Paper Menu >>
Journal Menu >>
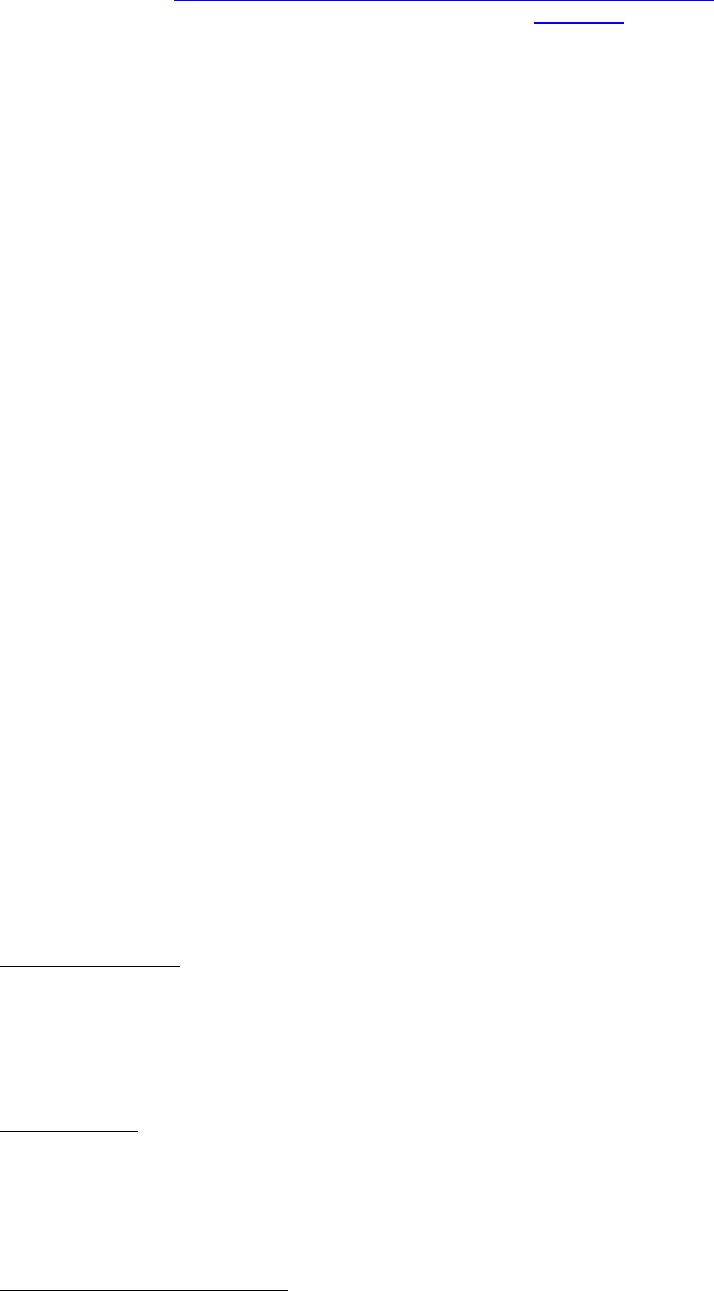 Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.1, pp 41-51, 2004 jmmce.org P rinted in t he USA . A ll rig hts re ser ved 41 Adsorption of Hg0 on the Unburned Carbon with HF Acid Leaching Jinjing Luo1, J.Y. Hwang2*, B. C. Greenlund3, Xiang Sun2, and Zhiyong Xu3 1Dept. of Civil & Environmental Engineering, 2Dept. of Materials Science & Engineering, 3Institute of Materials Processing, Michigan Technological University, Houghton, MI Unburned carbons from fly ash were leached with concentrated HF acid solutions in this study. The mercury adsorption abilities of the treated unburned carbons were examined. Effects of temperature, contact time, preloaded mercury emission and gaseous mercury concentration on adsorption behaviors were investigated. Leached by HF acid solution, unburned carbons were altered both physically and chemically. The influences of structure alteration on adsorption behaviors were also discussed. KEY WORDS: Unburned Carbon, HF Acid Leaching, Adsorption Capacity, Adsorption Rate. INTRODUCTION Originated from fly ash, a by-product of coal combustion power plant, unburned carbon contains various materials, most of which are located inside the pores or on the surface. Previous studies1,2 revealed that the impurities include trace elements and metal oxides, and among them aluminum silicate compounds take big portions. Due to the blockage of pores and the occupation of surfaces, it is assumed that the ability of the unburned carbon to remove Hg0 is reduced. Removal of the impurities may be a way to increase the Hg0 adsorption capacity of the unburned carbon samples. An acid leaching method was employed in this investigation to digest the impurities. EXPERIMENTAL DESIGN Carbon Preparation AEP unburned carbon and Pepco unburned carbon were extracted from AEP (American Electric Power) fly ash, and Pepco (Potomac Electric Power) fly ash by using the froth floatation method3, respectively. Both fly ashes belong to class F fly ash. F400 activated carbon was purchased from Calgon Carbon Corporation, Pittsburgh, PA, USA. Acid Screening Four acids were selected in this test, i.e. HCl, H 2SO4, HNO3 and HF acid. Equal portions of representative samples were digested in same volumes and concentrations (50%) of each acid. The leachates were analyzed by ICP for composition. SEM examination was provided by IMP, MTU. * Auth or to who m cor res pon de nce sh ou ld be add re sse d, Tel : 1-906-487-2600, Email: jhwang@mtu.edu 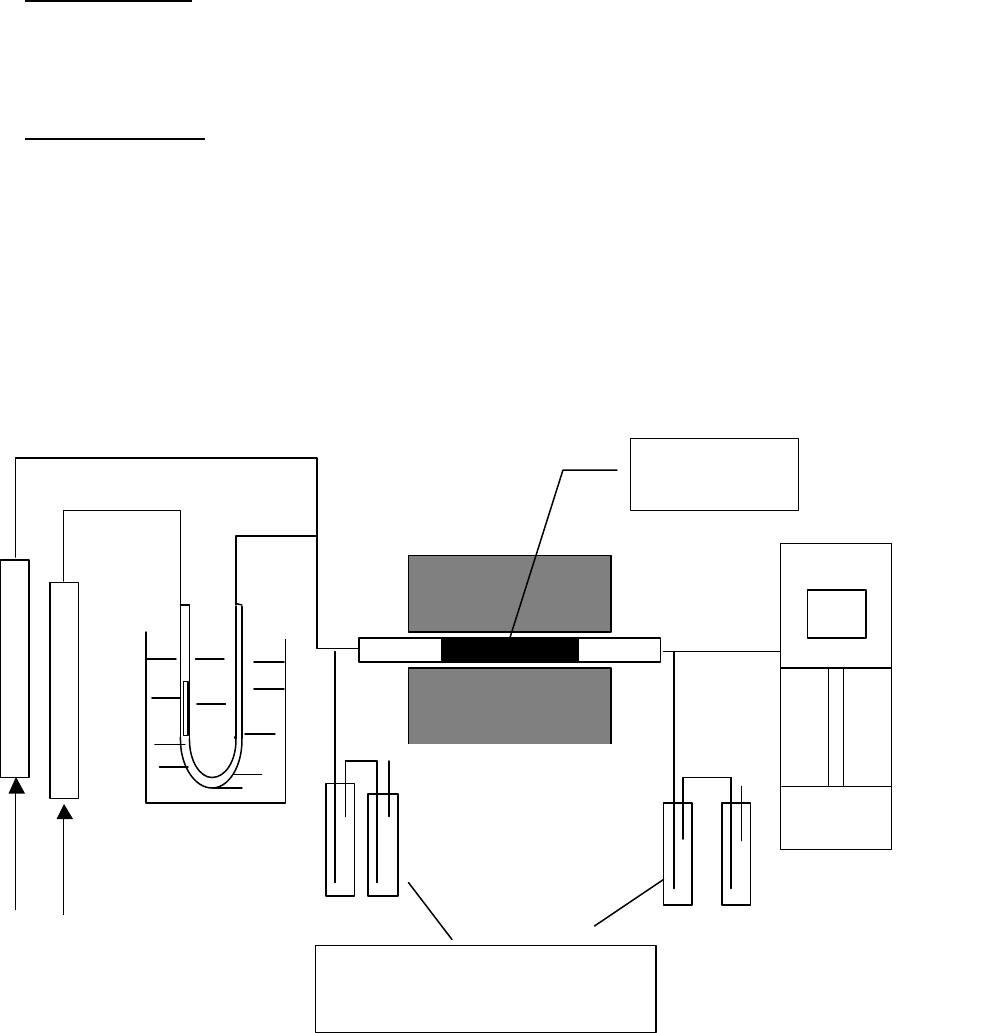 42 J. Luo, J.Y. Hwang, R. Gree n lund , X. S u n , and Z . Xu Vol. 3, No. 1 HF Acid Leaching AEP and Pepco unburned carbon were processed with a concentrate HF acid (49%) solution by volume ratio of 1:2 for 2 hours. After reaction, the treated samples were filtrated and washed by distilled water to remove residue HF acid solution and oven-dried at 105oC. Hg Adsorption Test Figure 1 illustrates the schematic diagram of mercury vapor adsorption apparatus. The Hg source was a 0.5 cm long mercury permeation tube (VICI Metronics. Inc., CA). A water bath maintained the required stable temperature. The carried gas was P.P. grade nitrogen gas. The concentrated mercury vapor was diluted with a bypass line of nitrogen gas before being introduced into carbon reactor. The carbon reactor was a 1cm I.D. (inside diameter), 22cm long glass column. The mixture of carbon sample and short glass fiber was packed in the middle of the column. The carbon bed temperature was regulated by a tube furnace. Tygon tubing from Saint-Gobain Performance Plastics was selected as connecting materials. Figure1. Schematic Diagram of Mercury Vapor Adsorption Apparatus Mercury vapor was collected using the one-liter Tedlar sampling bag at the site upstream and downstream of the carbon bed respectively. The concentration was determined by a gold film mercury vapor analyzer (JEROME 431-X, Arizona Instrument Corp)4. Mercury Analyzer Carbon Bed Tubular Furnace Mercury Source N2 1.5%KMnO4 + 10%H2SO4  Vol. 3, No. 1 Ad so r p t i o n o f H g0 on the Unburned Carbon with HF Acid Leaching 43 Exhaust vapor was introduced to the impinger solution before being expelled into the air. The impinger solutions were prepared daily by adding 1.5% potassium permanganate in 10% sulfuric acid5. A blank test was performed before each new adsorption experiment and after each test the entire system was purged with pure nitrogen gas to expel leftover Hg.6 The amount of mercury captured was determined by mass balance and normalized to the weight of sample. RESULTS & DISCUSSION Effect of Acid Leaching Table 1 presents leaching test result for AEP carbon. Hydrofluoric acid produced the best digestion among four tested acids. It readily removed the aluminum silicate compounds from carbon surface. Table 1 Laboratory Leaching Tests of AEP Unburned Carbon Leachate Impurity Composition (ppm) Acid Ca Mn Si Mg Na Zn Al HCl 3.82 0.04 1.62 0.93 0.29 0 9.89 H2SO4 3.75 0.05 0.34 1.38 0.46 0.02 14.19 HNO3 4.94 0.03 1.89 1.11 0.57 0 8.9 HF 1.38 0.08 117.5 2.92 0.04 49.1 SEM examinations of the AEP carbon before and after HF acid leaching were performed and the results are displayed in Figure 2 through Figure 5. The SEM photo taken at 250X is shown in figure 2. The light colored particles are the impurities. The LOI of this specimen is 69.75%. The same material is magnified to 3500X and presented in Figure 3. The photos show that the spherical fly ash was physically locked into the pores of the carbon matrix. The photo taken at 250X of HF leached AEP sample is pictured in Figure 4, and shows a significant reduction of impurity particles. The leached material possesses an assay of 97.34% LOI. The same material was magnified to 3500X and is shown in Figure 5. A noticeable absence of fly ash spheres is observed when compared with Figure 3. Hg Adsorption Test Adsorption curves of AEP-HF and Pepco-HF are presented in Figure 6. AEP, Pepco and F400 activated carbon are shown as references. With HF acid leaching, both unburned carbons improved their adsorption performance over their virgin carbons. The average adsorption rate of  44 J. Luo, J.Y. Hwang, R. Gree n lund , X. S u n , and Z . Xu Vol. 3, No. 1 AEP-HF is 2.26 times higher than that of AEP carbon. And although Pepco carbon did not show positive adsorption capacity, Pepco-HF indicated satisfactory performance that was near to that of AEP carbon. Furthermore, Pepco-HF and AEP-HF displayed almost similar adsorption behaviors and capacities within first 225 minutes of testing. This is important because the reaction time is very short if absorbents are used in ESP (electrostatic precipitator), which is the most popular pollutant control device in coal-fired power plants. Based on this point, hydrofluoric acid leaching may be a promising method to diminish the influence on Hg adsorption capacity from variations in carbon sources at the initial contact time. More unburned carbon samples will be examined in a future study. From Figure 7, the breakthrough profiles, AEP-HF did not reach its sorption equilibrium after approximately 2700 minutes of testing, where it still possessed 30% adsorption ability. Pepco-HF had reached its maximum adsorption capacity at the end of the experiment. The adsorption behaviors of unburned carbons with HF acid leaching were compared with that of F400 activated carbon and the curves are presented in Figure 6. Activated carbon performed the best among all samples, and its adsorption rate was around two times that of AEP- HF and about three times of that of Pepco-HF. Effect of Temperature Influence of temperature on the adsorption capacity of Pepco-HF carbon at a concentration of 0.05mg/m3 is displayed in Figure 8. With temperature increasing from 20oC to 150oC, its adsorption capacity was reduced over twelve times. With HF acid leaching treatment, Pepco-HF carbon still obeyed the physisorption theory. But AEP-HF carbon revealed a contradictory result. Figure 9 shows temperature effect for AEP-HF at the concentration of 0.05mg/m3. In the first 270 minutes, AEP-HF increased its adsorption capacity with temperature decreasing. It followed the physisorption theory during this period. But afterwards the adsorption rate of AEP-HF at 150oC was found to be around 1.5 times greater than that at 20oC. The physisorption theory cannot explain this phenomenon, which means the chemisorption may be the dominant factor. The adsorption mechanism of AEP-HF was controlled by physisorption and chemisorption respectively during the entire adsorption test. It can be concluded that with HF acid leaching the AEP carbon surface was altered both physically (more empty pores supplied) and chemically. Effect of Influent Hg Concentration The influence of gaseous Hg concentration on the adsorption capacity of the samples was studied and results are displayed in Figure 10. Pepco-HF and F400 activated carbon showed a greater adsorption rate with an increasing feed of Hg concentration, F400 sample having the faster rate. AEP-HF demonstrated similar behavior during the first 4 hours of experiment, but its adsorption rates were almost equal to each other afterwards, no matter whether the Hg concentration was high or low. Moreover, the equilibrium capacity of AEP-HF at a low influent Hg content was much better than that of AEP-HF at a high gaseous Hg content. These results imply that AEP-HF is more suitable for use at low Hg influent content condition. Effect of Preloaded Mercury A previous study6 demonstrated that the emission of preloaded mercury from carbon surface at 150oC impaired the Hg capturing ability of unburned carbons. In this study, the Vol. 3, No. 1 Ad so r p t i o n o f H g0 on the Unburned Carbon with HF Acid Leaching 45 amount of preloaded Hg held by HF acid leached unburned carbon was tested. Figure 11 presents the desorption curve and desorption amount of Pepco-HF carbon. Being purged mercury-free vapor at 150oC, Pepco carbon desorbed 0.2µgHg/gCarbon and this would be its entire preloaded mercury value since its desorbed mercury concentration reached zero at the end of experiment. This result suggests that preloaded mercury could be emitted from carbon surface during the adsorption test at 150oC, which causes a poorer mercury capturing ability. SUMMARY AND CONCLUSION With HF acid leaching, the pore structure on unburned carbon surface was changed. The impurity spheres, including mostly the aluminum silicate compound, were dissolved by HF acid solution, leaving more empty pores on carbon surface. In addition to physically altering the surface of the unburned carbon, HF acid leaching may have also changed the surface chemistry of the unburned carbon. This is supported by the observation that adsorption performance of AEP-HF at high temperature was better than that at low temperature. Pepco-HF carbon obeyed the physisorption mechanism, which is consistent with the performances of AEP, Pepco and F400 activated carbon6. Both AEP-HF and Pepco-HF demonstrated better adsorption behaviors than the virgin unburned carbon. Pepco-HF even increased its capturing capacity from negative to close to that of AEP unburned carbon. During the initial adsorption time, AEP-HF and Pepco-HF did not show significant difference between their adsorption behaviors and capacities. With HF acid leaching, the adsorption behavior depending on the carbon source was reduced in some extent. F400 activated carbon demonstrated the best adsorption behavior at 150oC temperature and Hg concentration of 0.05mg/m3. The influence of gaseous Hg content on adsorption behavior of carbon samples varied. Pepco-HF carbon and F400 activated carbon improved their adsorption behaviors with Hg influent concentration increasing. But AEP-HF carbon did not indicate significant difference in adsorption rate with mercury influent concentration changing. The preloaded mercury desorbed from Pepco-HF at 150oC when purged by mercury-free vapor. The desorption of preloaded mercury from carbon surface may be a reason that unburned carbon shows less adsorption capacity than the activated carbon.  46 J. Luo, J.Y. Hwang, R. Gree n lund , X. S u n , and Z . Xu Vol. 3, No. 1 Figure 2. AEP Carbon Product, 250X magnification. Figure 3. AEP Carbon Product, 3500X magnification  Vol. 3, No. 1 Ad so r p t i o n o f H g0 on the Unburned Carbon with HF Acid Leaching 47 Figure 4. Leached AEP Carbon Product, 250X magnification Figure 5. Leached AEP Carbon Product, 3500X magnification. 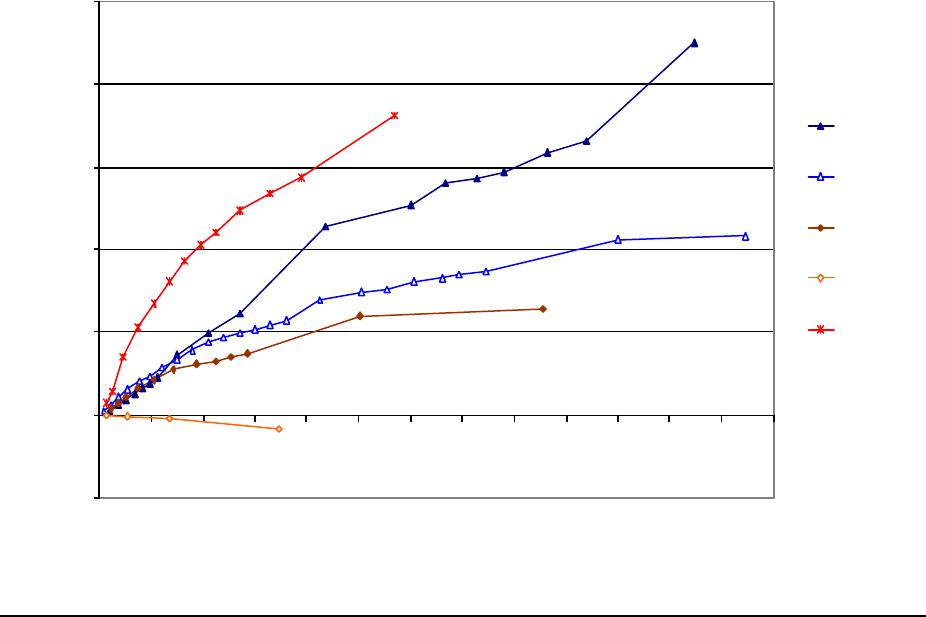 48 J. Luo, J.Y. Hwang, R. Gree n lund , X. S u n , and Z . Xu Vol. 3, No. 1 Fig. 6 HF Acid Effect on Hg Adsorption at 150 oC, 0 . 0 5 m g / m 3 -0.4 0 0.4 0.8 1.2 1.6 2 0200400 600 800 1000 1200 1400 1600 1800 2000 22002400 2600 Time (min) Adsorption (ug/gCarbon) AEP-HF AEP Pepco-HF Pepco F400 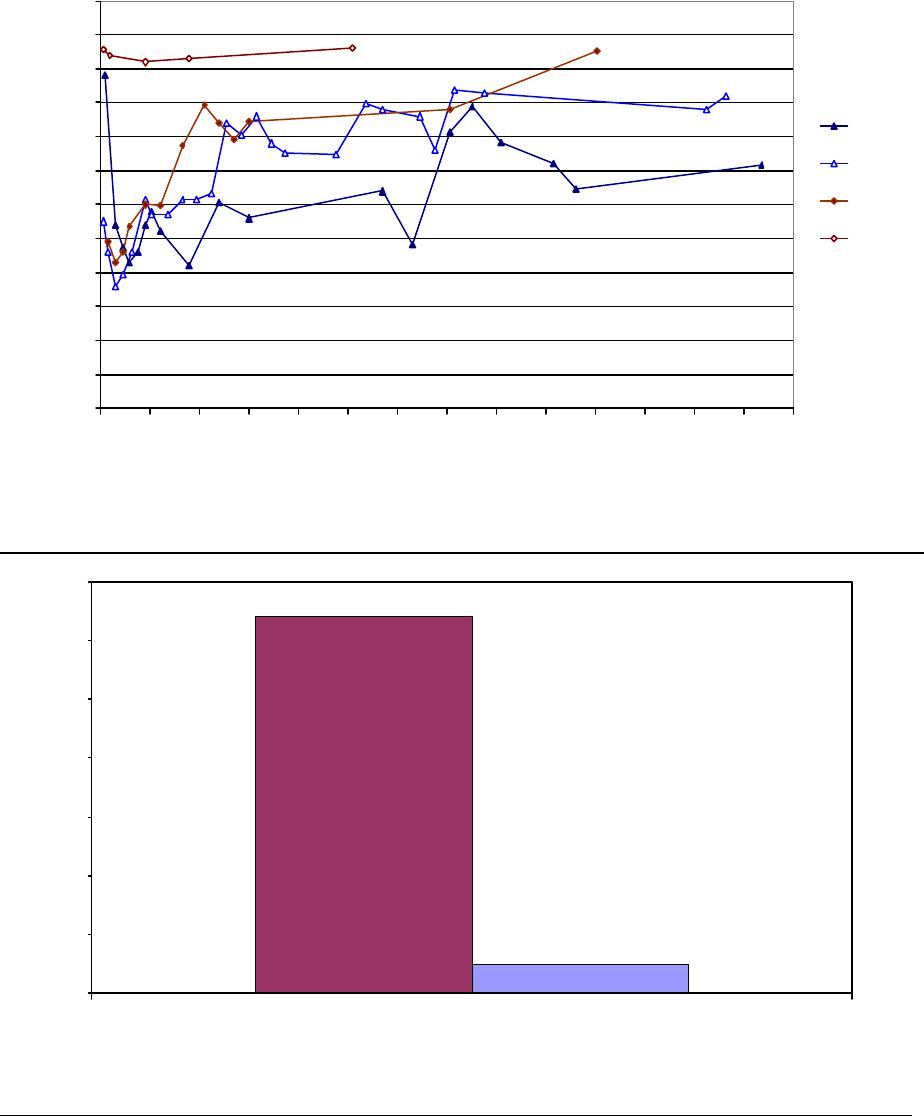 Vol. 3, No. 1 Ad so r p t i o n o f H g0 on the Unburned Carbon with HF Acid Leaching 49 Fig. 7 Breakthrough Profiles of Carbon Samples at 150oC, 0.05 mg/m3 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 1.1 1.2 0200400 6008001000 12001400 1600 1800 2000 2200 2400 2600 2800 Time (min) C/Co AEP-HF AEP Pepco-HF Pepco Fig. 8 Influence of Temperature on Hg adsorption 20oC 150oC 0 1 2 3 4 5 6 7 Pepco-HF Adsorption (ug/gCarbon) 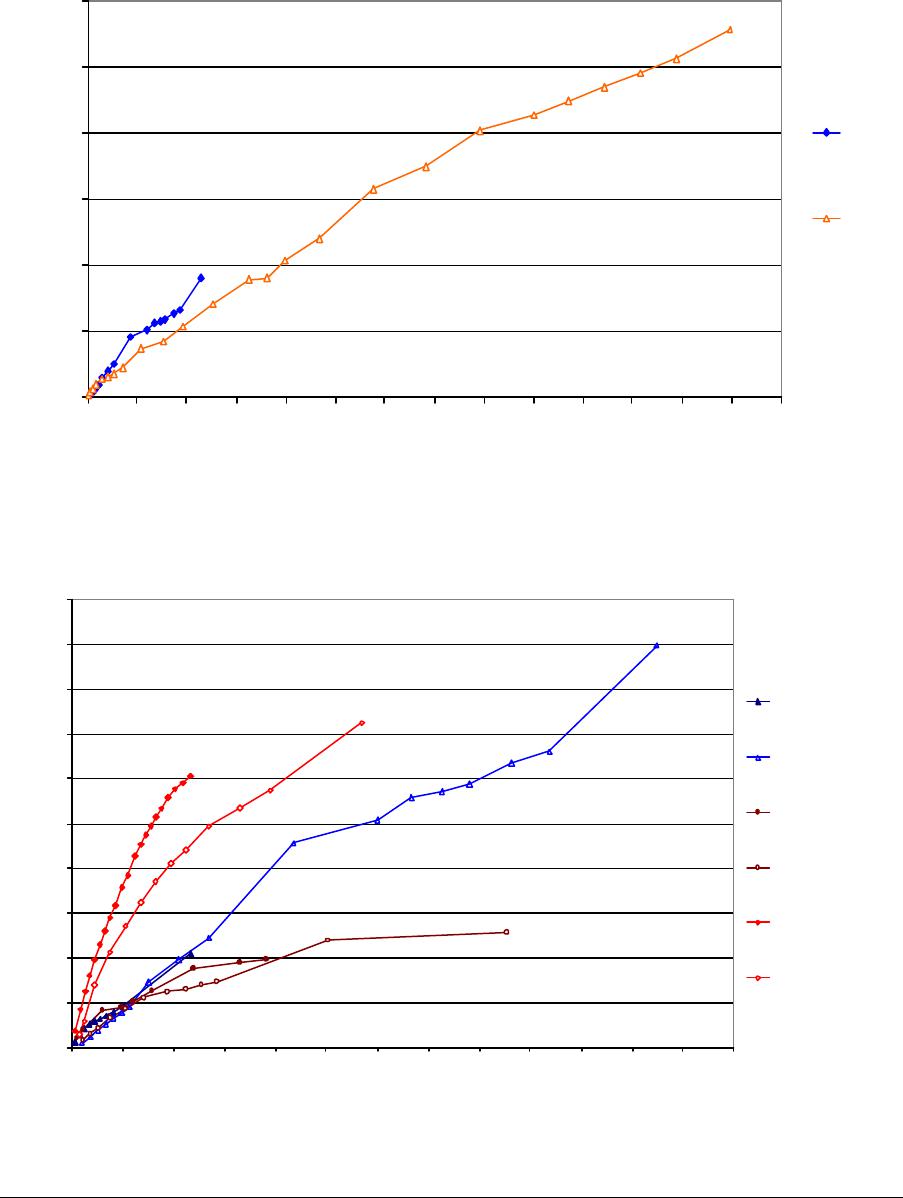 50 J. Luo, J.Y. Hwang, R. Gree n lund , X. S u n , and Z . Xu Vol. 3, No. 1 Fig. 9 Temperature Effect on Hg Adsorption for AEP-HF at 0.05mg/m 3 0 1 2 3 4 5 6 01000 20003000 4000 5000 6000 700080009000 1000 01100 01200 01300 01400 0 Time (min) Adsorption (ug/gCarbon) 150oC 20oC Fig. 10 Effect of Gaseous Hg Concentration on Adsorption Capacities at 150oC 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 2 0200 400600 800 1000 1200 14001600 1800 2000 2200 2400 2600 Time (min) Adsorption (ug/gCarbon) AEP-HF (High Content) AEP-HF (Low Content) Pepco-HF (High Content) Pepco-HF (Low Content) F400 (High Content) F400 (Low Content) 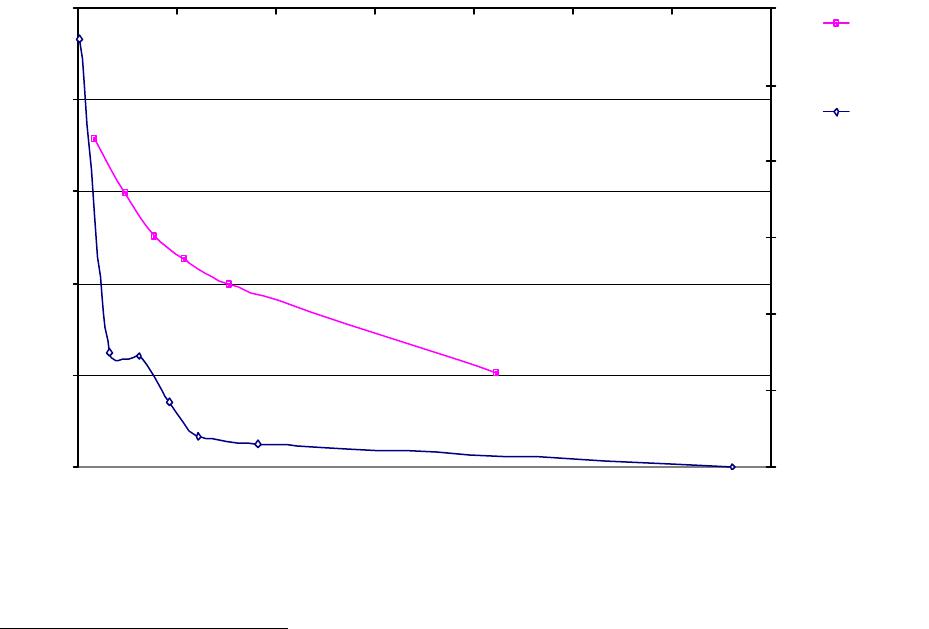 Vol. 3, No. 1 Ad so r p t i o n o f H g0 on the Unburned Carbon with HF Acid Leaching 51 Fig.11 Hg Preloaded in Pepco-HF Carbon -0.25 -0.2 -0.15 -0.1 -0.05 0 0200400 600 80010001200 1400 Time (min) Desorption (ug/gCarbon) 0 0.01 0.02 0.03 0.04 0.05 0.06 CHg (mg/m3) Desorption Amount Conc. of Preloaded Hg REFERENCE: 1 J.Y.Hwang, X.Sun, Z.Li, “Unburned Carbon from Fly Ash for Mercury Adsorption: I. Separation and Characterization of Unburned Carbon”, Journal of Minerals & Materials Characterization & Engineering, Vol1. No.1, p39-60 2 Dewey,M.VPI/ALRC Coal Refuse Leach Project Physical Characterization. U.S. Bureau of Mines. Albany, OR, Research Center, 1995 3 J.Y.Hwang, “Wet Process for Fly Ash Beneficiation”, U.S. Patent 5,047,145 (1991) 4 JEROME 431-X Mercury Vapor Analyzer Manual 5 Shendrikar, A.D.; Damle, A.; Gutknect, W.F. “Collection Efficiency Evaluation of Mercury Trapping Media for the SASS Train Impinger System”, U.S. Environmental Protection Agency. U.S. Government Printing Office: Washing, DC, 1984, EPA-600/7-84-089 6 J.Luo, J.Y.Hwang, “Adsorption of Vapor Phase Mercury on Various Carbons”, Journal of Minerals & Materials Characterization & Engineering, Vol.3, No.1, 2004 |

