Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.2, pp.115-122, 2010 jmmce.org Printed in the USA. All rights reserved 115 In-vitro Studies of Biologically Important Barium Strontium Chromium Magnesium Hydrogen Phosphate (BaSrCrMgHPO4) Mixed Crystal Growth in SMS Gel Medium at Ambient Temperature and Its Characterization Studies J. Kishore Kumar, P. Ramesh, P. Suresh and P. Sundaramoorthi* Department of Physics, Thiruvalluvar Govt. Arts College, Rasipuram, Namakkal, India-637401 *Corresponding Author: moorthi.sundara@gmail.com, Ph-04287-252785, Cell-9486902430) ABSTRACT Kidney stone consists of various compounds. Mineral oxalate monohydrate and di-hydrate are the main constituen t of kidn e y ston e s. However, the formation of calc ium oxalate kid n ey stones is still not clearly understood. In this field of studies, several new hypotheses are created, which includes nucleation, reduction of nucleation, crystal growth and or aggregation of formation of different crystals such as oxalate monohydrate and oxalate di-hydrate. The effect of some urinary species such as ammonium oxalate, calcium, citrate, proteins and trace elements were reported by the author. The kidney stone constituents along with trace minerals are grown in silica gel medium (SMS) which provides the necessary growth simulation (in-vivo). In the artificial urinary stone growth process, growth parameters within the different chemical environments were carried out and reported for several urinary crystals such as CaHP, SrHP, SrMHP, BaHP, BaMHP and MgHP. In the present investigation, BaSrCrMHP (Barium Strontium Chromium Magnesium Hydrogen Phosphate) crystals are grown in different growth faces to attain the total nucleation reduction. Extension of this investigation, many characterization studies have been carried out and compared with reported results. 1. INTRODUCTION SrHP (Strontium hydrogen phosphate), CaHP (Calcium hydrogen phosphate) and BaHP (Barium hydrogen phosphate) were grown in silica gel medium at room temperature. The next approach is to investigate and grow mixed crystal in silica gel medium at different environments, which contains one major elements (phosphate), three minor or trace elements (strontium, barium and chromium) and one inhibitor (Magnesium). BaSrCrMHP is a mixed crystal, which typically represents the biological crystals formed in the human urinary tracts called renal stones [1-3]. 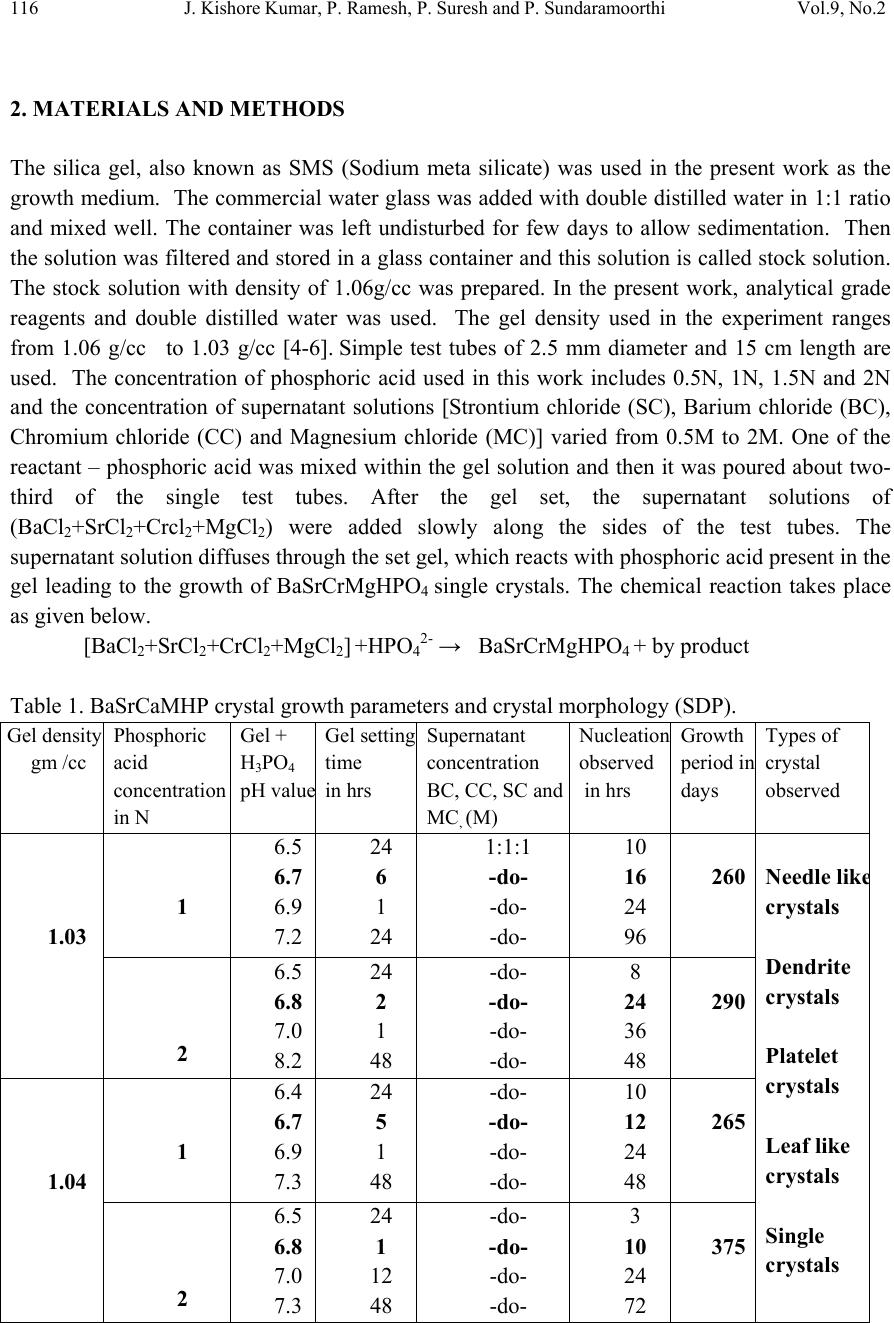 116 J. Kishore Kumar, P. Ramesh, P. Suresh and P. Sundaramoorthi Vol.9, No.2 2. MATERIALS AND METHODS The silica gel, also known as SMS (Sodium meta silicate) was used in the present work as the growth medium. The commercial water glass was added with double distilled water in 1:1 ratio and mixed well. The container was left undisturbed for few days to allow sedimentation. Then the solution was filtered and stored in a glass container and this solution is called stock solution. The stock solution with density of 1.06g/cc was prepared. In the present work, analytical grade reagents and double distilled water was used. The gel density used in the experiment ranges from 1.06 g/cc to 1.03 g/cc [4-6]. Simple test tubes of 2.5 mm diameter and 15 cm length are used. The concentration of phosphoric acid used in this work includes 0.5N, 1N, 1.5N and 2N and the concentration of supernatant solutions [Strontium chloride (SC), Barium chloride (BC), Chromium chloride (CC) and Magnesium chloride (MC)] varied from 0.5M to 2M. One of the reactant – phosphoric acid was mixed within the gel solution and then it was poured about two- third of the single test tubes. After the gel set, the supernatant solutions of (BaCl2+SrCl2+Crcl2+MgCl2) were added slowly along the sides of the test tubes. The supernatant solution diffuses through the set gel, which reacts with phosphoric acid present in the gel leading to the growth of BaSrCrMgHPO4 single crystals. The chemical reaction takes place as given below. [BaCl2+SrCl2+CrCl2+MgCl2] +HPO42- → BaSrCrMgHPO4 + by product Table 1. BaSrCaMHP crystal growth parameters and crystal morphology (SDP). Gel density gm /cc Phosphoric acid concentration in N Gel + H3PO4 pH valu e Gel setting time in hrs Supernatant concentration BC, CC, SC and MC, (M) Nucleation observed in hrs Growth period in days Types of crystal observed 1.03 1 6.5 6.7 6.9 7.2 24 6 1 24 1:1:1 -do- -do- -do- 10 16 24 96 260 Needle lik e crystals Dendrite crystals Platelet crystals Leaf like crystals Single crystals 2 6.5 6.8 7.0 8.2 24 2 1 48 -do- -do- -do- -do- 8 24 36 48 290 1.04 1 6.4 6.7 6.9 7.3 24 5 1 48 -do- -do- -do- -do- 10 12 24 48 265 2 6.5 6.8 7.0 7.3 24 1 12 48 -do- -do- -do- -do- 3 10 24 72 375  Vol.9, No.2 In-vitro Studies of Biologically Important BaSrCrMgHPO4 117 Figure 1. Growth of BaSrCrMHP medium with in laboratory (Gel density-1.04 g/cc). Figure 2. Harvested BaSrCrMHP crystal. 3. RESULT AND DISCUSSION The BaSrCrMgHPO4 crystals are grown in three different growth faces by applying various growth parameters. Table 1 gives the growth parameters of BaSrCrMgHPO4 crystals and the bold letters shows the optimum growth parameters. Among them, the laser exposed growth medium shows better nucleation reduction and no crystals were formed, because of the inability to attain super saturation. In the sun light exposed medium partial nucleation was observed, since exposure of sunlight to the growth medium was only in day time that is six hours per day and the growth period was ten months. 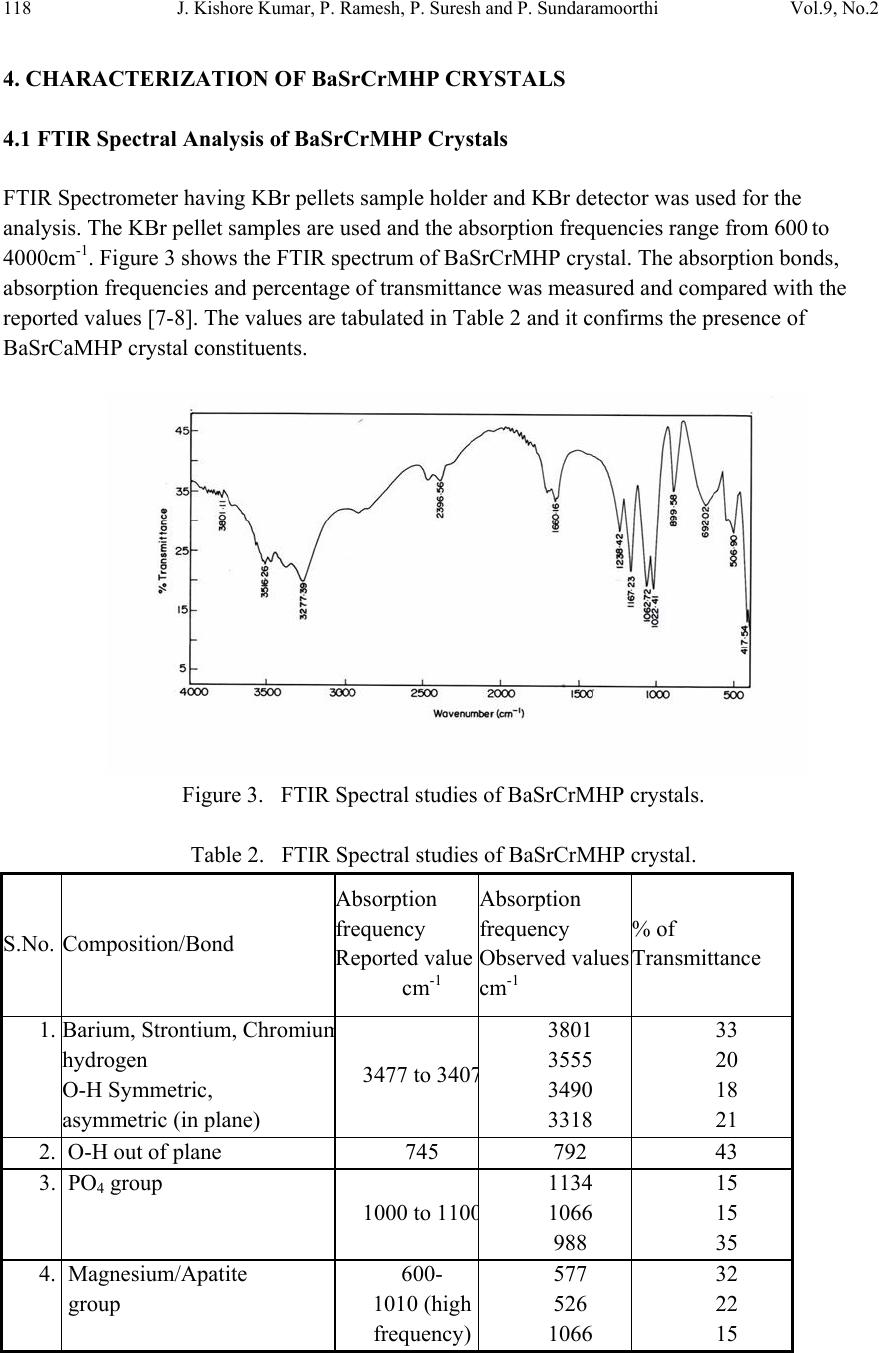 118 J. Kishore Kumar, P. Ramesh, P. Suresh and P. Sundaramoorthi Vol.9, No.2 4. CHARACTERIZATION OF BaSrCrMHP CRYSTALS 4.1 FTIR Spectral Analysis of BaSrCrMHP Crystals FTIR Spectrometer having KBr pellets sample holder and KBr detector was used for the analysis. The KBr pellet samples are used and the absorption frequencies range from 600 to 4000cm-1. Figure 3 shows the FTIR spectrum of BaSrCrMHP crystal. The absorption bonds, absorption frequencies and percentage of transmittance was measured and compared with the reported values [7-8]. The values are tabulated in Table 2 and it confirms the presence of BaSrCaMHP crystal constituents. Figure 3. FTIR Spectral studies of BaSrCrMHP crystals. Table 2. FTIR Spectral studies of BaSrCrMHP crystal. S.No. Composition/Bond Absorption frequency Reported value cm-1 Absorption frequency Observed values cm-1 % of Transmittance 1. Barium, Strontium, Chromiu m hydrogen O-H Symmetric, asymmetric (in plane) 3477 to 340 7 3801 3555 3490 3318 33 20 18 21 2. O-H out of plane 745 792 43 3. PO4 group 1000 to 110 0 1134 1066 988 15 15 35 4. Magnesium/Apatite group 600- 1010 (high frequency) 577 526 1066 32 22 15 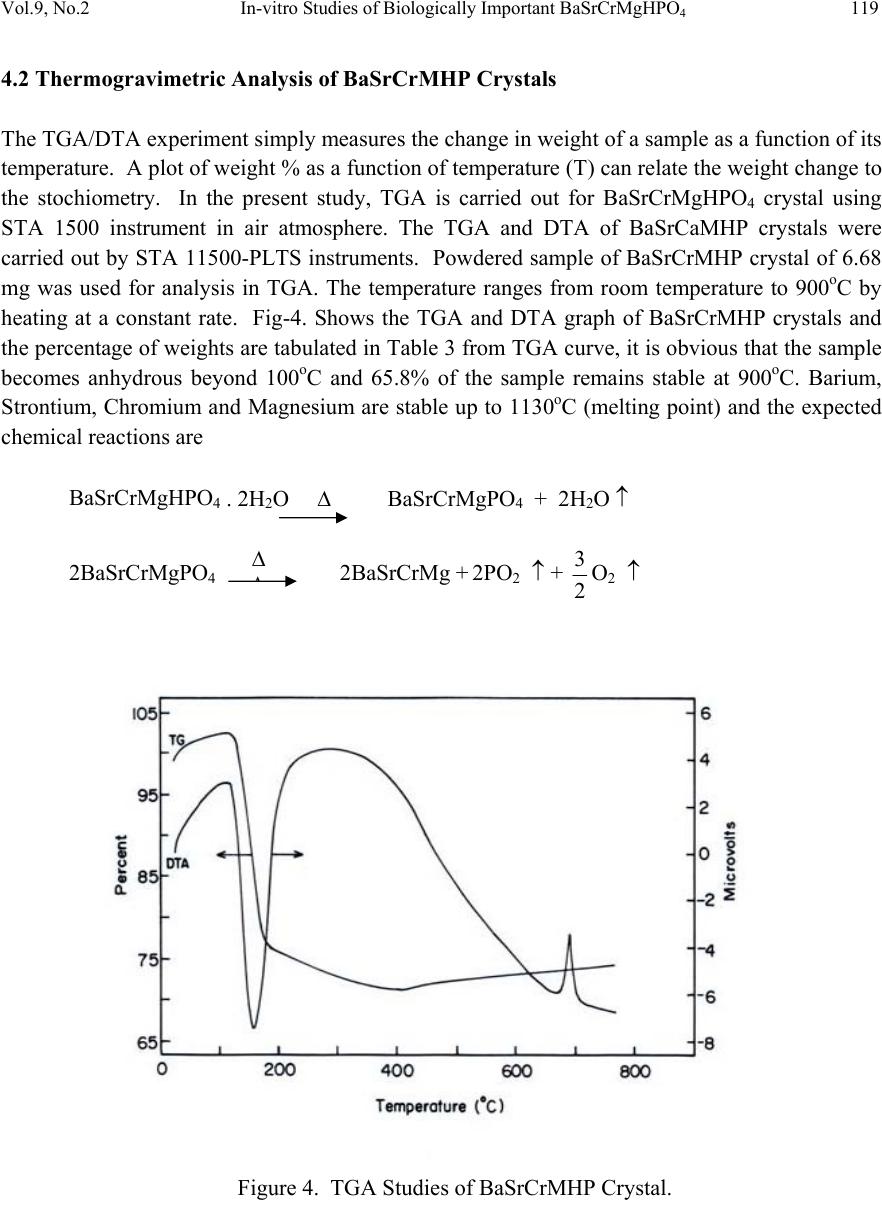 Vol.9, No.2 In-vitro Studies of Biologically Important BaSrCrMgHPO4 119 4.2 Thermogravimetric Analysis of BaSrCrMHP Crystals The TGA/DTA experiment simply measures the change in weight of a sample as a function of its temperature. A plot of weight % as a function of temperature (T) can relate the weight change to the stochiometry. In the present study, TGA is carried out for BaSrCrMgHPO4 crystal using STA 1500 instrument in air atmosphere. The TGA and DTA of BaSrCaMHP crystals were carried out by STA 11500-PLTS instruments. Powdered sample of BaSrCrMHP crystal of 6.68 mg was used for analysis in TGA. The temperature ranges from room temperature to 900oC by heating at a constant rate. Fig-4. Shows the TGA and DTA graph of BaSrCrMHP crystals and the percentage of weights are tabulated in Table 3 from TGA curve, it is obvious that the sample becomes anhydrous beyond 100oC and 65.8% of the sample remains stable at 900oC. Barium, Strontium, Chromium and Magnesium are stable up to 1130oC (melting point) and the expected chemical reactions are BaSrCrMgHPO4 . 2H2O Δ BaSrCrMgPO4 + 2H2O ↑ 2BaSrCrMgPO4 2BaSrCrMg + 2PO2 ↑ + 2 3O2 ↑ Figure 4. TGA Studies of BaSrCrMHP Crystal. Δ Δ 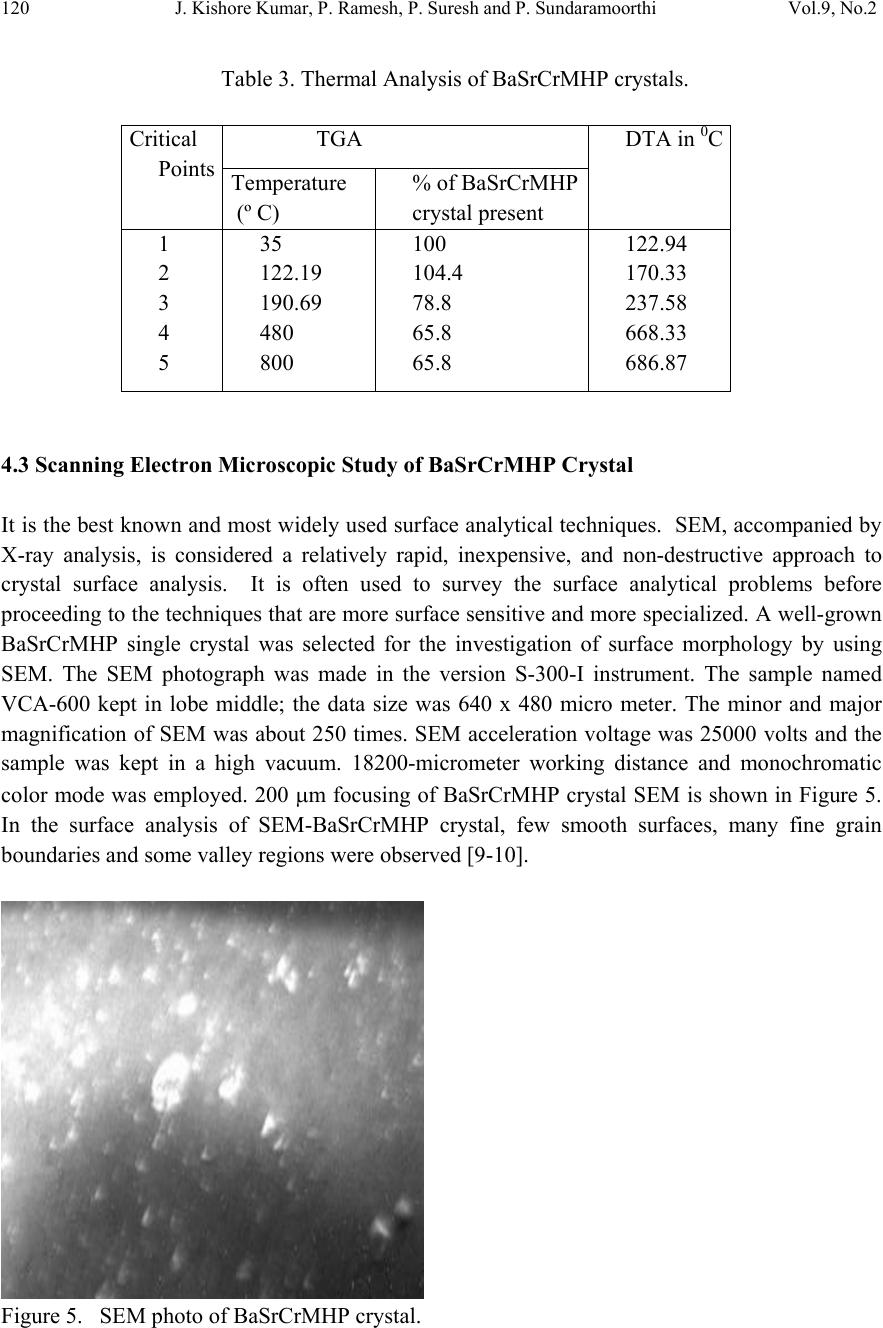 120 J. Kishore Kumar, P. Ramesh, P. Suresh and P. Sundaramoorthi Vol.9, No.2 Table 3. Thermal Analysis of BaSrCrMHP crystals. Critical Points TGA DTA in 0C Temperature (º C) % of BaSrCrMHP crystal present 1 2 3 4 5 35 122.19 190.69 480 800 100 104.4 78.8 65.8 65.8 122.94 170.33 237.58 668.33 686.87 4.3 Scanning Electron Microscopic Study of BaSrCrMHP Crystal It is the best known and most widely used surface analytical techniques. SEM, accompanied by X-ray analysis, is considered a relatively rapid, inexpensive, and non-destructive approach to crystal surface analysis. It is often used to survey the surface analytical problems before proceeding to the techniques that are more surface sensitive and more specialized. A well-grown BaSrCrMHP single crystal was selected for the investigation of surface morphology by using SEM. The SEM photograph was made in the version S-300-I instrument. The sample named VCA-600 kept in lobe middle; the data size was 640 x 480 micro meter. The minor and major magnification of SEM was about 250 times. SEM acceleration voltage was 25000 volts and the sample was kept in a high vacuum. 18200-micrometer working distance and monochromatic color mode was employed. 200 μm focusing of BaSrCrMHP crystal SEM is shown in Figure 5. In the surface analysis of SEM-BaSrCrMHP crystal, few smooth surfaces, many fine grain boundaries and some valley regions were observed [9-10]. Figure 5. SEM photo of BaSrCrMHP crystal.  Vol.9, No.2 In-vitro Studies of Biologically Important BaSrCrMgHPO4 121 4.6 Single Crystal X-ray Diffraction X-ray crystallography is one of the most useful methods for exploring the nature of matter. X- ray diffractometry is used to determine the phase content in many minerals and materials. As a result, it is used by researchers in a wide range of disciplines. It is used as an adjunct to chemical analysis in the identification of the constituents of mixtures of crystalline phases, such as minerals, cements and alloys; for measurements of the lattice parameters of artificially produced structures such as to identify changes in raw materials or methods that might alter a drugs efficacy. X-ray diffractory meter readings got from RSIC – IIT Chennai-25 in the BaSrCrMHP single powder crystals. The XRD pattern and diffraction indices of the grown crystal are calculated by Treor programme according to the values of 2θ in XRD [11-12]. The unit cell parameters are shown in Table 4. Table 4. Unit cell parameters of BaSrCrMHP. From the data it is confirmed that the crystal system is triclinic. 4.7 Etching Study of BaSrCrMgHPO4 Crystal A well-grown BaSrCrMHP crystal was immersed in HCl solution at a desired concentration. The dissolution of BaSrCrMHP crystal depends upon the etchant concentration, temperature, crystal morphology, etching time etc [13-14]. The etch pits are shown in Figure 6. The etch pits were observed as plane pit, leaf pit and step pits. Figure 6. Etch picture – (Etching time-7 minutes, 2N -HCl as an etchant). Primitives in Å Interfacial angle in degree a-10.013 α =90.08 b-10.216 β =90.02 c-10.684 γ=90.00 a≠b≠c α ≠ β ≠ γ  122 J. Kishore Kumar, P. Ramesh, P. Suresh and P. Sundaramoorthi Vol.9, No.2 5. CONCLUSION In the present work good transparent Barium Strontium Chromium Magnesium hydrogen phosphate (BaSrCrMHP) single crystals were grown with well developed faces. Different growth habits were observed with respect to the different pH values for gel density 1.03g/cc to 1.06g/cc. The effect of sunlight and laser on the growth of BaSrCrMHP crystals were studied and found that laser exposed medium produces better nucleation reduction. Characterization studies like XRD, .FTIR, TGA/DTA, SEM and etching were done and the results analyzed. Using XRD results, the grown crystal cell parameters are calculated and found the crystal system as triclinic. The chemical components present in the grown crystals were confirmed by FTIR. Using TGA/DTA graph, calculated the percentage of decomposition of grown crystals. The surface morphology of the grown crystal was studied by SEM. The study of chemical etching was carried out to obtain the etch pits pattern which gives the surface nature of grown crystals. REFERENCES [1] P. Sudaramoorthi, S. Kalainathan., Asia Journal of Chem., 16 (2007) 569-574. [2] P. Sundaramoorthi, S. Kalainathan., J. Biochem. Engg. ,34 ( 2007) 244-249. [3] P. Sundaramoorthi, S. Kalainathan, J, Min. and Mat. Char. & Eng., 6 (2007) 33-40. [4] H.K.Henisch, Crystals in Gel and Liesegang Rings, (Cambridge University Press) Cambridge (1986). [5] H.K. Henisch, J.M. Garcia-Ruiz, J. Crystal Growth, 75 (1986)195. [6] H.K. Henisch, J.M. Garcia-Ruiz, J. Crystal Growth , 75 (1985) 203. [7] G.Socrater, Infrared Cha. Group Friq. (John Willy Edi.) Chichester (1980). [8] Yean-Chin Tasay et al, J. Urology, 86 (1961) 838-854. [9] J.B. Kirk, In: Direct observation of Imperfection in crystals (Interscience Publishers) New York (1962). [10] H. Bethage, et al, Electron Microscopy in Solid State Physics (Elsevier Edi) Amsterdom (1987). [11] G. Machennan, C.A. Reevers, J.Acta Crystals, 18 (1955) 579. [12] N.A. Curry, D.W. Jones, J. Chem. Soc., A(1971) 3725. [13] H.C. Gates, Thirty years of progress in Surface Science, In: Crystal growth and characterization, North Holland. (1975) [14] K.Taukamot, J. Crystal. Growth, 61 (1983) 199. |

