Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 7, No.4, pp 331-346, 2008 jmmce.org Printed in the USA. All rights reserved Corrosion Behavior of Tin-Plated Carbon Steel and Aluminum in NaCl Solutions Using Electrochemical Impedance Spectroscopy B.O. Oni a , N.O. Egiebor b* , N.J. Ekekwe a , and A. Chuku a a Department of Electrical Engineering b Environmental Engineering Program, Department of Chemical Engineering Tuskegee University, Tuskegee, AL 36088, USA * Author for Communications: E-mail: Egiebor@tuskegee.edu Phone: 334-724-4265, Fax: 334-724-4397 Abstract The corrosion behavior of both tin-plated carbon steel and aluminum (Al 1100) in NaCl solutions were studied using electrochemical impedance spectroscopy (EIS). The study objectives were to compare their corrosion behavior in concentrated brine solution, establish the equivalent electrical circuit diagrams of their corrosion phenomena, and consequently develop steady-state corrosion models for estimating the corrosion rates in the given solution. The steady-state corrosion rates for the tin-plated carbon steel increased significantly when the concentration of NaCl was increased from 1.0M to 1.5M. The increase in corrosion rate of the tin-plated steel is attributed to reduced charge transfer resistance due to the breakdown of the surface tin plating at high chloride concentrations. In contrast, there was little incremental change in the corrosion rates of aluminum when the NaCl concentration was increased from 1.0M to 1.5M. This is attributed to the known formation of a stable, passivating surface oxide film on aluminum which inhibits corrosion. The surface passivating film did not show any significant evidence of degradation with increase in chloride concentration. Using MATHLAB and the experimental corrosion rate data, a mathematical corrosion model was derived for the two samples to describe their steady-state corrosion in NaCl electrolyte solutions. Key words: Corrosion, models, corrosion rates, carbon steel, aluminum, brine solution  332 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 1. INTRODUCTION Electrochemical Impedance Spectroscopy (EIS) has become a well established tool for the study of corrosion and degradation of metallic materials through electrochemical reactions. EIS is now the method of choice for characterizing any system in which the electrical behavior is dependent on several different physico-chemical processes occurring on material surfaces at different rates [1]. It also has the advantage of allowing the rapid determination of the corrosion rate of a sample without requiring long-term testing [2, 3, 4]. The corrosion of carbon steel and aluminum is a major infrastructure degradation problem in practically all industries, including the chemical, mineral, materials, and petrochemical industries world-wide [5, 6]. In order to improve the corrosion resistance of carbon steel, they are often alloyed or surface treated with more corrosion resistant metals such as Zn, Sn, Co, etc. Because of its excellent corrosion resistant properties, tin-plated carbon steel are frequently used for the production of beverage cans, food cans, and aerosol cans [7]. Similarly, aluminum alloys have long been regarded as corrosion resistant, but recent studies [8] have shown that they do also corrode readily in chloride rich solutions. However, the relatively cheaper cost of steel-based materials, as compared to aluminum, makes them more attractive for commercial applications in all industries. Therefore, the primary objective of this project was to conduct a comparative corrosion study of aluminum and tin-plated carbon steel and establish the equivalent electrical circuit diagrams of their corrosion phenomena. The results are used to develop corrosion models for estimating the corrosion rates in chloride solutions. Such data generally represent a vital resource for practicing engineers, especially those in the construction industries who desire tools and information to effectively estimate corrosion rates of metals in order to project the life expectancy of the metals and structures under different environmental conditions. A variety of coated steel, including tin coating, has been shown to exhibit high corrosion resistance in chloride solutions [9, 10, 11]. 2. BACKGROUND THEORY An electrode interface undergoing an electrochemical reaction is typically analogous to an electronic circuit consisting of a specific combination of resistors, capacitors, and inductors. In a galvanic cell, the initial corrosion current is defined by R EE I ac initial )( 00 − == R v 0 ∆ (1) and 000 ac EEv −=∆ (2) where 0 c E and 0 a E are the open circuit cathode and anode potentials respectively. R is the total resistance which in corrosion is made up mainly of polarization resistance, solution resistance,  Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 333 and charge transfer resistance to current flow. The corrosion current can be obtained from the polarization resistance via the Stern-Geary equation [12] given as: ] )(3.2 [ 1 ca ca p corr R I ββ β β = (3) Where I corr is the corrosion current density in A/cm 2 , ß a is the anodic Tafel coefficient, ß c is the cathodic Tafel coefficient and Rp is the polarization resistance (ohm.cm2). The Tafel coefficients are generated from the anodic and cathodic branches of the potential-current diagrams generally known as the Tafel plots. The corrosion rate is related to the corrosion current density by the equation: )( year mill C rate = k r ε corr I (4) where C rate is the corrosion rate in milli-inches or mills per year (mpy), k r is a constant which defines the corrosion rate units divided by the sample density and area, and ε is the equivalent weight of the material being studied. When a small potential is applied, electrochemical impedance spectroscopy (EIS) uses the impedance frequency response of a corroding system, to generate a plot of the magnitude of the real impedance against the imaginary impedance component. This is known as the complex plane plot or Nyquist plot. The impedance is generated in real and imaginary format according to the equation: Z = R – jX, (5) Where Z is the impedance response, R is the real impedance, X is the magnitude of the imaginary component, and j is a complex number equivalent to the square root of -1. Data from the Nyquist and Tafel plots are used by the EIS software to generate the equivalent electrical circuits (EEC) which approximates the physical phenomena occurring on the working electrode surface during corrosion. 3. EXPERIMENTAL The metal samples used for the experiments were obtained from the Metal Sample Company of Alabama, USA. The two samples were a tin-plated carbon steel and an aluminum-1100 metal strips. The 1.0M and 1.5M NaCl electrolytes used in the experiments were prepared by pouring 1000cm 3 of distilled water into two separate beakers each. 58.5grams of analytical grade NaCl salt, obtained from Fisher Scientific, was dissolved in the 1.0 liter distilled water for the 1.0M  334 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 solution, and 87.75grams of NaCl salt was dissolved in 1.0 liter distilled water for the 1.5M NaCl solution. The solutions were stirred until the salt particles were completely dissolved before use. The electrochemical equipment used in the experiment was a Princeton Applied Research (PAR) Potentiostat Model 263A, a Princeton Applied Research K0235 Flat electrochemical cell with a platinum counter electrode, and a Ag-AgCl/KCl (saturated) reference electrode. The software used for data acquisition and analyses was a Princeton Applied Research (PAR) PowerSuite electrochemical application software. Tests on the samples were setup and conducted in a three-electrode configuration as illustrated in Figure 1. Each alloy at the specified electrolyte concentration was studied for 14 days. The metal samples were exposed to the electrolyte solution through a fixed orifice of 1.0 cm 2 surface area. The reference electrode was submerged in the Ag-AgCl/KCl saturated solution. An alternating current of 10mV was applied to the electrode under study at sweeping frequencies of between 0.1Hz and 100 kHz. The frequency response of the electrode under the different test conditions were recorded and displayed as Nyquist plots for further analysis. The equipment software was also used to generate the anodic and cathodic polarization or Tafel plots and equivalent electrical circuits (EEC) diagrams based on the acquired experimental data. This is generally achieved by using mathematical equations for simple electrical circuits to estimate the EIS experimental data. The experimental software package regress equations representing appropriate electrical circuits around the experimental data by iteratively adjusting parameters, such as resistance and capacitance, until the best fit of an equation and equivalent circuit to the experimental data is obtained [12]. Working Electrode Reference Electrode Counter Electrode Electrolyte Galvanostat/Potentiostat Te st Sample Figure 1. Experimental Set-up with a Potentiostat and 3-Electrode Cell. 4. RESULTS AND DISCUSSION Figures 2 and 3 present the Tafel plots for the tin-plated steel and aluminum samples respectively in 1.0 M NaCl solution after 3 days of experimentation. The current axis displays the logarithm of the absolute current. The tin-plated sample shows a significantly higher corrosion potential (E corr ) of about -0.72 V than the aluminum sample with E corr of about -0.92 V. The corrosion current for the tin-plated steel is also proportionately higher, thus indicating a much higher corrosion rate for the tin-plated steel than for the aluminum sample. In addition, Figure 3 indicate  Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 335 a significant surface passivation between the potentials of -0.8 V a and -0.7 V where increasing potential does not lead to a significant increase in current flow. This is attributable to the well known formation of a passive oxide film on the aluminum surface. This phenomenon is not observed for the tin-plated steel. Figure 2. Tafel Plot for Tin-Plated Steel in 1.0 M NaCl after 3 days.  336 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 Figure 3. Tafel Plot for Aluminum in 1.0 M NaCl after 3 days. At a higher electrolyte concentration of 1.5 M NaCl, Figures 4 and 5 show the corresponding Tafel plots for tin-plated and aluminum samples respectively after 3 days of experimentation. Both samples show increased corrosion current as compared to the 1.0 M solution sample. This is indicative of increased corrosion rate at the higher electrolyte concentration. However, the aluminum samples steel displayed significant passivation from -0.85 V to about -0.72 V for the higher electrolyte concentration. Figure 4. Tafel Plot for Tin-Plated Steel in 1.5 M NaCl after 3 days.  Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 337 Figure 5. Tafel Plot for Aluminum in 1.5 M NaCl after 3 days. Figures 6 and 7 present the complex plane or Nyquist plots for both the steel and the aluminum samples respectively, in 1.0 M solution after 3 days. The solid circles in the plots indicate the experimental data while the rectangular points were obtained from the derived models based on the equivalent electrical circuit (EEC) diagrams as will be discussed later. The experimental data for the Nyquist plot of the tin-plated steel sample displays a single semi-circle which is indicative of a single time-constant. On the contrary, the experimental data for the aluminum sample shows an initial semi-circular pattern followed by a straight line displaying an angle of about 45 o to the real impedance axis at the low frequency range. Figure 7 illustrates a two time- constant situation for aluminum. The straight line observed at lower frequencies for aluminum complex plane plot indicates a constant phase element (CPE) which is most likely due to a Warburg impedance. This is attributable to infinite charge transfer diffusion limitation as a result of the effectiveness of the passivating oxide film on the aluminum surface. This diffusion limitation is not observed for the tin-plated steel sample in 1.0 M NaCl solution after 3 days of corrosion. Furthermore, notice that at the low frequency range of the Nyquist plots, the upper limits of impedance (resistance) for the tin-plated sample is observed to be about 1400 Ohm, while at the same frequency range, the upper limit of impedance for the aluminum sample is observed to be about 6000 Ohm. This is also indicative of higher resistance of aluminum to corrosion than tin-plated steel at the conditions studied. 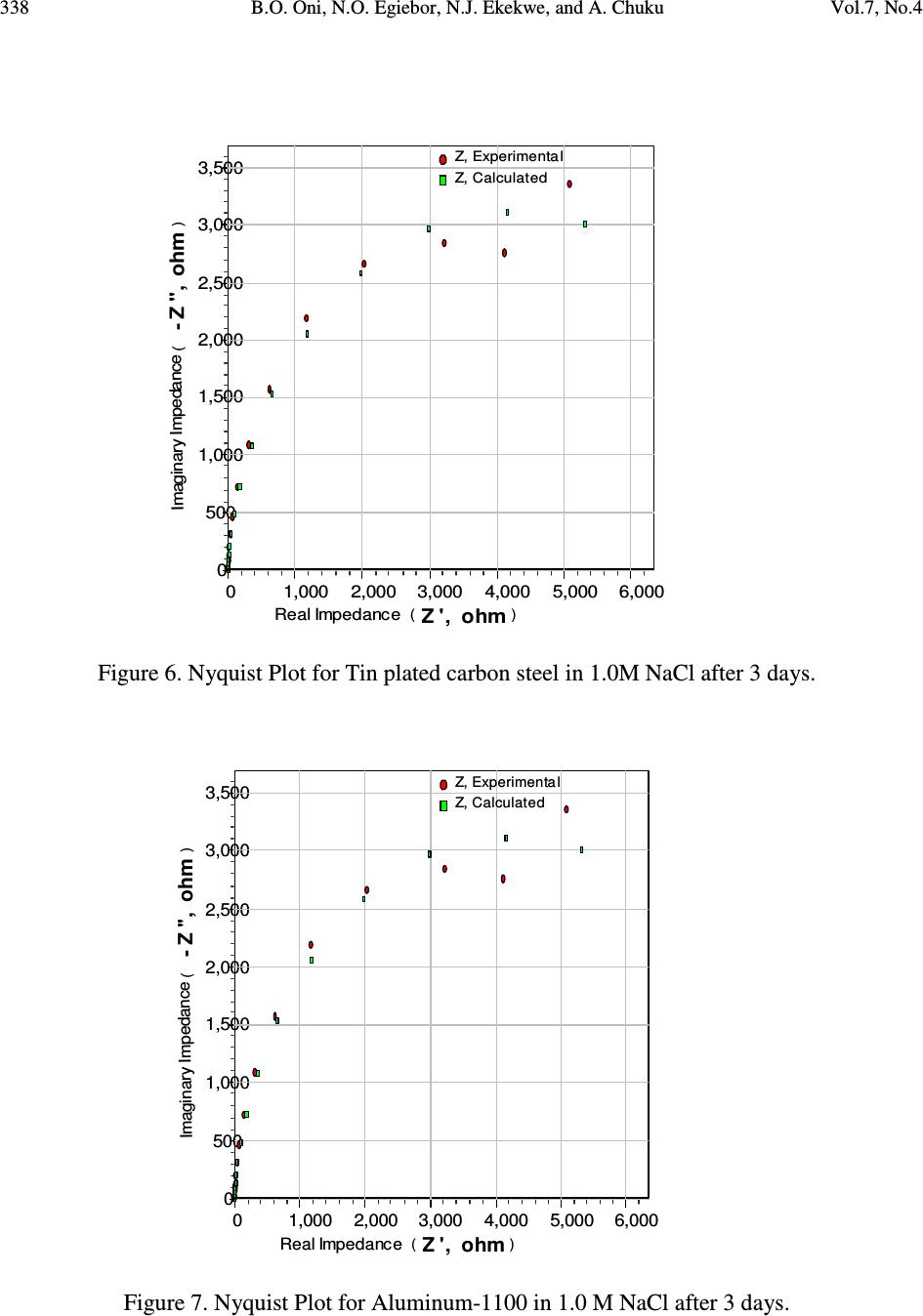 338 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 Z , Msd. Z , Calc. unknown.txt Model : R(Q(R(CR))) Wgt : Modulus Z ', ohm 6,0005,0004,0003,0002,0001,0000 - Z '', ohm 3,500 3,000 2,500 2,000 1,500 1,000 500 0 Z, Experimental Z, Calculated Real Impedance ( ) Imaginary Impedance ( ) Figure 6. Nyquist Plot for Tin plated carbon steel in 1.0M NaCl after 3 days. Z , Msd. Z , Calc. unknown.txt Model : R(Q(R(CR))) Wgt : Modulus Z ', ohm 6,0005,0004,0003,0002,0001,0000 - Z '', ohm 3,500 3,000 2,500 2,000 1,500 1,000 500 0 Z, Experimental Z, Calculated Real Impedance ( ) Imaginary Impedance ( ) Figure 7. Nyquist Plot for Aluminum-1100 in 1.0 M NaCl after 3 days. 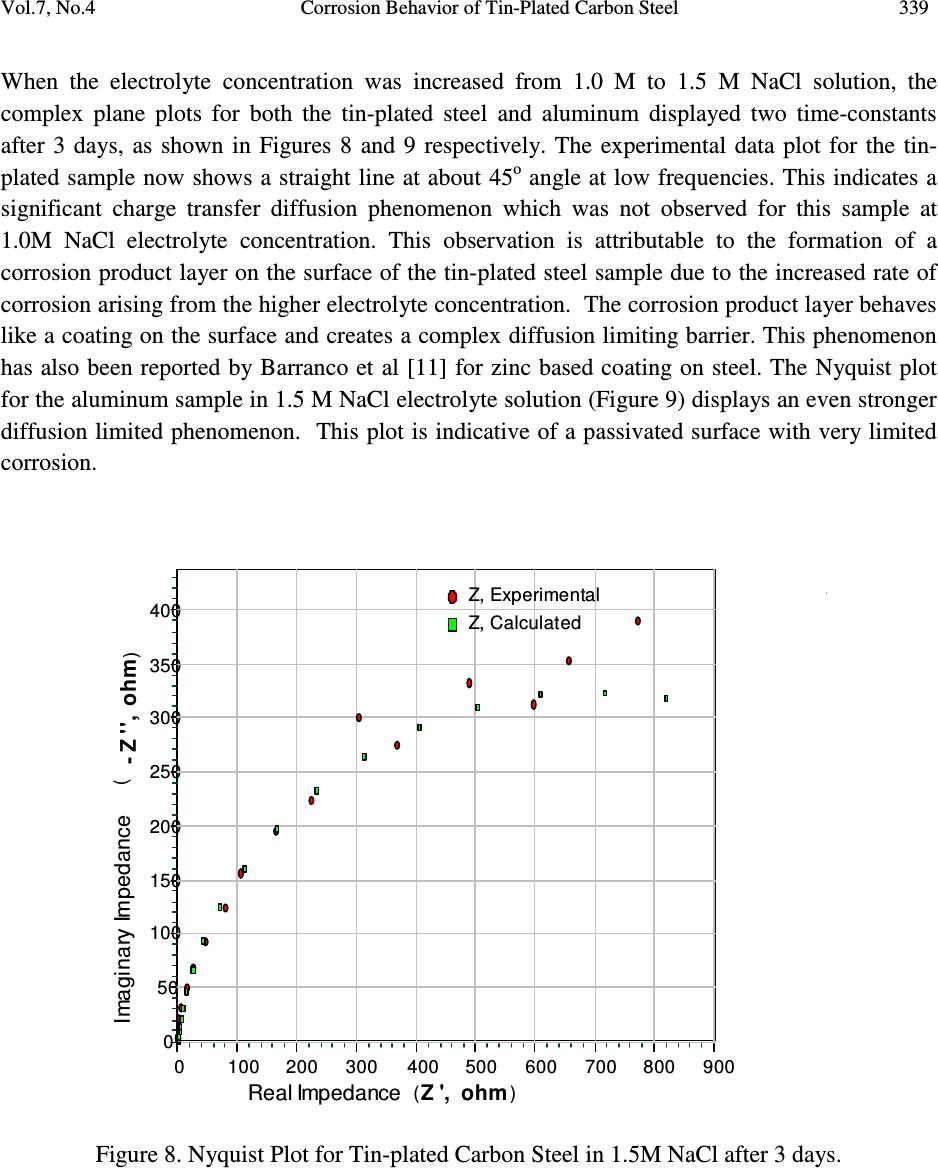 Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 339 When the electrolyte concentration was increased from 1.0 M to 1.5 M NaCl solution, the complex plane plots for both the tin-plated steel and aluminum displayed two time-constants after 3 days, as shown in Figures 8 and 9 respectively. The experimental data plot for the tin- plated sample now shows a straight line at about 45 o angle at low frequencies. This indicates a significant charge transfer diffusion phenomenon which was not observed for this sample at 1.0M NaCl electrolyte concentration. This observation is attributable to the formation of a corrosion product layer on the surface of the tin-plated steel sample due to the increased rate of corrosion arising from the higher electrolyte concentration. The corrosion product layer behaves like a coating on the surface and creates a complex diffusion limiting barrier. This phenomenon has also been reported by Barranco et al [11] for zinc based coating on steel. The Nyquist plot for the aluminum sample in 1.5 M NaCl electrolyte solution (Figure 9) displays an even stronger diffusion limited phenomenon. This plot is indicative of a passivated surface with very limited corrosion. Z , Msd. Z , Calc. u nk no wn.t x t Model : R(Q(R(CR))) Wgt : Modulus Z ', ohm 9 008 007 006 0050 04 003 0 020 010 00 - Z '', ohm 40 0 35 0 30 0 25 0 20 0 15 0 10 0 50 0 Z, Experimental Z, Calculated Real Impedance ( ) Imaginary Impedance ( ) Figure 8. Nyquist Plot for Tin-plated Carbon Steel in 1.5M NaCl after 3 days.  340 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 Z , Msd. Z , Calc. unknow n.tx t Model : R(Q(R(CR))) Wgt : Modulus Z ', ohm 10,0008,0006,0004,0002,0000 - Z '', ohm 5,500 5,000 4,500 4,000 3,500 3,000 2,500 2,000 1,500 1,000 500 0 Z, Experimental Z, Calculated Imaginary Impedance Real Impedance() ( ) Figure 9. Nyquist Plot for Aluminum-1100 in 1.5 M NaCl after 3 days. Based on the experimental corrosion data discussed above, equivalent electrical circuit (EEC) diagrams were generated for the tin-plated steel and aluminum samples as shown in Figures 10 and 11 respectively in 1.5 M NaCl solution after 3 days. Both circuits represent two time- constants with a characteristic constant phase element (CPE) as discussed earlier, but represented by Q on each circuit. In each circuit, R s is the solution resistance, R ct is charge transfer resistance, R p is the polarization resistance, C dl is the double layer capacitance, and C ol is the capacitance due to the oxide layer on the aluminum sample. The EEC for the aluminum sample indicates two capacitances due to the electrical double layers on the metal surface and on the passivating oxide film in addition to a diffusion resistance factor Q. The EEC for the tin-coated steel also displays two time constants occasioned principally by the corrosion product layer which gives rise to the constant phase element represented by Q. The validity of these EEC diagrams was tested by using them to calculating the frequency response of the real and imaginary magnitudes of the impedance. These numbers were plotted against the observed experimental values as shown in the rectangular points in Figures 6 – 9. In all cases, a reasonable fit is observed between the experimental and calculated values, except for the extreme low frequency regions of Figures 7 and 8. While the EEC diagrams may not exactly represent all of the physical phenomena occurring during corrosion, it is a reasonable approximation of the surface corrosion phenomena.  Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 341 C dl = capacitance of the double layer R ct = Charge transfer resistance R p = Polarization resistance R s = Solution resistance Q = Constant Phase Element (CPE) Figure 10. Equivalent Electrical Circuit Model of Tin plated carbon steel in 1.5M of NaCl. Figure 11. Equivalent Electrical Circuit Model of Aluminum in 1.5M of NaCl.  342 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 The corrosion currents measured during the experimental work were used to calculate the corrosion rates using Equation 4. A plot of the corrosion rate versus time for the 1.0 M and 1.5 M electrolyte solutions is shown in Figure 12 for the tin-plated samples. The experimental data are plotted as the thin grey lines in the figure. The figure for the 1.0 M solution (Figure 12A) shows an initial corrosion rate of about 5.5 mills per year (mpy) which declines continuously until about the 5 th day when steady-state corrosion was observed at about 1.5 mpy. In contrast, the 1.5 M solution sample (Fig. 12B) showed a much higher initial corrosion of over 100 mpy, declining to a steady state rate of slightly above 50 mpy after about 12 days. Similarly, Figures 13A and 13B present the corrosion rate versus time curves for the aluminum-1100 sample at 1.0 M and 1.5 M NaCl solutions respectively. The aluminum sample in 1.0 M NaCl electrolyte solution shows an initial corrosion rate of 2.5 mpy and declines over the next 10 days until a steady-state corrosion rate of less than 0.1 mpy is observed. This low corrosion rate is indicative of a highly passivated surface for which aluminum is a typical example. The 1.5 M NaCl electrolyte for the aluminum showed a slightly higher initial corrosion rate of 3.8 mpy as compared to the lower concentration electrolyte. The corrosion rate continued to decline throughout the 14-day experimental period, thus indicating significant passivation even for the higher electrolyte solution. At the end of the 14 days of experimental work, the corrosion rate had declined to about 0.5 mpy. Subsequent measurements beyond the 14 days of experimentation revealed a steady-state corrosion rate of about 0.3 mpy which is only slightly higher that the rate for the alumionum sample studied at 1.0 M NaCl solution. In order to develop a mathematical model for the corrosion rates of these samples under the conditions studied, the corrosion rate versus time data were fed into the analytical and plotting tools software of MATHLAB. The mathematical models derived from MATHLAB were used to plot the various corrosion rates versus time curves as shown in the dark solid lines in Figures 12 and 13. All the mathematical models showed good fit between the experimental data and the model. The various mathematical models are shown in Equations 6 – 9. Tin-plated in 1.0M Nacl: Y = 0.00062x 4 – 0.024x 3 + 0.33x 2 –2x+5.7 (6) Al 1100 1.0 NaCl: Y=0.000015x 6 -0.0008x 5 +0.016x 4 -0.15x 3 +0.68x 2 –1.4x+2.5 (7) Tin plated in 1.5M : Y = 0.00019x 5 +0.013x 4 -0.31x 3 + 3.6x 2 –21x+100 (8) Al 1100 in 1.5M : Y = 0.00013x 4 – 0.0054x 3 + 0.086x 2 –0.74x+3.9 (9) These equations will be useful in predicting the corrosion rates of tin-plated steel and aluminum- 1100 in salt water systems.  Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 343 0 2 46 8101214 1 1 .5 2 2 .5 3 3 .5 4 4 .5 5 5 .5 6 Corrosion Rate vs.Time Time (days) Corrosion Rate (mpy) y = 0.00062*x4 - 0.024*x3 + 0.33*x2 - 2*x + 5.7 Actual Data Fit Time (Days) Corrosion Rate (mpy) (A) 0 2 4 6 8101214 50 60 70 80 90 100 110 Time (days) Corrosion Rate (mpy) Corrosion Rate vs.Time y = - 0.00019*x 5 + 0.013*x 4 - 0.31*x 3 + 3.6*x 2 - 21*x + 1e+002 Actual Data Fit (B) Figure 12. Corrosion Rate vs. Time Curves for Tin-Plated Steel in (A) 1.0 M and (B) 1.5 M NaCl Electrolyte.  344 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 02 468101214 0. 5 1 1. 5 2 2. 5 3 3. 5 4 Corrosion Rate vs.Time Time (days) Corrosion Rate (mpy) y = 0.00013*x 4 - 0.0054*x 3 + 0.086*x 2 - 0.74*x + 3.9 Actual Data Fit Actual Data Fit (A) 02 4 6 8101214 0.5 1 1.5 2 2.5 3 3.5 4 Corrosion Rate vs.Time Time (days) Corrosion Rate (mpy) y = 0.00013*x 4 - 0.0054*x 3 + 0.086*x 2 - 0.74*x + 3.9 Actual Data Fit (B ) Figure 13. Corrosion Rate vs. Time Curves for Aluminum-1100 in (A) 1.0 M and (B) 1.5 M NaCl Electrolyte.  Vol.7, No.4 Corrosion Behavior of Tin-Plated Carbon Steel 345 5. CONCLUSIONS An investigation of the corrosion of tin-plated steel and aluminum-1100 in NaCl solutions by electrochemical impedance spectroscopy has revealed that while a one time-constant phenomenon is observed for the corrosion of tin-plated steel in 1.0 M NaCl solution, a two time- constant phenomenon is observed for the same sample at 1.5 M NaCl electrolyte solution after 3 days of corrosion. The occurrence of the two time-constants is attributable to charge transfer diffusion limitation arising from the formation of a corrosion product layer on the surface of the sample. In contrast, for the aluminum sample, two-time constants were observed for both the 1.0 M and 1.5 M NaCl solutions after 3 days. This is attributable to the effective surface passivation of aluminum by the formation of a stable oxide layer. The rates of corrosion of the aluminum samples were found to be consistently much lower than those of the tin-plated steel samples at the NaCl electrolyte concentrations studied. Finally, the corrosion rate versus time data were used to derive mathematical corrosion models for the two samples that may be useful for predicting the corrosion behavior of these samples under NaCl or salt water solutions. References [1] Cottis, R. and Turgoose, S.. 1999. Electrochemical Impedance and Noise. Corrosion Testing Made Easy, (Syrett, B.C, Series Editor), NACE International, Houston, TX, 1999. [2] Nigam, A.K., Balasubramaniam, R., Bhargava, S. and Baligidad, R.G. 2006. Electrochemical Impedance Spectroscopy and Cyclic Voltametry Study of carbon-Alloyed Iron Aluminides in Sulfuric Acid. Corrosion Science , 48, 1666-1678. [3] J. Suay, E. Gimenez, T. Rodriguez, K.Habbib and J.J. Saura. 2003. Characterization of anodized and sealed aluminum by EIS. Corrosion Science , 45, 611-624. [4]. P. Yadav, A. Nishikata and T. Tsuru. 2004. Electrochemical impedance study on galvanized steel corrosion under cyclic wet-dry conditions-influence of time of wetness, Corrosion Science , 46. 169-181. [5]. Soprano, M., Bennardo, A., and Gabetta, G. (1998). EIS Measurements on Burried Pipelines Cathodically Protected. Corrosion 98, paper #618, NACE International, Houston, TX., pp. 618/1-618/14. [6]. Buchheit, R.G., Montes, L.P., Maestas, L.M., and Hinkebein, T.E. (1998). Corrosion Monitoring of Concrete-Lined Brine Service pipelines Using AC and DC Electrochemical Methods. Corrosion 98, paper #81, NACE International, Houston, TX., pp. 81/1-81/12. [7]. Wake, R. and Ezure, K (1991). Thinly Tin Coated Steel Sheets Having Excellent rust Resistance and weldability. US Patent # 4999258.  346 B.O. Oni, N.O. Egiebor, N.J. Ekekwe, and A. Chuku Vol.7, No.4 [8]. Odegard, C. and Bronson, A. (1998). Evaluation of Pitting Corrosion with EIS for Alumina/Aluminum alloy Composites. Corrosion 98, paper #319, NACE International, Houston, TX., pp. 319/1-319/16. [9]. Umebayashi, R., Akao, N., Hara, N. and Sugomoto, K. (2003). Corrosion and Electrochemical Properties of Sn-8% Zn Alloy-Coated Steel in Methanol Containing Water, NaCl, and HCOOH. Journal of the Electrochemical Society , 150 (7), B295-B305. [10]. Masadeh, S. (2005). Electrochemical impedance spectroscopy of Epoxy-Coated Steel exposed to dead Sea Water. Journal of Minerals & Materials Characterization & Engineering , 4 (2), 75-84. [11] Barranco, V., Feliu, S. Jr., and Feliu, S. (2004). EIS Study of the Corrosion Behaviour of Zinc-based Coatings on Steel in Quiescent 3% Nacl Solution. Part 1: Directly Exposed Coatings. Corrosion Science , 46, 2203-2220. [12]. Tait, W.S. (1994). An Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists, PairODocs Publishers, Racine, Wisconsin 1994. |

