Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.2, pp 105-108, 2004 jmmce.org Printed in the USA. All rights reserved 105 An Investigation Into T he Sint e ring Of Magnesium Fluoride Optical Material By Microwave Shangzhao Shi, Jiann-Yang Hwang, Bowen Li, Xiaodi Huang Michigan Technological University Houghton, MI 49931 Abstract: The presented work was an investigation on the sintering of magnesium fluoride with microwaves. The sintering was conducted in a 2.45-GHz microwave applicator under an argon atmosphere. Sintering shrinkage and density were measured. The microstructure of the sintered samples was examined. Feasibility and advantages regarding microwave sintering of magnesium fluoride were discussed. Introduction Magnesium fluoride is an optical material utilized in fabrication of infrared transmission windows. The conventional techniques to produce magnesium fluoride ceramics include single crystal growth1, pressureless sintering2, hot pressing3 as well as hot isostatic pressing4. Microwave sintering is a volumetric and fast densification technique, and has demonstrated many advantages in ceramics sintering. This method, however, has not been attempted in the sintering of Magnesium fluoride. The presented study is the first reported attempt of microwave sintering of the MgF2 ceramics. Experimen tal As-received Mg F2 powder was calcinated in argon at 600°C for 2 hours and then ground with a mortar to pass a 325-mesh screen. Th e ground powder w as mixed with 2% gum as binder. Distilled water of appropriate amount was added to the binder to develop strength. Uniaxial compacting was performed with a steel die of 0.5 inch in diamet er, and the compaction pressure was 5000 psi. The disk dimensions were φ½” × ¼”. Microwave sinteri ng was performed with a 4-kW microwav e furnace. M gF2 disks were placed at the center of the hot chamber surrounded by SiC susceptors. Zirconia beads were placed on the bottom of the chamber, in order to avoid possible reactions of the refractory insulation with the specimen as well as the SiC susceptor. A k-type thermocouple was used to measure the chamber temperature. The distance from the specimen top to the thermocouple tip was ½”. The hot chamber of the furnace was airtight, and had ports to connect a vacuum pump and an argon cylinder. For each run, the chamber was vacuumed and then filled with argon to reach atmospheric pressure. The procedure of vacuuming/argon-filling was repeated several times to ensure complete removal of oxygen. 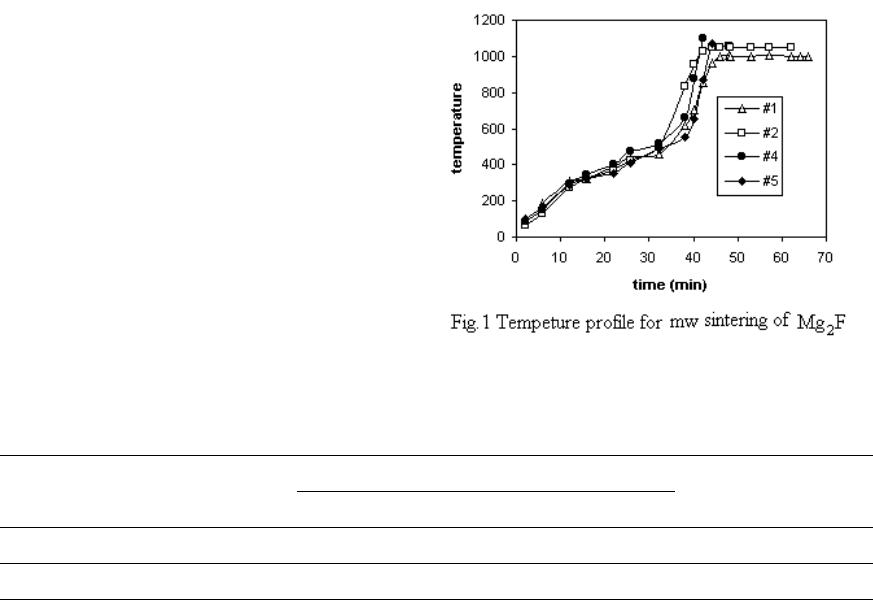 106 Shangzhao Shi, Jiann-Yang Hwang, Bowen Li, Xiaodi Huang Vol. 3, No.2 The sintering temper ature profi le was determined with the consideration of binder decomposition, which may otherwise result in collapsing, cracking or other problems. The temperature was controlled with the controller, which is capable of continuously adjusting the microwave power intensity. The specimen dimensions were measured with a caliper before and after sintering. Linear shrinkage was calculated by comparing of the data. The sintered specimens were weighed. Densities were calculated based on the weight and volume of the sintered specimens. SEM was employed to examine the microstructures. Results and Discussion Fig.1 shows the sintering temperature profile. The shrinkage and density of #1 and #2 specimens are given in Table 1. Compared to the theoretical density (3.18 g.cm-3), the densities of #1 and #2 specimens are substantially low. Sintering at 1100°C (#4) and 1075°C (#5) was therefore attempted in order to improve the density. However, the sintering behavior was so sensitive to temperature, that these two specimens were partially melted even though no holding time was used. Table 1 Shrinkage (%) specimen sSintering conditions Heightdiameter Density (g.cm-3) #1 1000°C×20min -9.79 -9.702.22 #2 1050°C×20min -11.11 -11.472.34 Figure 2 shows the SEM images taken from the MgF2 samples sintered at different temperatures and holding times. Image a and b were taken from sample #1 and #2, respectively. The images indicate that the higher degree of sintering and denser microstructures were achieved when sintering was performed at 1050°C for 20min. The SEM observation is in good agreement with the shrinkage and density measurement as shown in Table 1. Compared to Image a, Image b features straight grain boundari es, less porosity and substantial grain growth. The transgranular cracking indicates the strong bonding between grains had been developed.  Vol.3, No.2 Investigation of Sintering of Magnesiu m Fluoride Op t ical M aterial b y Microwave 107 Image c and d were taken form simple #4 and #5, respectively. They indicate substantial liquids formed in sintering at these temperatures. Grains in d have almost lost their shape and merged into a shapeless matrix. Although there are a few distinguishable grains, their shape chan ged i nto spheri cal. Im age c reve als a number o f cr aters distribut ed in the liquid matrix. It indicates gaseous species formed during sintering at 1100°C. There are also rod-like pa rticles, one of which is shown in higher magnification in Fig.3. Their formation is believed to involve with an evaporation-condensation process. Conclusions Sintering of magnesium fluoride is difficult. We have searched several well- documented literature databases for microwave sintering of magnesium fluoride, but have found no publication dealing with this subject. Even for conventional sintering, very few references can be found dealing with this subject. It seems that successful microwave sintering of pure MgF2 requires delicate sintering conditions, which needs extensive research.  108 Shangzhao Shi, Jiann-Yang Hwang, Bowen Li, Xiaodi Huang Vol. 3, No.2 The result from this study suggests that the optimum sintering temperature would be 1050°C. Lowering the sintering temperature would not produce a densified microstructure. Increase the sintering temperature would result in melting. At the optimum sintering temperature, a prolonged holding period seems necessary for higher density. However, structural coarsening appears significant. In order to obtain a fine microstructure while achieving full densification, an additional densification method, such as hot pressing, needs to be added. Reference 1. Recker, Kurt; Leckebusch, R.; “Vapor phase growth of single crystals of high-melting fluorides. I. Magnesium fluoride”; Journal of Crystal Growth, (1969), 5(2), 125-31. 2. Rice, Hal H.; Garey, Maurice J.; “Sintering of magnesium fluoride”; American Ceramic Society Bulletin, (1967), 46(12), 1149-53. 3. Mal'tsev, M. V.; Udalova, L. V.; Goryachev, A. Ya.; Levina, N. K.; Perminova, N. B.; “Fabrication of optical-ceramic preforms without mechanical treatments”; Opticheskii Zhurnal, (1993), (1), 69-72. 4. Shirakawa, Youichi; Harada, Tamotsu; Sashida, Norikazu; Miyata, Noboru; “Preparation of MgF2 sintered body by normal sintering combined with capsule-free hot-isostatic pressing treatment”, Nippon Seramikkusu Kyokai Gakujutsu Ronbunshi/Journal of the Ceramic Society of Japan, v 107, n 1252, Dec, 1999, p 1137-1139. |

