Effect of the Pre-Treatment Severity on the Antioxidant Properties of Ethanol Organosolv Miscanthus x giganteus Lignin 33
Products
Coupling reactions
MeO
LO
2
H+
LO
2
OH
OMe
MeO OMe
O.
LO
2
+
LO
2
LO
2
+
Products
LOOH + LO
2
AIBN + LH + O
2
LO
2
+ LH + O
2
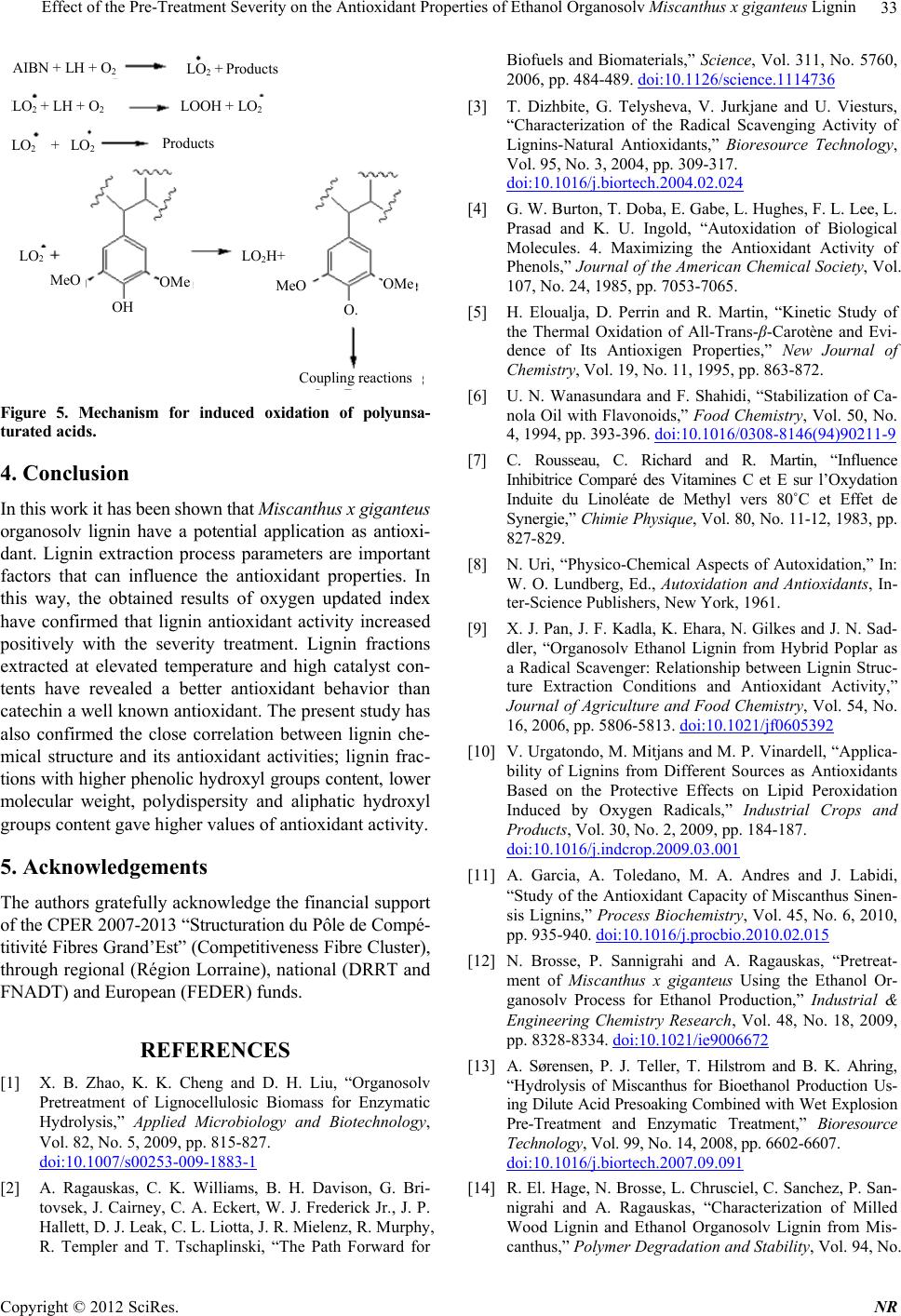
Figure 5. Mechanism for induced oxidation of polyunsa-
turated aci ds.
4. Conclusion
In this work it has been shown that Miscanthus x giganteus
organosolv lignin have a potential application as antioxi-
dant. Lignin extraction process parameters are important
factors that can influence the antioxidant properties. In
this way, the obtained results of oxygen updated index
have confirmed that lignin antioxidant activity increased
positively with the severity treatment. Lignin fractions
extracted at elevated temperature and high catalyst con-
tents have revealed a better antioxidant behavior than
catechin a well known antioxidant. The present study has
also confirmed the close correlation between lignin che-
mical structure and its antioxidant activities; lignin frac-
tions with higher phenolic hydroxyl groups content, lower
molecular weight, polydispersity and aliphatic hydroxyl
groups content gave higher values of antioxidant activity.
5. Acknowledgements
The authors gratefully acknowledge the financial support
of the CPER 2007-2013 “Structuration du Pôle de Compé-
titivité Fibres Grand’Est” (Competitiveness Fibre Cluster),
through regional (Région Lorraine), national (DRRT and
FNADT) and European (FEDER) funds.
REFERENCES
[1] X. B. Zhao, K. K. Cheng and D. H. Liu, “Organosolv
Pretreatment of Lignocellulosic Biomass for Enzymatic
Hydrolysis,” Applied Microbiology and Biotechnology,
Vol. 82, No. 5, 2009, pp. 815-827.
doi:10.1007/s00253-009-1883-1
[2] A. Ragauskas, C. K. Williams, B. H. Davison, G. Bri-
tovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr., J. P.
Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy,
R. Templer and T. Tschaplinski, “The Path Forward for
Biofuels and Biomaterials,” Science, Vol. 311, No. 5760,
2006, pp. 484-489. doi:10.1126/science.1114736
[3] T. Dizhbite, G. Telysheva, V. Jurkjane and U. Viesturs,
“Characterization of the Radical Scavenging Activity of
Lignins-Natural Antioxidants,” Bioresource Technology,
Vol. 95, No. 3, 2004, pp. 309-317.
doi:10.1016/j.biortech.2004.02.024
[4] G. W. Burton, T. Doba, E. Gabe, L. Hughes, F. L. Lee, L.
Prasad and K. U. Ingold, “Autoxidation of Biological
Molecules. 4. Maximizing the Antioxidant Activity of
Phenols,” Journal of the American Chemical Society, Vol.
107, No. 24, 1985, pp. 7053-7065.
[5] H. Eloualja, D. Perrin and R. Martin, “Kinetic Study of
the Thermal Oxidation of All-Trans-β-Carotène and Evi-
dence of Its Antioxigen Properties,” New Journal of
Chemistry, Vol. 19, No. 11, 1995, pp. 863-872.
[6] U. N. Wanasundara and F. Shahidi, “Stabilization of Ca-
nola Oil with Flavonoids,” Food Chemistry, Vol. 50, No.
4, 1994, pp. 393-396. doi:10.1016/0308-8146(94)90211-9
[7] C. Rousseau, C. Richard and R. Martin, “Influence
Inhibitrice Comparé des Vitamines C et E sur l’Oxydation
Induite du Linoléate de Methyl vers 80˚C et Effet de
Synergie,” Chimie Physique, Vol. 80, No. 11-12, 1983, pp.
827-829.
[8] N. Uri, “Physico-Chemical Aspects of Autoxidation,” In:
W. O. Lundberg, Ed., Autoxidation and Antioxidants, In-
ter-Science Publishers, New York, 1961.
[9] X. J. Pan, J. F. Kadla, K. Ehara, N. Gilkes and J. N. Sad-
dler, “Organosolv Ethanol Lignin from Hybrid Poplar as
a Radical Scavenger: Relationship between Lignin Struc-
ture Extraction Conditions and Antioxidant Activity,”
Journal of Agriculture and Food Chemistry, Vol. 54, No.
16, 2006, pp. 5806-5813. doi:10.1021/jf0605392
[10] V. Urgatondo, M. Mitjans and M. P. Vinardell, “Applica-
bility of Lignins from Different Sources as Antioxidants
Based on the Protective Effects on Lipid Peroxidation
Induced by Oxygen Radicals,” Industrial Crops and
Products, Vol. 30, No. 2, 2009, pp. 184-187.
doi:10.1016/j.indcrop.2009.03.001
[11] A. Garcia, A. Toledano, M. A. Andres and J. Labidi,
“Study of the Antioxidant Capacity of Miscanthus Sinen-
sis Lignins,” Process Biochemistry, Vol. 45, No. 6, 2010,
pp. 935-940. doi:10.1016/j.procbio.2010.02.015
[12] N. Brosse, P. Sannigrahi and A. Ragauskas, “Pretreat-
ment of Miscanthus x giganteus Using the Ethanol Or-
ganosolv Process for Ethanol Production,” Industrial &
Engineering Chemistry Research, Vol. 48, No. 18, 2009,
pp. 8328-8334. doi:10.1021/ie9006672
[13] A. Sørensen, P. J. Teller, T. Hilstrom and B. K. Ahring,
“Hydrolysis of Miscanthus for Bioethanol Production Us-
ing Dilute Acid Presoaking Combined with Wet Explosion
Pre-Treatment and Enzymatic Treatment,” Bioresource
Technology, Vol. 99, No. 14, 2008, pp. 6602-6607.
doi:10.1016/j.biortech.2007.09.091
[14] R. El. Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. San-
nigrahi and A. Ragauskas, “Characterization of Milled
Wood Lignin and Ethanol Organosolv Lignin from Mis-
canthus,” Polymer Degradation and Stability, Vol. 94, No.
Copyright © 2012 SciRes. NR