Paper Menu >>
Journal Menu >>
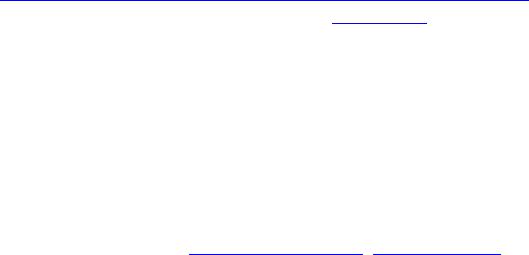 Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.2, pp 91-98, 2004 jmmce.org Printed in the USA. All rights reserved 91 Melt Infi ltration Casting Of Zr57Al10Nb5Cu15.4Ni12.6 Amorphous Matrix Composite K.Q. Qiu and Y.L. Ren School of Materials Science and Engineering, Shenyang University of Technology, 58 South Xinghua Street, Shenyang 110023, China. E-mail: kqqiu@yahoo.com.cn, ghc@sut.edu.cn Tel.: 86-24-25691315; Fax: 86-24-25694028 Abstract: The Zr57Al10Nb5Cu15.4Ni12.6 bulk metallic glass-forming alloy is reinforced with tungsten fiber. Cylindrical samples up to 6.3 mm in diameter and 7cm in length have been synthesized using melt infiltration casting method. The structure and microstructure of the composites processed in typical conditions were analyzed by x-ray diffraction and scanning electron microscopy. The suitable interface reaction and fully amorphous matrix can only be obtained through the combination of a suitable infiltration temperature and time. The processing conditions were more rigorous compared to those of making Zr-Ti-Ni-Cu-Be matrix composite due to intermetallic compound nucleation when exposed to the tungsten fibers. A technique map, including infiltrating temperature and time is presented in this paper. Keywords: melt infiltration casting composite bulk metallic glass 1. Introduction The high glass-forming ability and high thermal stability against cr ystallization of recentl y developed glassy alloys [1-3] allow their use for fundamental investigations and practical applications. The development of bulk metallic glass matrix composites especiall y opens new opportunities for application of metallic glass. The multicomponent Zr57Al10Nb5Cu15.4Ni12.6 (Vit106) is known to be one of the best glass-formers with a critical cooling rate of ~10Ks-1[4]. Such a glassy alloy has promising properties such as high yield strength and high elastic strain limit combined with relatively high fracture toughness, fatigue, and corrosion resistance [5,6]. However, it has almost no ductility in tension or compression. The lack of ductility could be a serious drawback in many applications. Thus, one of the motivations for adding second phase particles or continuous metal fibers to the metallic glass is to hinder propagation of shear bands and encourage the formation of multiple shear bands, which can increase plastic deformation of bulk metallic glasses. Many researchers [7-10] have focused on the processing of fiber or particulate reinforced composites with a metallic glass matrix to improve the mechanical properties. It was reported that bulk metallic glass Zr-Ti-Ni-Cu-Be [7] and Zr-Nb-Al-Ni-Cu [8] alloys reinforced with a ductile metal (W, Ta or steel) fiber or ceramics (SiC, WC, or TiC) have improved their mechanical properties. The Inoue group has successfully synthesized ZrC particle reinforced Zr55Al10Ni5Cu30 composites by in- situ reaction [11] or by adding SiC particle to Cu47Ti34Zr11Ni 8 metallic glass [12]. In this 92 K.Q.Qiu and Y.L.RenVol. 3, No.2 paper, the techniques for melt infiltration casting of Zr57Al10Nb5Cu15.4Ni12.6 alloy and the influence of interface reaction on fabrication of the composites are investigated. When the second reinforced medium is dispersed in the glassy matrix, there is a high risk of partial crystallization at the interface between the glassy matrix and reinforcing phase. The important factors in preparation of above-mentioned composite materials, one can list a high glass-forming ability of the glassy matrix, low reactivity, small difference in thermal expansion coefficient and good wettability between reinforcement and matrix. If these factors are achieved and the second reinforcing phase is homogeneously dispersed without agglomeration or segregation in the glassy matrix, the reinforcement is likely to suppress the propagation of shear bands and might increase the toughness and fatigue resistance of the composi te materi als. Long fibe r reinfo rcement homogeneously distributed in the glassy matrix is a good method for that purpose. Tungsten fiber is used as reinforcement not only because it has limited reactivit y with the matrix material due to its high melting temperature but also the composite is a candidate material used as W-base kinetic energy penetrators due to its high density and self-sharpening behavior during dynamic deformation. In addition, the matrix alloy is a beryllium-free Zr-based glass former, which has no harmful to health. 2. Experimental In this study the Zr57Al10Nb5Cu15.4 Ni 12.6 alloy was selected as the matrix material. The master alloy was prepared by alloying together the constitutive elements in an arc furnace under a titanium-gettered argon atmosphere. The starting materials were high- purity (99.8% metals basis or better) research grade metals. The oxygen content of the starting material was in the range of 30~35ppm in weight percent. Tungsten fiber with a nominal diameter of 250 µm was used as t he reinforcement in the composite. The apparatus and methods used for the composite specimen fabrication are almost the same as the one described in the literature [7]. The reinforcement material was placed in the sealed end of a 6.3mm inner diameter quartz tube which was evacu ated to a pressure o f no more than 1×10-3 Pa. Then the sample tube was heated in a vertical resistive tube furnace with a temperature feedback controller. Two heating stages were used in the melt infiltration processing. One was the initial heating stage held at 1330±20°K, well above the liquidus temperature (1100°K) of the glass-forming alloy. The liquidus temperature was measured by the instrument of NETZSCH DSC 404°C at a heating rate of 20K/min. The sample was held at the initial heating temperature for 12 min. The temperature was then lowered to the second stage held at different temperature from 1150 to 1300°K according to preset value. When the furnace reach ed the preset temperature, a positive pressure of 260kPa purified argon was applied above the melt and held for different time ranging from 5~20min to allow infiltration of the molten matrix material into the reinforcement. Then the sample was quickly quenched in a supersaturated NaCl/H2O solution. 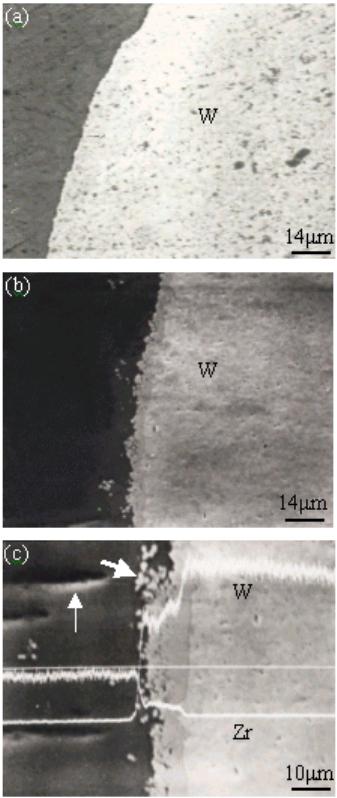 Vol. 3, No.2 Melt Infiltration Casting of Zr57Al10Nb5Cu15.4Ni12.6 Amorphous Matrix Composite93 The specimens were first ground to a 6mm diameter and then sliced normal to the fiber orientation. X-ray diffraction patterns of the slices were obtained using a RIGAKU D/max-rA diffractometer and Cu Kα radiation. The fiber/matrix interface in selected samples was examined by EPM-810Q scanning electron microscopy with electron microprobe. In this paper, we generally fabricated 6.3mm diameter samples with nominal reinforcement volume fractions of 20~30%, because the samples with higher volume fraction of wires the XRD patterns of the matrix pattern is obscured due to the large intensity difference between the Bragg peaks of the reinforcement and the broad amorphous bands. The nominal volume fraction of tungsten fiber is determined by overall cross-area of fibers divided by the internal cross-area of the quartz tube. Whether the fiber distribution is even or not does not affect the questions we concern. 3. Results 3.1 Temperature dependence of interface reaction Fig.1 shows SEM micrographs of interfacial region of the composite. The samples were held for 10min at temperature of 1150°K, 1225°K and 1300°K, respectively. The white part in each picture is tungsten fiber while the black one the matrix. At lower temperature of 1150°K, no interfacial reaction is seen as shown in Fig. 1 (a). Increasing the temperature to 1225°K, a reaction layer was formed around the tungsten wires and some white particles were eroded away from the wires. When the temperature was increased to 1300°K, the reaction layer becomes thicker and more particles eroded away from the wires. Fig. 2 shows typical x-ray diffraction patterns of two cross-sectioned composite samples with tungsten volume fraction of 25% as shown in Fig.1. For comparison, the pattern from an unreinforced sample produced at 1225°K is also included. The unreinforced matrix pattern shows broad diffraction peaks typical of a fully amorphous structure (Fig.1(a)). The same broad Figure 1. SEM micrograph of W fiber/ Zr57Al10Nb5Cu 15. 4Ni12. 6 composites processed at temperature of (a) 1150K, (b) 1225K and (c) 1300K, respectively, for the same in filtration time period of 10min. Lighter regions are W wires. Darker regions are matrix. Gray regions are interfacial reaction layers  94 K.Q.Qiu and Y.L.RenVol. 3, No.2 peaks are also visible in the pattern processed at temperature of 1225°K as shown in Fig.2(b). The difference is that the higher intensity of W diffraction peaks superimpose on them, but the intensity of amorphous peak is reduced for the one processed at high infiltration temperature of 1300°K as shown in Fig.2(c). This indicated that the quantity of the amorphous phase in the matrix is reduced even though we do not obviously observe the diffraction peaks of crystalline phases due to the high intensity of W phase or the quantity of crystalline phases is not high enough to show them. But we do observe some crystalline phases formed as indicated by a vertical arrow as shown in Fig1(c). The sample processed at 1150°K gives almost the same patterns as shown in Fig.1(b). From the line scans (Fig. 1c) the amount of tungsten in the reaction layer decreased and the zirconium increased compared with original composition. At temperature of 1300°K, the average amount of zirconium measured by electron microprobe in the white particles (marked with a declining arrow as shown in Fig. 1(c)) was 28.3at. %. The W content reduced to 54.70 at. %. The balance is Al (2.1 at. %), Cu (7.4 at. %), Ni (4.3 at. %) and Nb (3.2 at. %). This result constitutes the W-based alloy that is im possible t o be a glass form e r. The dissolved W did not precipitate as nearl y pure bcc W on cooling as in the case of Be-b earing alloy [13]. T he relativel y high Nb content in the eroded particles gives us an idea that it is easy to be disturbed into W based alloy. Away from the interface region or the eroded particle, the composition for the oth er parts (the black part) in the matrix was not changed significantly. Therefore not much more other crystalline phases observed on the XRD pattern as shown in Fig2(c). 3.2 Time dependence of infiltration The influence of the infiltration time at 1225°K was also studied. Fig.3 shows a sample that was held for 20 min at 1225°K. An interface reaction occurred just as the sample processed at temperature of 1300°K for 10 min. There is a little difference from W reinforced Zr55Al10Ni5Cu30 metallic glass matrix composites in which time is very important in controlling eroding and wetting process [14]. Here the time and temperature may play a similar role in controlling such a process. 20 3040 50 6070 80 σσ σ Intensity/a.u. 2θ; deg. (a) (b) (c) σ : W Cu Kα Figure 2. X-ray diffraction patterns for cross- sectioned sa mples of unreinforced bulk metallic glass (a) and tungsten reinforced bulk metallic glass matrix composites processed at temperature of 1225K (b) and 1300K(c) respectively Figure 3. SEM micrograph of metal matrix composite processed at temperature of 1225K for 20min 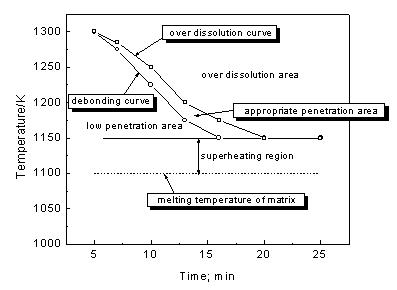 Vol. 3, No.2 Melt Infiltration Casting of Zr57Al10Nb5Cu15.4Ni12.6 Amorphous Matrix Composite95 3.3 Technique map for melt infiltration casting A batch of samples was made at different infiltrating temperature and time. Apart from the reinforcement, the overall crystallites in the matrix should not be above 5 vol.%, we consider this value as an upper threshold value for determining appropriate infiltrating temperature and time, above which interface reaction will cause intermetallic compounds formation just as the vertical arrow indicated in Fig.1(c). When the fibers are separated from the matrix of the sample during tension or not wetted completely by liquid metal [15], we consider the infiltrating temperature or time of this case as a lower threshold value, below which the binding between fibers and matrix is weak. Fig.4 shows the results of the experiments. A suitable reaction area was formed between the low wetting area and the over reaction area. It becomes narrow at higher temperatures and less time and wider at lower temperatures and over a longer time span. When the infiltrating temperature is below 1150°K, however, it is difficult to ensure melting to infiltrate the fibers, especially when the volume fraction of fiber is over 40%. This indicates that there is not enough flowing ability or infiltrating ability for the melt at such conditions. Therefore the lowest temperature and longest time for melt infiltrating are 1150°K and 20min, respectively. The narrowest superheating region of melt infiltration temperature is about 50°K. 4. Discussions To take advantage of the mechanical properties of the metallic glass in the composite, it is important to avoid crystallization of brittle intermetallics [13]. In fact, an interfmetallic such Zr2Cu is formed in the matrix as the vertical arrow indicated in the Fig.1(c) when interface reaction occurred. This intermetallic bar is normal to the interface. Therefore it is important to avoid interface reaction during processing. During the initial stage of processing, prior to infiltration, the alloy was preheated to a certain temperature required to remelt some residual crystalline particles present in the starting ingots. It was found that some inclusions were included and crystalline phases were formed in the matrix without such a sta ge. Dandliker [ 7] and Lin [16] found in other Zr-based bulk glass-formers that preheating a few hundred degrees above the melting temperature was necessary to achieve a larger undercooling for the melt. Preheating can cause the nucleation sites to be inactive and that nucleation is impossible for some Figure 4. Melt infiltratio n map for Zr57Al10Nb5Cu 15. 4Ni12. 6 bu lk metallic glass matrix composites 96 K.Q.Qiu and Y.L.RenVol. 3, No.2 impurities present in the melt. In Be-bearing bulk metallic glass composite, it was reported [13] that the atom of tungsten diffuses out from the wires over a rel atively narrow z one owing to an apparentl y very low diffusion constant. In a several micron thick layer near the glass/W-wire interface, the matrix is found to contain about 12 at. % W after being processed. This W precipitates as nearly pure bcc W on cooling and also forming a dispersion of W nanocrystals in the glass matrix. The glass forming ability of the matrix is otherwise unaffected. Those results are a little different from the case of what we know about Zr57Al10Nb5Cu15.4Ni12.6 metallic glass matrix composite. Considering that the W content in the reaction layer is decreased with the processing temperature increasing, we can therefore infe r that the element s, such as Zr, Al, Cu, Ni and Nb, diffuse into the tungsten wires forming thick layers due to their relatively high diffusion constants during W diffusing out of tungsten wires. The reaction layers or penetrated layers were W-based layers. When the W in the reaction layers reduced to a certain amount, the strength of grain boundaries of tungsten is too low to sustain its grains under thermal destruction. The grains were ver y much eroded away from the fibers forming W-rich particles which were shifted away from fibers with increasing processing time and temperature. The quantity of Zr and other elements in reaction layers were increased with temperature, therefore, the matrix composition, especially the composition in the reaction layers, was beyond the composition of glass former or the interf aces between the matrix and re action layers stimulated the formation of some crystalline nuclei, which grow directly into the matrix formed vertical arrays of intermetallic bars. 5. Conclusions Bulk Zr57Al10Nb5Cu15.4Ni12.6 metallic-glass matrix-composite up to 6.3mm in diameter and 7cm in length can successfully be fabricated by melt infiltration of the reinforcement of tungsten fibers. The suitable interface reaction and fully amorphous matrix can only be obtained through the combination of the suitable infiltration temperature and time. A technique map for p rocessing condition relating temperature and time is presented. The lowest temperature and longest time for melt infiltrating are 1150°K and 20min, respectively. The narrowest superheating region of melt infiltration temperature is about 50°K. Acknow l ed gments The financial support from the Shenyang University of Technology is greatly acknowledged. References 1. Inoue, A., Zhang, T., Nishiyama, N., Ohba, K., and Matsuda, T., 1993, Vol. 3, No.2 Melt Infiltration Casting of Zr57Al10Nb5Cu15.4Ni12.6 Amorphous Matrix Composite97 “Preparation of 16mm Diameter Rod of Amorphous Zr65Al7.5Ni10Cu17.5 Alloy”. Materials Transactions JIM. Vol. 34, No.12, pp.1234-1237. 2. Inoue, A., and Zhang, T., 1996, “Fabrication of Bulk Glass Zr55Al10Ni5Cu30 Alloy of 30mm in Diameter by Suction Casting Method”. Materials Transactions JIM. Vol. 37, No.2, pp.185-187. 3. Peker, A., and Johnson, W. L., 1993, “A Highly Processable Metallic Glass: Zr41.25Ti13.75Ni10Cu12.5Be22.5”. Applied Physics Letters. Vol. 63, No. 17, pp.2342-2344. 4. Choi-Yim, H., Conner, R. D, Szuecs, F., and Johnson, W. L., 2001, “Qu asistatic and Dynamic Deformation of Tungsten Reinforced Zr57Al10Nb5Cu15.4Ni12.6 Bulk Metallic Glass Matrix Composites”. Scripta Materialia. Vol.45, pp.1039-1045. 5. Gilbert, C. J., Schroeder, V., and Ritchie, R. O., 1999, “Mechanisms for Fracture and Fatigue-Crack Propagation in a Bulk Metallic Glass”. Metallurgical & Materials Transactions A: Vol. 30, pp.1739-1754. 6. Pang, S. J., Zhang, T., Kimura, H., Asami, K., and Inoue, A., 2000, “Corrosion Behavior of Zr-(Nb-)Al-Ni-Cu Glass Alloys”. Materials Transactions JIM. Vol. 41, No. 11, pp.1495-1500. 7. Dandliker, R. B., Conner, R. D., and Johnson, W. L., 1998, “Melt Infiltration Casting of Bulk Metallis-Glass Matrix Composites”. Journal of Materials Research. Vol. 13, No. 10, pp.2896-2901. 8. Choi-Yim, H., Conner, R. D., and Johnson, W. L., 2002, “Processing, Microstructure and Properties of Ductile Metal Particulate Reinforced Zr57Al10Nb5Cu15.4Ni12.6 Bulk Metallic Glass Composites”. Acta Materialia. Vol. 50, No.10, pp.2737-2745. 9. Hufnagel, T. C., Fan, C., Ott R, T., Li, J., and Brennah, S., 2002, “Controlling Shear Band Behavior in Metallic Glasses Through Microstructure Design”. Intermetallics. Vol. 10, pp.1163-1166. 10. Conner, R. D., Dandliker, R. B., and Johnson, W. L., 1998, “Mechanical Properties of Tungsten and Steel Fiber Reinforced Zr41.25Ti13.75Cu12.5Ni10Be22.5 Metallic Glass Matrix Composites”. Acta Materialia. Vol. 46, No.17. pp.6089-6102. 11. Hirano, T., Kato, H., Matsuo, A., Kawamura, Y., and Inoue, A., 2000, “Synthesis and Mechanical Properties of Zr55Al10Ni 5Cu30 Bulk Metallic Glass Composites Containing ZrC Particles Formed by the In-Situ Reaction”. Materials Transactions JIM. Vol. 41, No.11, pp1454-1459. 12. Choi-Yim, H., Busch, R., and Johnson, W. L., 1998, “Th e Effect of Silicon on the Glass Forming Ability of the Cu47Ti34Zr 11Ni8 Bulk Metallic Alloy During Processing of Composites”. Journal of Applied Physics. Vol. 83, No. 12, pp.7993-7997 13. Johnson, W. L., 1999, “Bulk Glass-Forming Metallic Alloys: Science and Technology”. MRS BULLETIN. Vol. 10, pp.42-56 14. Qiu, K.Q., Wang, A.M., Zhang, H.F., Ding, B.Z., and Hu, Z.Q., 2002, “Melt Infiltration casting of Zr55Al10Ni5Cu30 bulk metallic glass Matrix Composite”. Chinese Journal of materials research. Vol.16, No. 4, pp.389-394. 15. Qiu, K.Q., 2002, “The Glass-Forming Ability, Composites and Properties of Bulk metallic Glasses”. Ph.D Dissertation, Institute of Metal Research, Chinese Academy of Sciences. pp.101-105. 16. Lin, X. H., Johnson, W. L., and Rhim, W. K., 1997, “Effect of Oxygen Impurity on Crystallization of an Undercooled Bulk Glass Forming Zr-Ti-Cu-Ni-Al 98 K.Q.Qiu and Y.L.RenVol. 3, No.2 Alloy”. Materials Transactions JIM, Vol.38, No.5, pp.473-477. |

