKNOWLEDGEMENTS We acknowledge the financial support (Research grant) for the study presented from Biochemistry Department, Faculty of Pharmacy-Zagazig University. REFERENCES [1] Boldt, D.H. (1999) New perspectives on iron: An intro- duction. American Journal of the Medical Sciences, 318(4), 207-212. [2] Houglum, K., Filip, M, Witztum, J.L. and Chojkier, M. (1990) Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. Journal of Clinical Investigation, 86(6), 1991-1998. [3] Toyokunis, J. (2002) Iron and carcinogenesis: From fen- ton reaction to target genes. Redox Report, 7(4), 189-197. [4] Swanson, C.A. (2003) Iron intake and regulation: Impli- cations for iron deficiency and iron overload. Alcohol, 30(2), 99-102. [5] Papanikolaou, G. and Pantapoulos, K. (2005) Iron me- tabolism and toxicity. Toxicology and Applied Pharma- cology, 202(2), 199-211. [6] Richard, A., Johnson, A.C. and Hanson, S. (2005) Par- enteral iron compounds sensitize mice to injury-initiated TNF-α mRNA production and TNF-α release. American Journal of Physiology. Renal Physiology, 57(2), F290- F297. [7] Brown, K.E., Meleah, M., Keimberly, A. and Weydert, J. (2006) Chronic iron overload stimulates hepatocyte pro- liferation and cyclin D1 expression in rodent liver. Translational Research, 148(2), 55-62. [8] Dunger, A., Cunningham, J.M., Delaney, C.A. and Lowe, E. (1990) Tumor necrosis factor-α and Interferone-y in- ibit insulin secretion and cause DNA damage in un- weanled rat islets. Diabetes, 45(2), 183-189. [9] Alderton, W., Cooper, C. and Knowles, R. (2001) Nitric oxide synthases: Structure, function and inhibition. Bio- chemical Journal, 357(Pt 3), 593-615. [10] Cornejero, P., Fernandez, V., Vial, M. and Videla, L.A. (2007) Hepatoprotective role of NO in an experimental model of chronic iron overload. Nitric Oxide, 16(1), 143-149. [11] V ogt, H.J., Weidenbach, T., Marqart, K.H. and Vogel, G. (1987) Idiopathic hemochromatosis in a 45 year old in- fertile man. Androlgia, 19(5), 532-538. [12] Gupta, R.S., Gupta, E.S., Dhakal, B.K. and Thakur, A.R. (2004) Vitamin C and Vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol cells, 17(1), 132-139. [13] Elmegeed, G.A., Ahmed, H. and Hussein, J.S. (2005) Novel synthesized aminsteroidal hetrocycles intervention for inhibiting iron induced oxidative stress. European Journal of Medicinal Chemistry, 40(12), 1283-1294. [14] Burits, C.A. and Ashwood, E.R. (1999) Methods for the determination of serum iron, iron binding capacity, and transferrin saturation. In: Burtis, C.A., Edward, R., Ash- wood, M.D. and Tietz, N.W., Eds., Tietz Textbook of Clinical Chemistry, 3rd Edition, AACC, 1701-1703. [15] Moshage, H., Kok, B., Huizenga, J.R., Jansen, P.L.M. (1995) Nitrite and nitrate determination in plasma: A critical evaluation. Clinical Chemistry, 41(6 Pt 1), 892- 896. [16] Chen, W., Jin, W., Cook, M., Weiner, H.L. and Wahl, S.M. (1998) Oral delivery of group a streptococcal cell walls augments circulating TGF-beta and suppresses strepto- coccal cell wall arthritis. The Journal of Immunology, 161(11), 6297-6304. [17] Wheeler, M.J. (1995) The determination of bio-avail- able testosterone. Annals of Clinical Biochemistry, 32(Pt 4), 345-357. [18] Lotz, J., Hafner, G. and Prellwitz, W. (1997) Reference study for ferritin assays. Kurzmitteilung Clinical Labo- ratory, 43(11), 993-994. [19] Buege, J. and Aust, S. (1978) Microsomal lipid peroxida- tion. Methods Enzymology, 52, 302-306. [20] Ahmed, A.E., Gamal, I.H., Loh, J. and Abdel-Rahman, S.Z. (1991) Studies on mechanism of haloacetonitrile induced gasyrointestinal toxicity, interaction of dibro- moacetonitrile with glutathione as glutath ione-s-tran- sferase in rats. Journal of Biochemical Toxicology, 6( 2), 115-121. [21] Basset, M.L., Halliday, J.W. and Powell, L.W. (1986) V alue of hepatic measurement in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology, 6(1), 24-29. [22] Fujita, M., Shannon, J.M., Morikawa, O., Gauldie, J., Hara, N. and Mason, R.J. (2003) Overexpression of tu- mor necrosis factor-α diminishes pulmonary fibrosis in- duced by bleomycin or transforming growth Factor-β. American Journal Respiratory Cell and Molecular Biol- ogy, 29(6), 669-676. [23] Zhao, Y., Li, H., Gao, Z. and Xu, H. (2005) Effects of dietary baicalin supplementation on iron overload in- duced mouse liver oxidative injury. European Journal of Pharmacology, 509(2-3), 195-200. [24] Silvana, M.L., Ribeiro, T., Sliva, M.E., Chianca, D.A., Paula, H.D., Cardoso, L.M., Colombari, E. and Pedrosa, L. (2003) Iron overload in hypercholesterolemic rats af- fects iron homeostasis and serum lipids but not blood pressure. Journal of Nutrition, 133(1), 15-20. [25] Pietrangelo, A., Rocchi, E., Schiaffonati, L., Ventura, E. and Cairo, G. (1990) Liver gene expression during chronic dietary iron overload in rats. Hepatology, 11(5), 798-804.  M. M. El-Seweidy et al. / Natural Science 2 (2010) 551-556 Copyright © 2010 SciRes. OPEN ACCESS 556 [26] Valerio, L.G.Jr. and Petersen, D.R. (2000) Characteriza- tion of hepatic iron overload following dietary admini- stration of dicyclopentadienyl iron (ferrocene) to mice: Cellular, biochemical, and molecular aspects. Experi- mental and Molecular Pathology, 68(1), 1-12. [27] Teresa, L., Brissot, P., Ma, W.-L. and Weisiger, R.A. (1986) Characterization of non-transferrin-bound iron clearance by rat liver. The Journal of Biological Chemis- try, 261(23), 10909-10914. [28] Lucesoli, F. and Fraga, C.G. (1999) Oxidative stress in testes of rats subjected to chronic iron intoxication and α-tocopherol supplementation. Toxicology, 13 2(2- 3), 179- 186. [29] Galleano, M. and Puntarulo, S. (1997) Dietary α-toco- pherol supplementatiom on antioxidant defenses after in vivo iron overload in rats. Toxicology, 124(1), 73-81 [30] Sylvester, S.R. and Griswold, M.D. (1993) Molecular biology of iron transport in the testis. In: De Krester, D., Ed., Molecular Biology of the Male Reproductive System, Academic Press, San Diego, 311-323. [31] Harandi, A.A., Allameh, A. and O’Brien, P.J. (2005) In vivo effects of iron overload on toxicological parameters in isolated hepatocytes obtained from adult rats. FEBS Journal, 272(s1), 1-650. [32] Jagetia, G.C., Reddy, T.K., Venkatesha, V.A. and Kedlaya, R. (2004) Influence of naringin on ferric iron induced oxidative damage in vitro. Clinica Chimica Acta, 347(1- 2), 189-197. [33] Alidoost, F., Gharagozloo, M., Bagherpour, B., Jafarian, A., Sajjadi, S.E., Hourfar, H. and Moayedi, B. (2006) Effects of silymarin on the proliferation and glutathione levels of peripheral blood mononuclear cells from be- ta-thalassemia major patients. International Immuno- pharmacology, 6(8) , 1305-1310. [34] Zhang, Y., Li, H., Zhao, Y. and Gao, Z. (2006) Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. European Journal of Pharmacology, 535(1-3), 263-269. [35] Poli, G. and Parola, M. (1997) Oxidative damage and fibrogenesis. Free Radical Biology and Medicine, 22(1- 2), 287-305. [36] Lucesoli, F., Caligiuri, M., Roberti, M.F., Perazzo, J.C. and Fraga, C.G. (1999) Dose-dependent increase of oxi- dative damage in the testes of rats subjected to acute iron overload. Archives of Biochemistry and Biophysics, 372(1), 37-43. [37] Tsukamoto, H., Lin, M., Ohata, M., Giulivi, C., French, S.W. and Brittenham G. (1999) Iron primes hepatic ma- crophages for NF-kB activation in alcoholic liver injury. American Journal of Physiology, 277(6 Pt 1), G1240- G1250. [38] Flohe´, L., Brigelius-Flohe, R., Saliou, C., Traber, M.G. and Packer, L. (1997) Redox regulation of NF-kB activa- tion. Free Radical Biology and Medicine, 22(6), 1115- 1126. [39] Li, S., Li, X. and Rozanski, G.J. (2003) Regulation of glutathione in cardiac myocytes. Journal of Molecular and Cellular Cardiology, 35(9), 1145-1152. [40] Dunger, A., Cunningham, J.M., Delaney, C.A., Lowe, J.E., Green, M.H.L., Bone, A.J. and Green, I.C. (1996) Tumor necrosis factor-α and interferone-γ inhibit insulin secretion and cause DNA damage in unweanted-rat islets. Diabetes, 45(2), 183-189. [41] Galleano, M., Simontacchi, M. and Puntarulo, S. (2004) Nitric oxide and iron: Effect of iron overload on nitric oxide production in endotoxemia. Molecular Aspects of Medicine, 25(1-2), 141-154. [42] Kleinert, H., Pautz, A., Linker, K. and Schwarz, P. (2004) Regulation of the expression of inducible nitric oxide synthase. European Journal of Pharmacology, 500(1-3), 255-266. [43] Seracchioli, R., Porcu, F. and Colombi, C. (1994) Trans- fusion-dependent homozygous β-thalassemia major suc- cessful twin pregnancy following in-vitro fertilization and tubal embryo transfer. Human Reproduction, 9(10), 1964-1965. [44] Sparacia, G., Iaia, A., Banco, A., D’Angelo, P. and La- galla, R. (2000) Transfusional hemochromatosis: Quan- titative relation of MR imaging pituitary signal intensity reduction to hypogonadotropic Hypogonadism. Radiol- ogy, 215(3), 818-823. [45] Bergeron, C. and Kovacs, K. (1978) Pituitary siderosis: A histologic, immunocytologic and ultrastructural study. American Journal of Pathology, 93(2), 295-310. [46] Wang, C., Tso, S. and Todd, D. (1989) Hypogonadotropic hypogonadism in severe beta-thalassemia: Effect of che- lation and pulsatile gonadotropin-releasing hormone the- rapy. The Journal of Clinical Endocrinology and Me- tabolism, 68(3), 511-516. [47] van der Poll, T., Romijn, J.A., Endert, E. and Sauerwein, H.P. (1993) Effects of tumor necrosis factor on the hypo- thalamic-pituitary-testicular axis in healthy men. Me- tabolism, 42(3), 303-307.
|