Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 6, No.1, pp 17-24, 2007 jmmce.org Printed in the USA. All rights reserved 17 Crystal Growth of Some Rena l S tones Constituents: I. In vitro Crystallization of T ra c e E l e m en t an d It s Ch a r ac t e r ization Studies P. Sundaramoorthi and S. Kalainathan* Department of physics, Mahendra Engg College, Mallasamuduram, Namakkal, TamilNadu, India-637503. (sundara78@rediffmail.com) *Department of Physics, Vellore Institute of Technology, Deemed University Vellore, India. Abstract The urinary calculi formation is still now unknown problems and seems to be very complex phenomenon. The in vitro studies are carried out by artificial renal stone formation in silica gel medium. In general, it appeared that trace element (TE) contents in mixed structure are always between values of respective pure phases. It is also found that some of the toxic elements such as barium are related to the structure but not necessarily to environment influences as in the case of lead. Barium hydrogen phosphate crystals are grown in gel method then identified the optimum growth parameters. TE mixed crystals are grown in different growth parameters. The grown crystals are analyzed by XRD, TGA/DTA, and SEM.The results are recorded and reported. Key words- Renal stones, TE-BHP, renal calculi, gel growth, Surface morphology, Growth parameters, trace elements, SDM, DDM. 1. INTRODUCTION A trace element (TE) with the organic, semi organic and organic mixed mineral deposition in urinary tract is less frequent in human history. Strontium hydrogen phosphate stones account for less than 5% of all urinary stones [1], but occur in individuals with rare genetic disorder in a renal tubular transport system. The defective absorption of organic and inorganic minerals in renal tubular system called Fanconi syndromes is believed to exist in associated with the mineral deposition in the renal systems. Renal stone (RS) diseases are due to biomineralisation. RS grows in a gel like medium, which is why they have radially striated growth [2]. The crystal growth by gel methods, provide to simulation of synovial cartilage and biological fluids [3]. The gel growth [in vitro study] of some urinary stone constituents COM, COD, CHP, HAP and the inhibitory role are reported [4]. Still there is no such an exact evidence to grow the trace element mixed RS [in vitro and in-vivo] growth. These types of TE-RS are in less percentage available within the urinary tract deposition. However, the TE-RS types produce major toxic effect in the urinary tract and induce human pathological causes. In 18 P. Sundaramoorthi and S. Kalainathan Vol.6, No.1 BHP crystal, barium is a toxic element, which may be absorbed in three ways. One is via air- by breathing. The average consumption rate is one microgram per day. The second is via liquid -by liquid /water in take. The average consumption rate was 1-20 micrograms per liter. The third one is via solid-by food intake. The average consumption of barium present is 600-900 micrograms per day. For a normal man, the barium consumption bar limit is 1240 micrograms per day, which is the tolerance level, if exceeds the specific tolerance level of human physical system, automatic RS deposition occurs. Authors have grown different RS crystals in several series of experiments with silica gel at different pH values. The dissociation of phosphoric acid system can be represented by three- dissociation equilibrium and the presence of various ions at various pH values are reported [5]. Because of these results, the gel pH in the range from 5.5 to 11 has been used (Milwaukee QS-MN pH-600, packet digital pH-meter are used for measurements) in which the HPO42- ions dominates or alone exist. This decreases the possibility of BMP crystals occurring during the BHP growth. 2 EXPERIMENTAL STUDY (CRYSTALLIZATION) The crystallization apparatus employed are glass test tube of 25 mm diameter and 150 mm long for single diffusion method (SD) and. thick walled 30 mm diameter and 180mm long glass U tubes for double diffusion (DD) method. The chemicals used are Excelar- Qualigens (E-Q) AR grade BaCl2 (MW-244.28) and E-Q, AR grade orthophosphoric acid (Sp.gr.1.75). The SMS gel or water glasses are prepared as per the literature [6] .The reactant orthophosphoric acid is mixed with silica gel at desired gel density and elevated temperatures. After the gel set, the supernatant mixture (Barium chloride) at a required mole solutions are slowly added along the walls of the growth columns (test tubes, U- tubes) over the set gels, then tightly closed to prevent evaporation. The growth systems are allowed to react within the gel medium and the following chemical reaction takes place. BaCl2 + H3PO4 + gel Æ BaHPO4 + by products. The crystal growth experiments are carried out at room temperature (average of 290C. 3. RESULT AND DISCUSSION 3.1 The following observations have been made in the present work. 1. The reaction starts immediately after the addition of supernatant solutions. However, the nucleation is observed only after 14 hours and the growth process took a period of two to three months for completion. 2. Leaf shape crystals, fiber and dendrites are observed along with Lies gang rings in the first half of the gel column from the top of the test tube.  Vol.6, No.1 Crystal Growth of Some Renal Stones 19 3. Some well-developed single crystals are observed in the DD growth columns (U-tubes) after one month. The growth processes are allowed for six months but the crystal size did not increase. 4. The gel density above 1.07g/cm3 and with pH above 9 yielded no crystals, but mean time reaction takes place. 5. Some of the test tubes of gel density 1.03g/cm3 with pH around 7 and below 7 are allowed for the reactions to take place. After a period of six months, a less number of nucleation were observed near the bottom of the test tubes. Some of them grew in needle and well transparent platelet crystals. 6. From the investigation, the optimum growth parameters of BHP crystals are identified and reported in Table-1, 2. 7. An extension of the reaction period at up to ten months did not improve the size of these crystals. 8. The growth columns of BHP crystals are shown in the Fig-1, 2 9. Some of the harvested BHP crystals are shown in the Fig-3-4. 10. The maximum dimension of the crystals obtained is 3 mm x 5 mm x 6 mm. 3.2 X-ray studies of BHP crystals. The crystals of BHP are dried and used for analysis. X-ray diffractograms (XRD) are recorded using Siemens X-ray diffaractometer with CuKα.-Radiation (wave length 1.5418Å). The single crystal XRD of grown BHP matched well with the chemical crystals database. The lattice parameters are a=10.0115Å, b=10.2089Å, c=10.677210Å, α=90.0031˚, β= 90.0257 ˚, γ=90.0050˚. From this data, the BHP crystal system is triclinic [7, 8]. 3.3. Thermo gravimetric (TGA and DTA) analysis of BHP crystals. The TGA and DTA of BHP crystals are carried out by STA 11500-PLTS instruments. The BHP crystals of 2.439mg sample are taken to the TGA process. The TGA are started from room temperature to 10000 C by heating at a constant rate. Fig.5, shows the TGA and DTA graph of BHP crystals. The percentages of weight present in the BHP sample at a particular temperature are tabulated in Table- 3 [9]. The TGA results show BHP crystals are decomposed (67.9%of samples) at temperatures up to 453 0C due to the presence of hydrogen and phosphorus with oxygen atoms. Above the temperature, the remaining samples are stable up to the end of the analysis. The expected chemical reactions BaHPO4.XH2O ▲ BaHPO4 + X H2O (Vapour) 2BaPO4 ▲ 2 Ba + P2O4 (Vapour) Barium is a stable compound with respect to the temperature up to 1230 0C (melting point). About 67.9% of the BHP crystals by weight are evaporated and the remaining 32.1% of the samples are stable at temperatures up to 1000 0C. 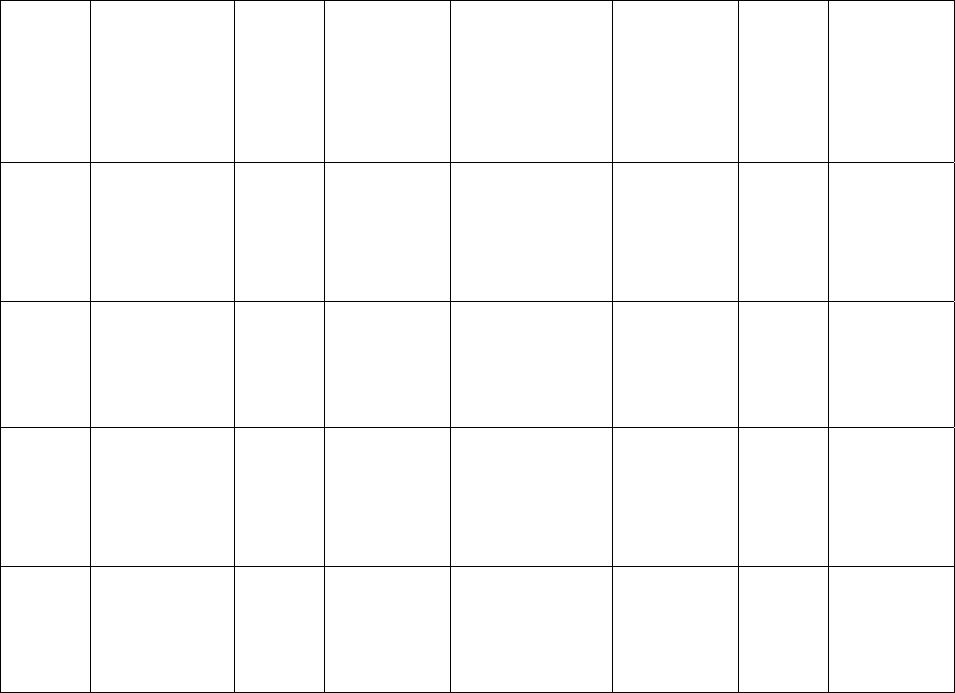 20 P. Sundaramoorthi and S. Kalainathan Vol.6, No.1 Table-1 Single diffusion (SD) growth process. (Reaction test tubes) Growth parameters. Gel density gm /cc3 Phosphoric acid concentration (Inner reactant mixed with gel) Gel+ HPO4 pH value Gel setting time Supernatant Concentration Bacl2 (M) Nucleation observed in hrs Growth period in days Growth appearances Inside the growth medium 1.03 1.03 1.03 1.03 1N 1N 1N 1N 6.4 6.8 6.9 7.1 26 hrs 16 hrs 6 mins. 18 hrs 1 -do- -do- -do- 16 hrs 17 hrs 32 hrs 89 hrs 136 SC, PC 1.03 1.03 1.03 1.03 1.5N 1.5N 1.5N 1.5N 6.6 6.7 7.1 8.0 28 hrs 1 hrs 3 hrs 46 hrs -do- -do- -do- -do- 26 hrs 16 hrs 46 hrs 66 hrs 90 SC 1.04 1.04 1.04 1.04 1 N 1 N 1 N 1 N 6.3 6.8 6.9 7.4 34 hrs 6 hrs 45 min 68 hrs -do- -do- -do- -do- 14 hrs 22 hrs 28 hrs 68 hrs 140 SC,PC 1.04 1.04 1.04 1.04 2N 2N 2N 2N 6.6 6.8 7.1 7.5 24 hrs 1 hour 12 hrs 48 hrs -do- -do- -do- -do- 13 hrs 14 hrs 24 hrs 72 hrs 75 SC SC- Good transparent single crystals are observed. PC-Poly crystals are observed. 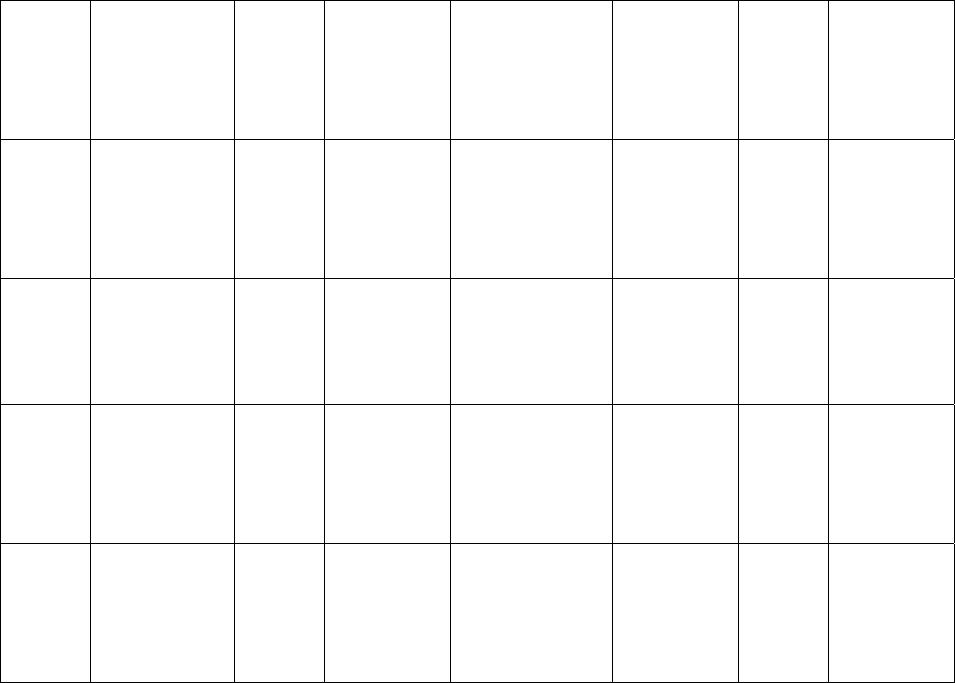 Vol.6, No.1 Crystal Growth of Some Renal Stones 21 Table-2. Double- diffusion (DD) growth process. (U- tubes) Growth parameters Gel density gm /cc3 Phosphoric acid concentration (Inner reactant mixed with gel) Gel+ HPO4 pH value Gel setting time Supernatant Concentration Bacl2 (M) Nucleation observed in hrs Growth period in days Growth appearances Inside the growth medium 1.05 1.05 1.05 1.05 1 N 1 N 1 N 1 N 6.0 6.7 6.9 7.1 48 hrs 16 hrs 15 Mts. 26 hrs 1 -do- -do- -do- 45 hrs 26 hrs 22 hrs 90 hrs 90 SC 1.05 1.05 1.05 1.05 2N 2N 2N 2N 6.4 6.9 7.2 8.1 36hrs 4 hrs 1 hrs 98 hrs -do- -do- -do- -do- 20 hrs 22 hrs 86 hrs 98 hrs 70 SC, PC 1.04 1.04 1.04 1.04 1 N 1 N 1 N 1 N 6.2 6.8 7.2 7.6 46 hrs 5 hrs 30 mts 28 hrs -do- -do- -do- -do- 40 hrs 22 hrs 64 hrs 88 hrs 110 SC 1.04 1.04 1.04 1.04 2N 2N 2N 2N 6.2 6.9 7.5 7.9 88 hrs 1 hour 10 hrs 58 hrs -do- -do- -do- -do- 20 hrs 15 hrs 32 hrs 82 hrs 90 SC, PC SC-good transparent single crystals are observed. PC-Poly crystals are observed 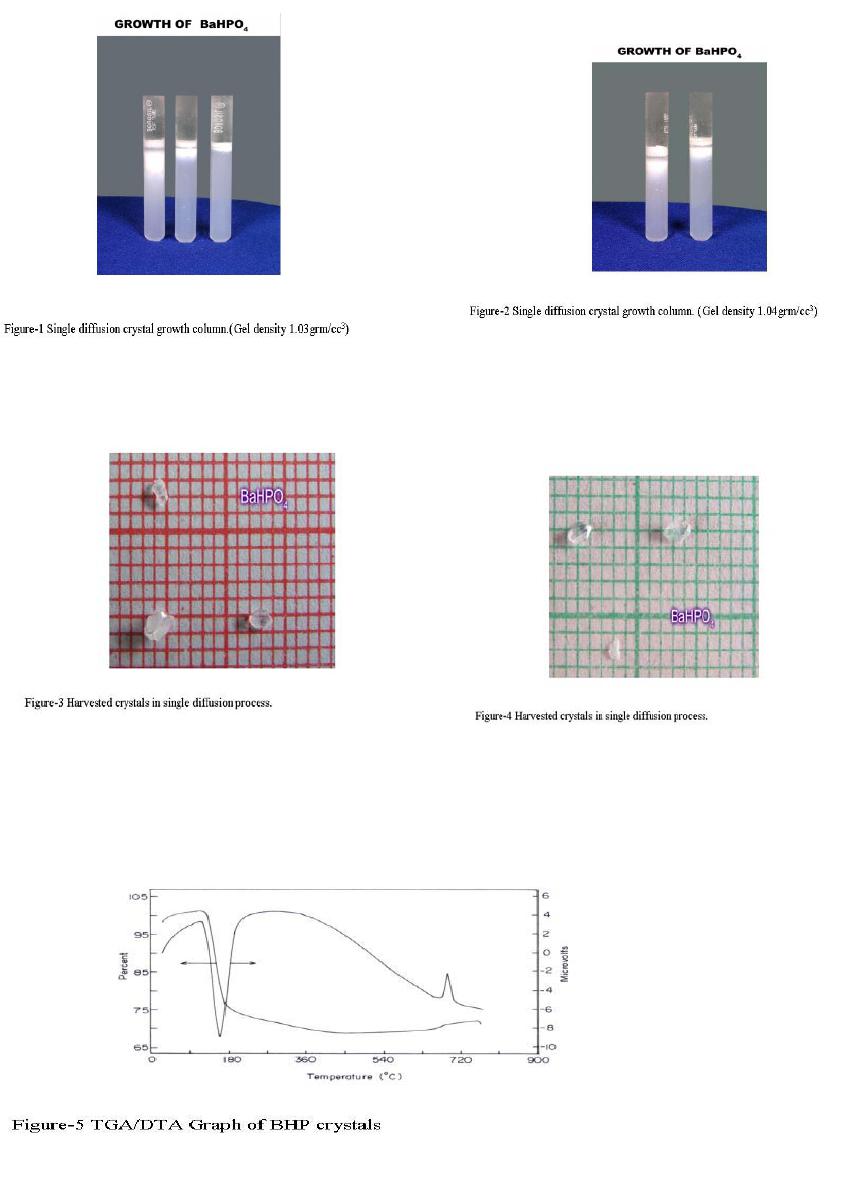 22 P. Sundaramoorthi and S. Kalainathan Vol.6, No.1 . 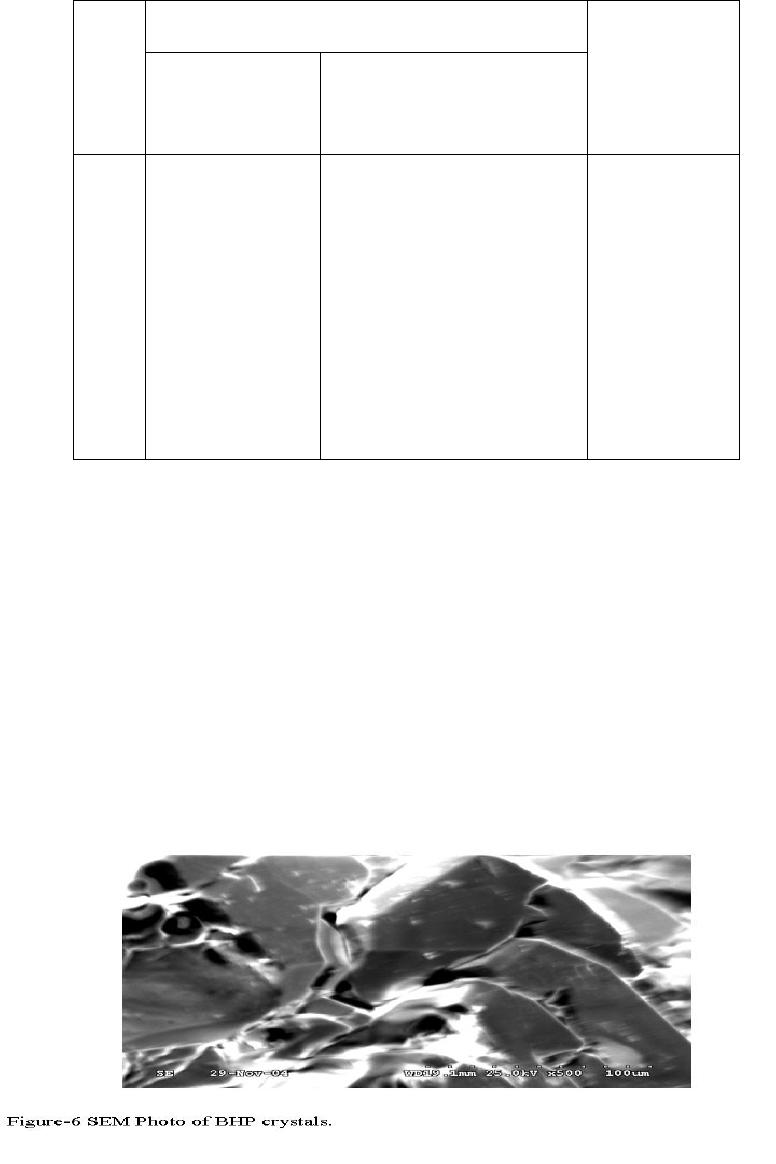 Vol.6, No.1 Crystal Growth of Some Renal Stones 23 Table 3. Thermal Analysis of BHP crystals TGA Point s Temperature º C % Of BHP crystal present DTA in 0C 1 2 3 4 5 35 131.50 199.76 452.24 850 100 101.24 74.95 67.941 67.9 128.47 182.53 249.77 669.10 691.28 3.4. Scanning Electron Microscopy study of BHP crystals Well-grown BHP single crystals are selected for the investigation of surface morphology crystal by using SEM. The SEM photographs are taken in the version S-300-I instrument. The sample named as VCA-600 kept in lobe middle; the data size are 640 x 480 micrometer. The minor (minimum) and major (maximum) magnification of SEM was about 250 times. SEM acceleration voltage is 25000 volts and kept the sample in highly vacuumed. 18200-micrometer working distance and monochromatic color mode are employed. 100µmeter focusing of BHP crystal SEM is shown in the Fig-6. The surfaces of the BHP crystal are smooth, few valley patterns are observed in the crystal plane [10- 13]. 24 P. Sundaramoorthi and S. Kalainathan Vol.6, No.1 Conclusion The BHP RS constituents are crystallized by single and double diffusion methods in sodium meta silicate gel. The single crystal XRD studies confirmed the structural identity of grown BHP single crystal. The TGA/DTA studies are carried out, and then calculated the losses of weight of the BHP crystals versus temperature up to 9000C. The BHP surface morphology is identified by SEM studies. References [1] Richard.W.Norman,in 74th annual refresher course in practical aspect in the medical management of urinary stone diseases, Dalhosic University, November 2000. [2] Iwata.H.,Abe.Y.,Nishin.S.,Wakatsuki.A.,Ochi.KandTakeuchi.M., J,Urol,135,(1986) 397. [3] Archilles.W.,Freltag.R.,Kiss.B, and Riedmiller.H.,J.Urol154,(1995) 1552. [4] Natrajan.S.,Ramachendran.E and Blisin suja.D.,Cryt.Res.Technol.32 (1997) 551. [5] Pecsok.R.L., Shields.L.D., Cairns.T., McWillian.I.G., Modern methods of chemical analysis, John Willy&Sons Inc.,Newyork (1976) 438-442. [6] Henisch.H.K., Crystals in Gel and Liesegang Rings, Cambridge University press, Cambridge (1986) 11. [7]. Machennan.G., Reevers.C.A., Acta crystals, 8 (1955) 579. [8]. Curry.N.A., Jones.D.W., J. Chem. Soc. A (1971) 3725. [9].Matsuzaki.S.,Matsushita.K.,Tanykawa.K.,.Masuda.A.,and Matsunaga.F.,Ind.J.Urol.2 (1965) 235-237. [10].Taukamot.K., J. Cry.Growth, 61 (1983) 199. [11]. Gates.H.C., Thirty years of progress in Surface Science, in: Crystal growth and characterization (North Holland, 1975) [12].Bethage.H., etal., Electron Microscopy in Solid State Physics (Elsiever, Amsterdam, 1987) [13]. Albon etal.N., in: Growth and Perfection of Crystals (Wiley, New York 1958) 446 |

