Journal of Cancer Therapy
Vol.2 No.5(2011), Article ID:16648,18 pages DOI:10.4236/jct.2011.25099
CuradermBEC5 for Skin Cancers, Is It? An Overview
![]()
Australasian Medical Research, Port Vila, Republic of Vanuatu.
E-mail: Tania.R.Chase@gmail.com
Received October 15th, 2011; revised November 13th, 2011; accepted November 30th, 2011.
Keywords: nonmelanoma skin cancers, solasodine rhamnosyl glycosides, curaderm, solamargine, solasonine
ABSTRACT
Skin cancer incidence is increasing at alarming rates and is considered by some as an epidemic. Its incidence is higher than all other cancers combined. The developments of new treatments have not parallelled the increased incidences of this disease. A variety of treatments are available with differing outcomes. More recently a novel topical treatment, consisting of the antineoplastic compounds solasodine rhamnosyl glycosides, solamargine and solasonine, which are derived from plant material, has been described that claims to have many advantages over the currently used skin cancer therapies. This review investigates such claims.
1. Introduction
Skin cancer incidence is increasing at alarming rates. In the US alone, more than two million people develop over 3.5 million nonmelanoma skin cancers every year. This translates to more than 300 percent increase in cancer incidence since 1992 [1,2]. This observation does not only apply to the US, but is a worldwide phenomenon. Skin cancer incidence is considered by some as an epidemic and its incidence is higher than all other cancers combined.
Nonmelanoma skin cancers, such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are the most common forms of skin cancer. Malignant melanomas are the least common but most serious type of skin cancer. Although malignant melanomas only constitute less than 5 percent of the incidences of all skin cancers, over the past 30 years incidences rates of malignant melanoma in Britain alone, have increased more rapidly than any of the top ten cancers in males and females. Increased rates of over 500 percent have been reported for malignant melanoma from 1975 to 2008 [3].
A variety of treatments are available for nonmelanoma skin cancers with good outcomes, especially if the cancers are detected and treated in the early stages of development. However, there are some serious disadvantages with the most common treatments. Some disadvantages of current treatments [2] include:
• margin around cancer may not be free of cancer;
• moderately painful;
• slow healing;
• scarring;
• specialized training by health professionals with appropriate facilities;
• expensive;
• activity restriction after surgery if skin graft or flap is needed;
• limited cosmetic results often with disfigurement;
• treatments are nonspecific, they do not distinguish between the killing and removal of cancerous tissues and the killing and removal of normal tissues.
In addition high recurrence rates of treated skin cancers have been reported [4].
Each Currently Used Skin Cancer Treatment Follows Specific Procedures
Surgical Excision. This surgical procedure is used to treat primary and recurrent tumours under local anesthesia. The tumour and an area of healthy looking skin (margin) around the tumour are removed surgically. The resulting wound is usually closed with stitches. Often, skin from another area of the body (skin graft), or healthy skin moved from a nearby area (skin flap) is used to complete the treatment. After surgery, the excised tissue is examined under a microscope to see if any cancer cells were present in the skin that appeared cancer free. The cure rates range from good to bad [4]. A limitation of this method is that this procedure does not distinguish between tumours and normal skin. The major shortcoming of surgical excision is the pain and discomfort and the potential for long term scarring and quality of life. This method of treatment is by its nature invasive, and at times disfiguring.
Mohs Micrographic Surgery. Mohs surgery has the highest reported cure rate for both basal cell and squamous cell carcinoma. This specialised surgical technique removes the visible tumour first and then removal of successive layers of skin one at a time until microscopic examination no longer reveals cancer cells. Mohs surgery is carried out while the patient is under local anesthesia. Removing and examining each layer takes a long time, usually over one hour. Once skin cancer is no longer visible under the microscope, the surgical wound is treated as needed and varies from stitches to skin flap. This treatment is invasive, and at times disfiguring. Mohs surgery is a treatment for most nonmelanoma skin cancers. However, the length and intensity of this surgical procedure limit its use to treating recurrent skin cancer, larger tumours, areas where it is essential to preserve as much skin as possible (such as an ear, eyelid, nose, lip or hand), tumours in which it is difficult to establish when the cancer ends, and sites prone to recurrence. This surgery when in process does not distinguish cancer cells from normal cells.
Curettage and Electrodesiccation. This method is used to treat small basal cell and squamous cell carcinomas in non-crucial areas such as the trunk and extremities.
The procedure consists of scooping out the cancer by using a spoon-like instrument called a curette and then using an electric needle to burn or “cauterize” the remaining cancer cells and to control bleeding. The scraping and cauterizing is typically repeated 3 times, and the wound tends to heal without stitches. This procedure does not distinguish cancer cells from normal cells.
Cryotherapy. Cryotherapy is a treatment in which surface skin lesions are frozen. Liquid nitrogen is the most common method used to freeze skin lesions. Cryotherapy also known as cryosurgery is mostly used to treat solar keratoses. However, it is sometimes also used to freeze small skin cancers such as superficial basal cell and in situ superficial squamous cell carcinomas (Bowen’s disease), but this is not always successful and careful follow-up is necessary. Cryotherapy stings and may be painful. There may be immediate swelling and redness. The treated area is likely to blister within a few hours. Within a few days a scab forms and the blister gradually dries up. The blister dries to a scab. The scab peels off after 5 - 10 days on the face and 3 weeks on the hand. A sore or scab may persist as long as 3 months on the lower leg because healing at this site is often slow. Cryotherapy may result in a white mark (hypopigmentation) or a scar, particularly when freezing has been deep or prolonged, as is required for cancerous lesions. Skin lesions may fail to clear or may recur at a later date, necessitating further cryotherapy, surgery or other treatment. Cryotherapy does not distinguish between normal or abnormal skin. Whatever area on the skin the liquid nitrogen (temperature –196˚C) touches, it kills.
Radiation Therapy. Radiation treatments for skin cancer are used in areas that are difficult to treat with surgery. It is used for large tumours, tumours that cover a large area, or tumours that are difficult to surgically remove because of location, such as eyelids, ears or noses. To obtain a good end result, the procedure involves many treatment sessions, usually 25 - 30. This procedure does not distinguish tumours from normal tissue.
Laser Therapy. Laser therapy may be used to vaporize superficial and multiple basal cell carcinomas and to excise or destroy squamous cell carcinoma. This therapy does not destroy cancer cells found deeper in the skin, so close follow-up with the patient is essential. This therapy does not distinguish tumours from normal tissue.
Topical Cream Treatment. Medical therapy using creams that contain anticancer agents (5-fluorouracil, 5-FU Efudex, Fluoroplex) or stimulate the immune system (imiquimod) are used to treat skin lesions. These creams are applied several times a week for several weeks. They produce brisk inflammation and irritation. Their limitations include discomfort, with many side effects, which may be severe, and a low cure rate, which makes medical treatment unsuitable for treating most skin cancers on the face.
Currently, surgical excision is the most common form of treatment for skin cancers. The goal of reconstructive surgery is restoration of normal appearance and function. The choice of technique in reconstruction is dictated by the size and location of the defect. Excision and reconstruction of facial cancers is generally more challenging due to the presence of highly visible and functional anatomic structures in the face.
The treatment and management of nonmelanoma skin cancers cost the USA health care system more than US $1.4 billion per year and this value is increasing dramatically each year. In the current world-wide economic situation this poses a financial burden and because of this, many patients afflicted with skin cancers may not seek proper treatment resulting in increased morbidity and mortality [2].
Unfortunately, the developments of new treatments for skin cancers have not parallelled the increased incidences of skin cancers.
Over the last few years a new treatment for nonmelanoma skin cancers has been described in the scientific literature. Any proposed new treatment for whatever purpose, must be at least as good or preferably better than current treatments.
This review examines whether the evidence of this new treatment lives up to its claims. The purportedly new treatment is a topical cream, CuradermBEC5, that is used to treat nonmelanoma skin cancers.
Treatment of nonmelanoma skin cancers with CuradermBEC5 claims the following:
• is derived from plant extracts;
• kills cancer cells by an unique procedure;
• kills cancer cells whether they are resting or multiplying, ensuring that all cancer cells are eliminated rapidly;
• at therapeutic doses kill cancer cells only and not normal cells;
• is more effective and safer than other well established anti-cancer drugs;
• is non invasive by simple application of a cream;
• shows superior cosmetic outcomes compared with other available treatments.
2. Discussion
What evidence is available to justify the claims?
2.1. The Therapeutic Agents in CuradermBEC5 are Derived from Plant Extracts
In 1987 it was reported that a standard mixture of glycoalkaloids (BEC) was extractable from the fruit of Solanum sodomaeum also known as S. Linnaeanum (Devil’s Apple). BEC is a mixture of solasodine glycosides consisting of the triglycosides solasonine (β-solatriose), solamargine (β-chacotriose), and di and monoglycosides. All the glycosides contain the same aglycone, solasodine [5-9].
Figure 1 shows the chemical structures of the two main glycoalkaloids, solasonine and solamargine [5-8] and figure 2 shows their mass spectra [9].
It was subsequently shown that the identical replica of BEC is also present in the eggplant or aubergine [10].
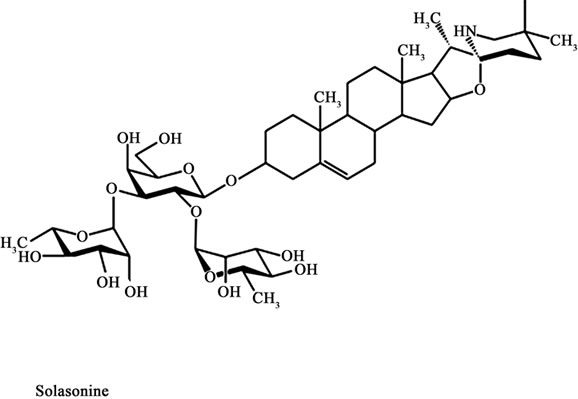
Figure 1. Chemical structures of solasonine and solamargine.
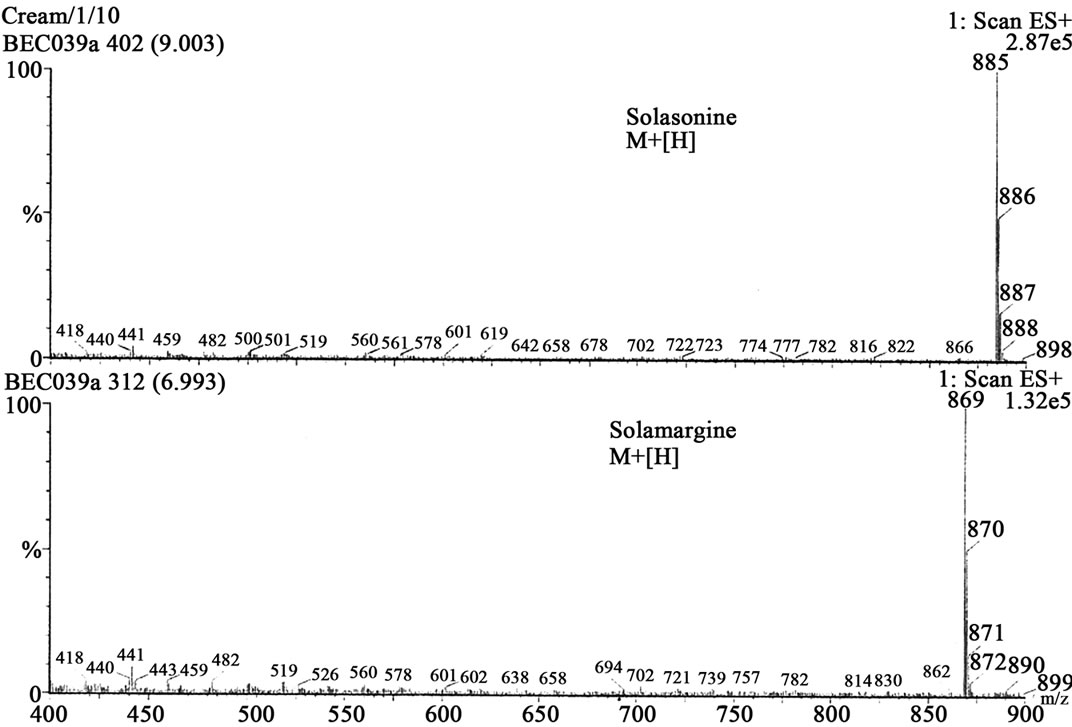
Figure 2. Mass spectra of solasonine and solamargine.
2.2. The Therapeutic Agents in CuradermBEC5 Kill Cancer Cells by an Unique Procedure
BEC, at the appropriate dose, only interacts with cancer cells and not with normal cells [6,11-28].
A specific protein has been identified on cancer cells. This protein, known as rhamnose binding protein, is not, or very limited, on normal cells [22]. The rhamnose binding protein is a receptor on cancer cells and binds BEC. After internalization in the cancer cell by receptormediated endocytosis through “coated pit endocytosis” a receptorsome or endosome is formed. Gradual transformation of endosomes results in the formation of lysosomes [29]. BEC then triggers extrinsic and intrinsic apoptotic pathways in the cancer cells by up-regulating the expressions of external death receptors, such as tumour necrosis factor receptor 1 (TNFR-1), Fas receptor, TNFR-1 associated death domain and Fas-associated death domain. BEC enhances the intrinsic ratio of Bax to Bcl-2 by up-regulating Bax and down-regulating Bcl-2 and Bcl-xL expressions. These effects result in activation of Caspase -8, -9 and -3 in cancer cells, indicating that BEC triggers extrinsic and intrinsic apoptotic pathways in cancer cells [6,30-37].
Apoptosis or programmed cell death is a highly organized physiological process that results in the removal of unwanted cells. Induction of apoptosis in cancer cells or malignant tissues is accepted as an efficient approach for cancer chemotherapy.
Figure 3 shows that BEC rapidly kills cancer cells by the characteristic apoptotic pathway of cell shrinkage, condensation of chromatin and nuclear fragmentation [32,38].
A recent study has shown that solamargine, the main component of BEC, also kills cancer cells by oncosis. After interaction of solamargine with cancer cells, marked changes in cell shape and volume occur. The cells get blebs on the membrane, the mitochondria swell, the contents of the nuclei clump and the cells die. It has been proposed that apoptosis and oncosis share certain mechanisms and alterations within the cell before they die by bursting. A study has shown that solamargine at low concentrations kill cancer cells by apoptosis and at higher doses solamargine kill cancer cells by oncosis, and both types of cell death are induced by intermediate concentrations of solamargine [39].
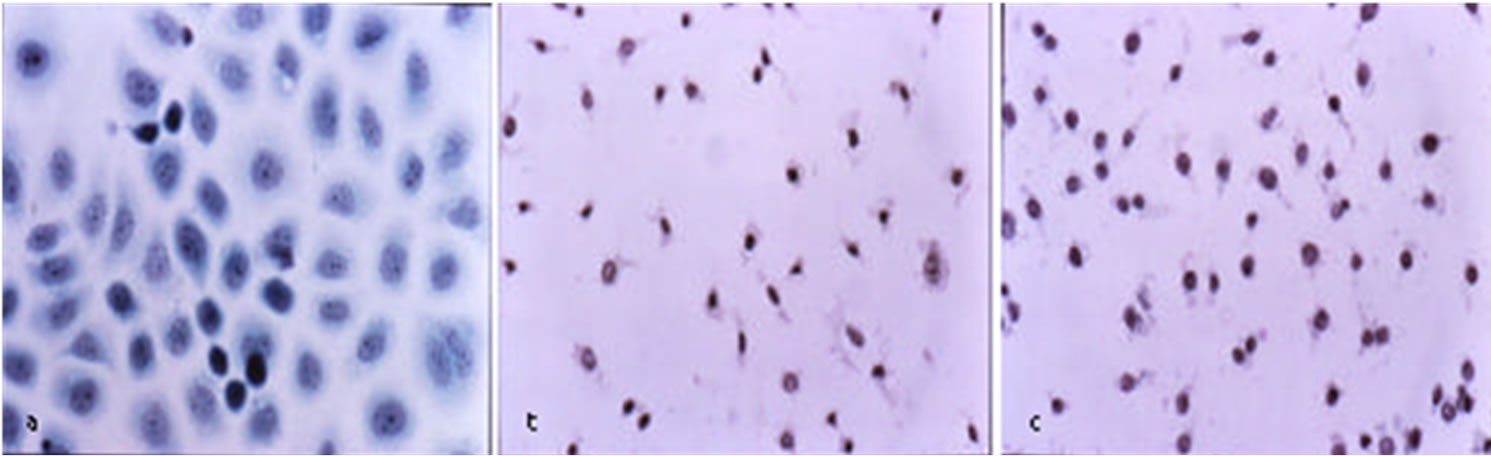 (a)
(a)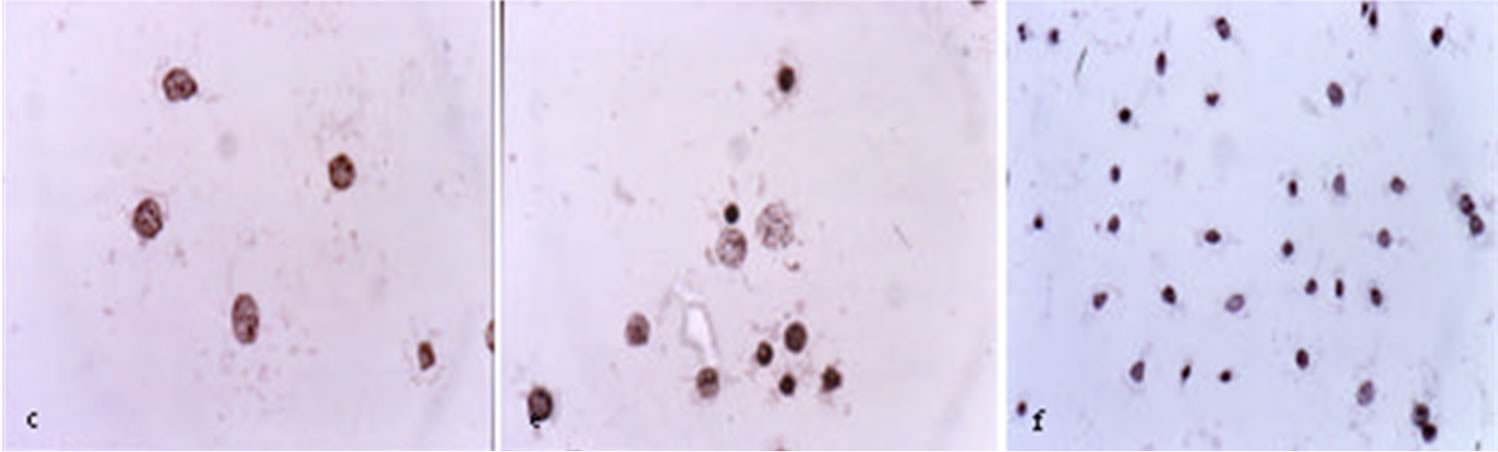 (b)
(b)
Figure 3. Untreated ovarian cancer cells, the cells are all viable (a) BEC causes the cytoplasm of the cancer cells to undergo dissolution, the nuclei contract and become dark staining; (b) nuclei then enlarge; (c) the chromatin (contents of nucleus) clumps; (d) and finally the nuclei disintegrate; (e) Only cellular debris is left after the interaction of the cancer cells with BEC; (f) This cell death is characteristic of apoptosis which is also known as programmed cell death.
2.3. The Therapeutic Agents in CuradermBEC5 Kill Cancer Cells in Proliferative and Non-Proliferative Stages Ensuring Rapid Removal of All Cancer Cells
A very important reported aspect of BEC therapy is the killing of cancer cells at their proliferative and nonproliferative stages. These observations are in stark contrast to other well established anti-mitotic chemotherapy drugs that only kill cancer cells while they are dividing and also kill normal cells when they too are dividing [40-48].
Traditional chemotherapy drugs lack specificity, as they enter both cancer and normal cells mainly through diffusion. Due to their DNA reactivity the widely used anticancer drugs can cause a second tumour which may be different than the one originally treated several years after “curative” treatment. BEC ruptures lysosomes and also affects the mitochondria in cancer cells which lead to apoptosis. BEC therapy lacks the mutagenic and carcinogenic potential of currently used chemotherapy drugs [7,29,34,42].
2.4. The Therapeutic Agents in CuradermBEC5 Kill Cancer Cells Only and Not Normal Cells
BEC and its individual components solamargine and solasonine kill cancer cells in a dose-dependent manner and, at therapeutic doses, do not affect normal cells [5,32,34].
Ex Vivo studies have demonstrated that BEC is effecttive against a wide spectrum of human cancers and that BEC is, in a dose dependent manner, selectively killing cancer cells without harming normal cells [11-16,32,34, 47,49-52].
Figure 4 illustrates the specificity of BEC on cancer cells and lack of effect on normal cells. This figure shows BEC at a concentration of 10 µg/mL eliminates various cancer cell lines but has no effect on normal cells such as the sensitive bone marrow cells.
BEC and its individual components solamargine and solasonine have been shown to be very effective in killing other human cancer cells such as Ehrlich carcinoma, K562 leukaemia, colon (HI29) cancer, liver (Hep G2) cancer cells, promyelocytic leukaemia (HL-50) and lung cancer cells but not normal cells [11-20, 50-53].
Table 1 shows at what concentrations solamargine kills cancer cells and normal cells. The safety margin of killing cancer cells relative to normal cells, the Therapeutic Index (TI) is also shown [11-20,50-53]. The higher the TI is, the more effective and safer the anticancer drug is.
MDR refers to multidrug resistant cancer cells which means that these cancer cells are no longer killed by existing anticancer drugs. Solamargine (BEC) does kill these MDR cancer cells! The higher the safety margin, the more effective solamargine is in killing cancer cells relative to normal cells. Safety margins of above 2 are regarded highly beneficial for anticancer drugs.

Figure 4. Effect of BEC on various primary cell lines and cell cultures. This figure shows that at a concentration of 6 ug/ml of BEC, virtually all Ovarian cancer cells are killed but no Bone Marrow cells are affected.
In Vivo single dose studies in mice with the lethal Sarcoma 180 cells have similarly shown that the LD50 and ED50 resulted in a TI of 3.3.
Figure 5 shows the lethal toxicity in normal untreated mice of single i.p. doses of BEC. The LD50 of BEC is 29 mg/kg [1].
Figure 6 illustrates the effect of single doses of varying concentrations of BEC on the absolute survival of mice which had the Sarcoma 180 tumour. The ED50 of BEC for this tumour is 9 mg/kg [1]. Data from Figures 5 and 6 show that the TI (LD50/ED50) is 3.3.
It was further shown that the effectiveness of BEC on the lethal Sarcoma 180 tumour in mice was dependent on the number of administrations [1]. Multiple doses at low concentrations of BEC resulted in much greater efficacy. Figure 7 shows that two doses of BEC cured 42% whereas three and four doses cured 92% of the mice that originally had terminal cancer. All untreated mice with the cancer died at day 20.
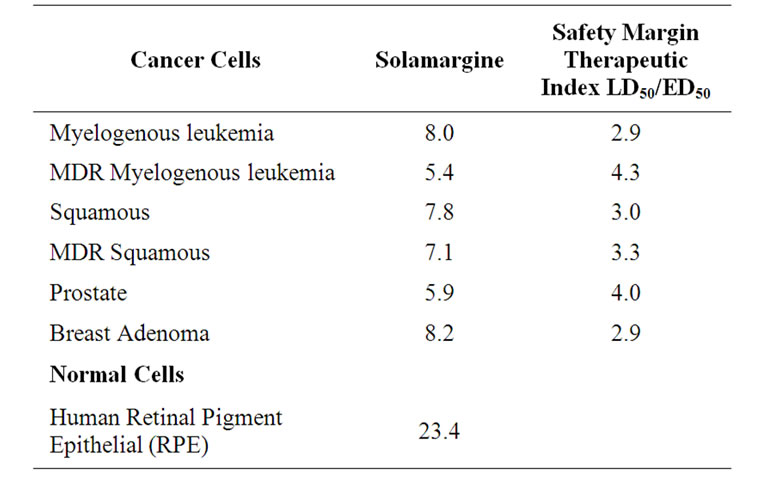
Table 1. Concentrations of solamargine (main component of BEC) [microM] to kill 50% of cancer and normal cells and their safety margins.
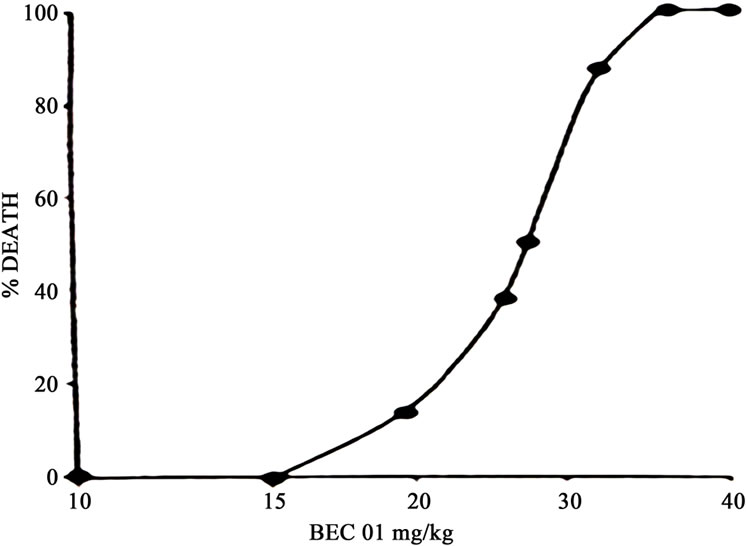
Figure 5. Single dose toxicity studies of BEC01 in mice. n = 12/group.
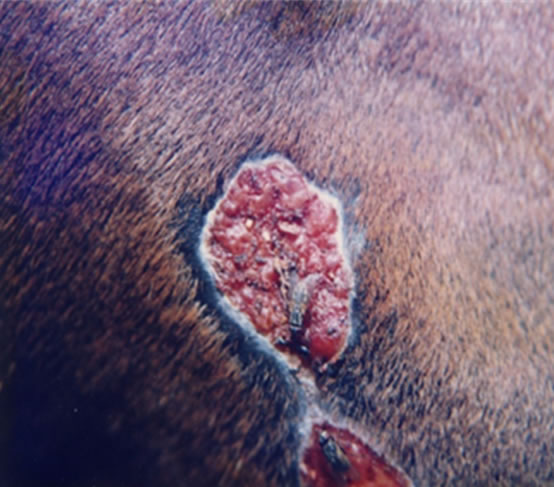
Intralesion injection of BEC in large tumours in horses resulted in complete removal of such tumours with no adverse effects on the surrounding normal tissue which was also injected with BEC (Figure 8). At the end of the BEC therapy the large tumours of up to 0.5 kg were replaced with normal tissue [9,41,42].
Figure 8 illustrates BEC intralesion therapy on squamous cell carcinoma on a horse [9].
2.5. The Therapeutic Agents in CuradermBEC5 are More Effective and Safer than Other Well Established Drugs
It has been reported that solamargine the main constituent of BEC as an anticancer agent is far more effective than taxol, cisplatin, gemcitabine, camptothecin, vinblastine, methotrexate, 5-fluorouracil, epirubicin and cyclophoshamide [21,22,42].
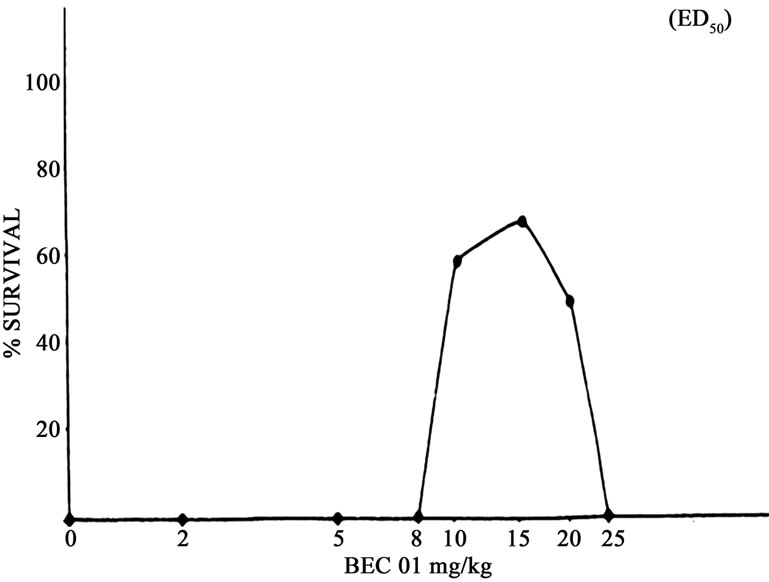
Figure 6. Survival of mice with Sarcoma 180 treated with single doses of BEC01. n = 12/group.
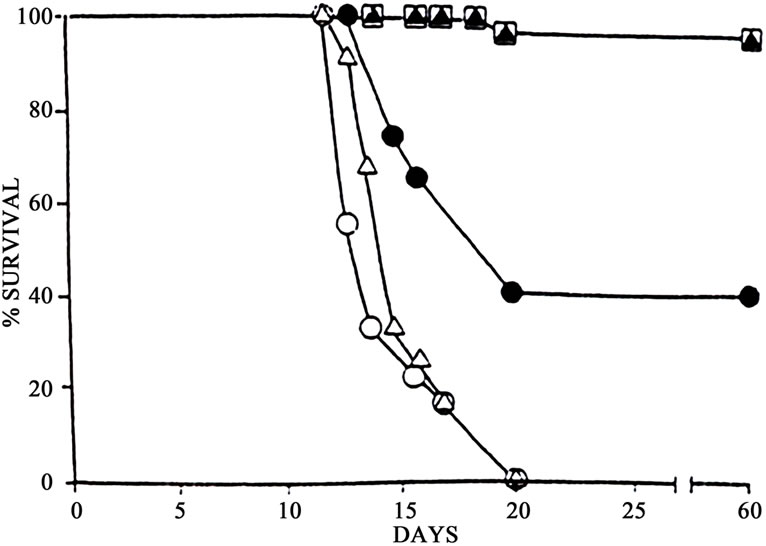
Figure 7. Survival of mice with Sarcoma 180 treated with varying doses of 8mg BEC01/kg. --Ο--, control; --Δ--, 1 dose; --●--, 2 doses; --□--, 3 doses; --▲--, 4 doses. n = 12/group.
 (a)
(a)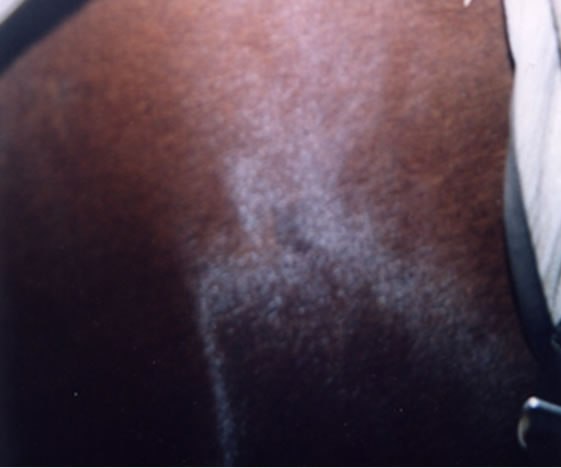 (b)
(b)
Figure 8. Two SCCs joined by a bridge on the neck of a horse before BEC injection (a) and after treatment was completed; (b) When the treated area was completely healed it was indistinguishable from the horse’s normal skin.
Figure 9 shows that very low concentrations of solamargine kill virtually all lung cancer cells whereas taxol at equimolar concentrations only kill 20 percent of cancer cells and cisplatin or gemcitabine kill no cancer cells. More than tenfold taxol is needed to have the same effect as solamargine. This figure also shows that cisplatin, a well utilized anticancer drug and gemcitabine even at high concentrations have no effect on lung cancer cells [21]. Published studies show that solamargine combined with cisplatin results in the effective killing of cisplatinresistant cancer cells, particularly lung cancer cells [21, 22,42].
The observations in Figure 9 compare well with other reported studies with ovarian cancer [32]. Table 2 shows that only a very low concentration of solamargine is required to kill ovarian cancer cells, the concentration of vinblastine to kill ovarian cancer cells is 6 times higher than solamargine and for cisplatin it is 40 times higher. The table also shows that solamargine is much safer than vinblastine and cisplatin, and vinblastine kills more normal cells (fibroblasts) than ovarian cancer cells [32].

Figure 9. The effect of solamargine (BEC component)(●), taxol (Ñ), cisplatin (▼), or gemcitabine (○) on the survival of lung cancer cells [21].
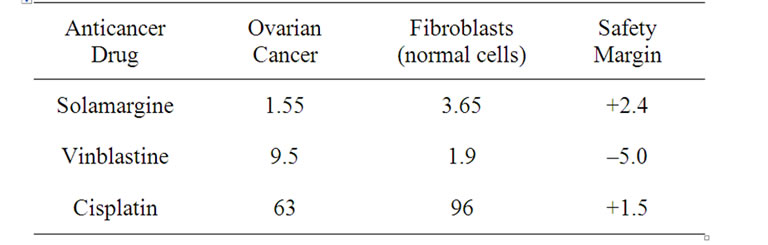
Table 2. Dose (micro mol/L) of anticancer drug to kill ovarian cancer and normal cells.
The higher the safety margin, the more effective solamargine is in killing cancer cells relative to normal cells. Safety margins of above 2 are regarded highly beneficial for anticancer drugs. A negative safety margin, means that the anticancer drug kills more normal cells than cancer cells.
Importantly, the initial studies with Curaderm utilized concentrations of BEC up to 50% in cream formulations. At these high concentrations of BEC, no significant toxic effects were observed. Subsequently clinical trials using 10% of BEC in a cream formulation determined that this formulation was effective for the treatments of keratoses, BCCs and SCCs [46]. More recently clinical trials with CuradermBEC5 containing 0.005% BEC showed impressive results when, even very large nonmelanoma skin cancers were treated [2,27,34,38,41-44,54-56]. BEC present in CuradermBEC5 is at a much lower concentration than is found naturally in the eggplant [10]. However, the eggplant also contains anticancer inhibitors which must be removed in order to obtain the anticancer benefits shown with CuradermBEC5 therapy. Accordingly, and as shown by many studies, BEC in CuradermBEC5 is very safe [2,9,27,34,38,41-44,54-56].
It was possible to effectively use these very low concentrations of BEC in CuradermBEC5 because of the cream formulation excipients such as salicylic acid and urea which increased the bioavailability of the BEC glycoalkaloids. Thus the degree of which BEC becomes available to the target cancer cells tissues after the CuradermBEC5 cream application was increased by orders of magnitudes. This resulted in BEC binding to the specific rhamnose receptors on cancer cells. The concentration of BEC interacting and eliminating the skin cancer cells were in the same order of concentrations of BEC that were effectively used in the cell culture and internal cancer treatments [2].
Studies with CuradermBEC5 have demonstrated that there are no adverse effects on the liver, kidneys or haematopoietic system during treatment [2,7,26,34,41,43,44, 47-49,54-56].
When CuradermBEC5 cream is applied to lesions on the skin, the action of CuradermBEC5 produces the following pattern of response: first erythema, then, usually, erosion, ulceration, dying of treated targeted cells and regrowth of normal cells [9,38,41,43,56]. No systemic absorption of BEC occurs [56].
The reported local adverse reactions are itching, pain and occasionally burning sensation at the site of application. The local adverse reactions which are transient, are due to excipients salicylic acid and urea in the CuradermBEC5 cream [2].
2.6. CuradermBEC5 Is Noninvasive by Simple Topical Application of a Cream
The reported recommended CuradermBEC5 treatment procedure [38] is as follows.
The lesion and surrounding area is washed with a mild, non-irritating, soap, rinsed with water and dried thoroughly. A thin layer of approximately 2 mm thick of CuradermBEC5 is applied to the lesion. The lesion is then covered with an occlusive dressing such as micropore tape. During treatment the lesion is continually covered with the micropore dressing as the lesion is not allowed to dry out to form a scab. Application of CuradermBEC5 is continued until the lesion is completely ablated and replaced with normal skin. At least twice daily application is necessary. Treatment period depends on the type and size of the lesion and accordingly, varies from a few days to several months. Clinical observations reveal that initially the lesion size appears to increase significantly and as treatment progresses the size of the lesion decreases until it is completely ablated and replaced with normal skin cells.
Figure 10 illustrates observable lesion changes in diameter during CuradermBEC5 therapy up to week 6 and after cessation of therapy. In those studies CuradermBEC5 therapy was stopped after 8 weeks. From weeks 2 to 6, CuradermBEC5 treatment resulted in significant increases in the lesions’ diameter. The reason of the initial increase in lesion size is because CuradermBEC5 is seeking and destroying the cancer cells that are initially not visible to the bare eyes. After 6 weeks of treatment the lesions size was reduced. Follow-up studies for over 1 year showed clearly that all the lesions had completely resolved [38].
Figure 11 illustrates a schematic representation of the sequential events of skin cancer treatment with CuradermBEC5 [38].
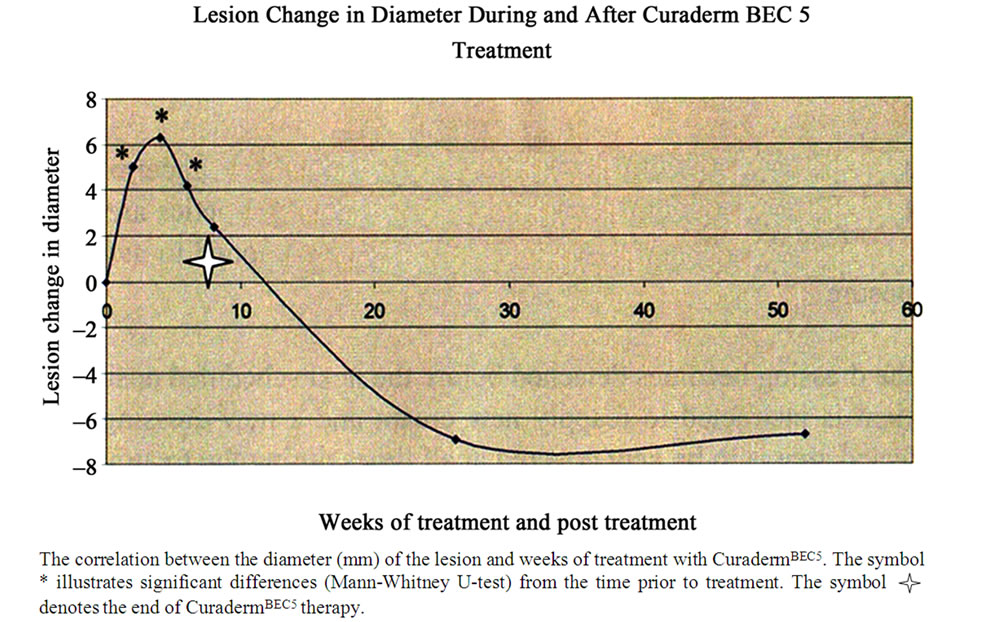
Figure 10. Changes in lesion diameter during and after CuradermBEC5 treatment.
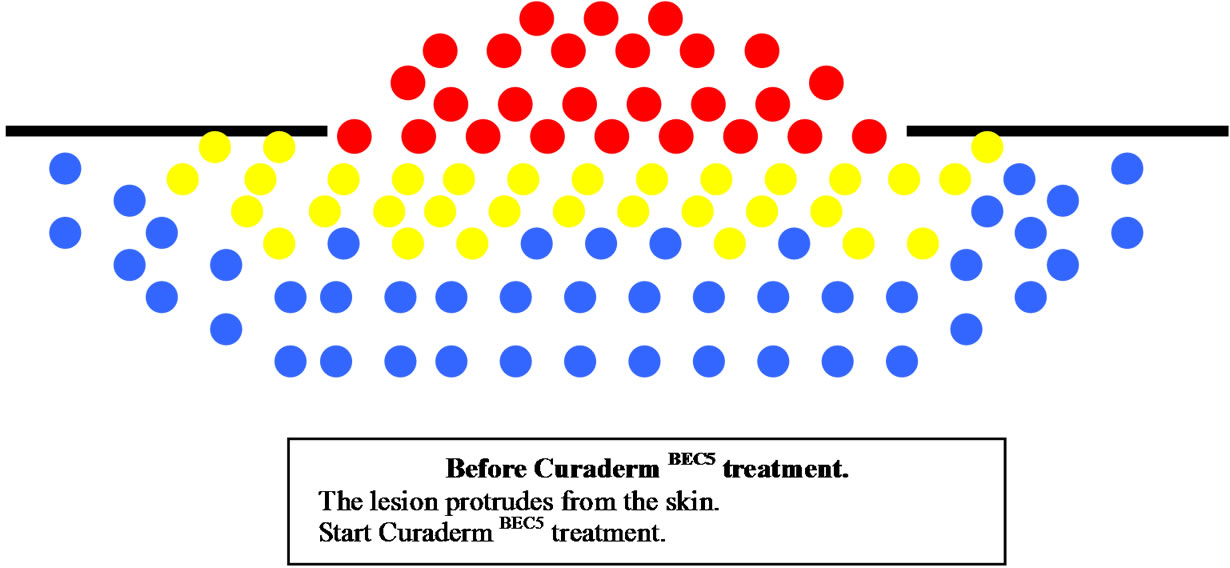
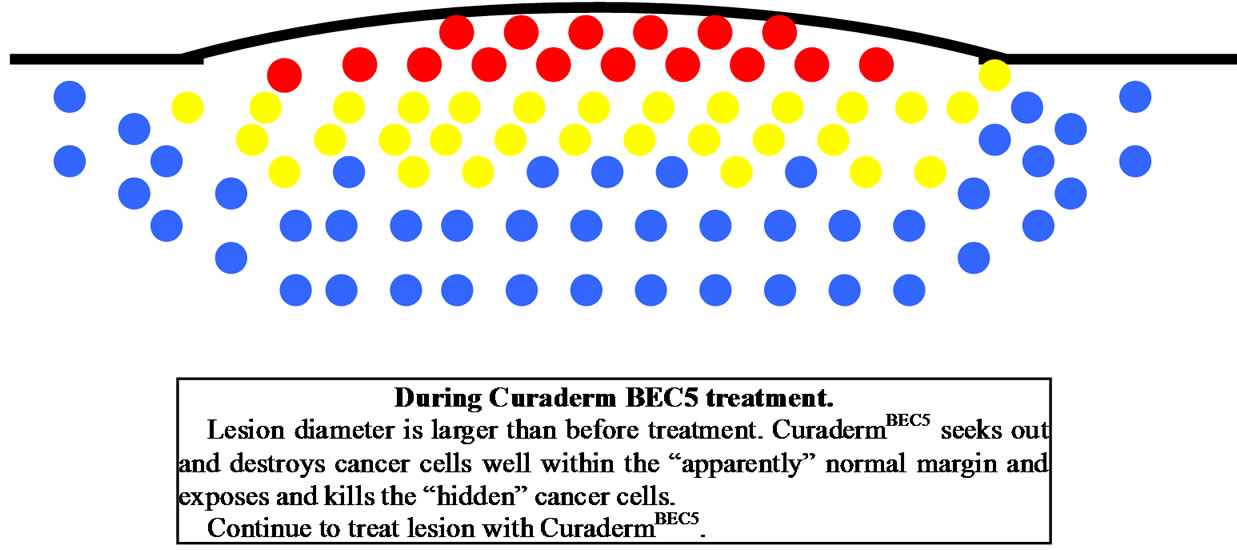
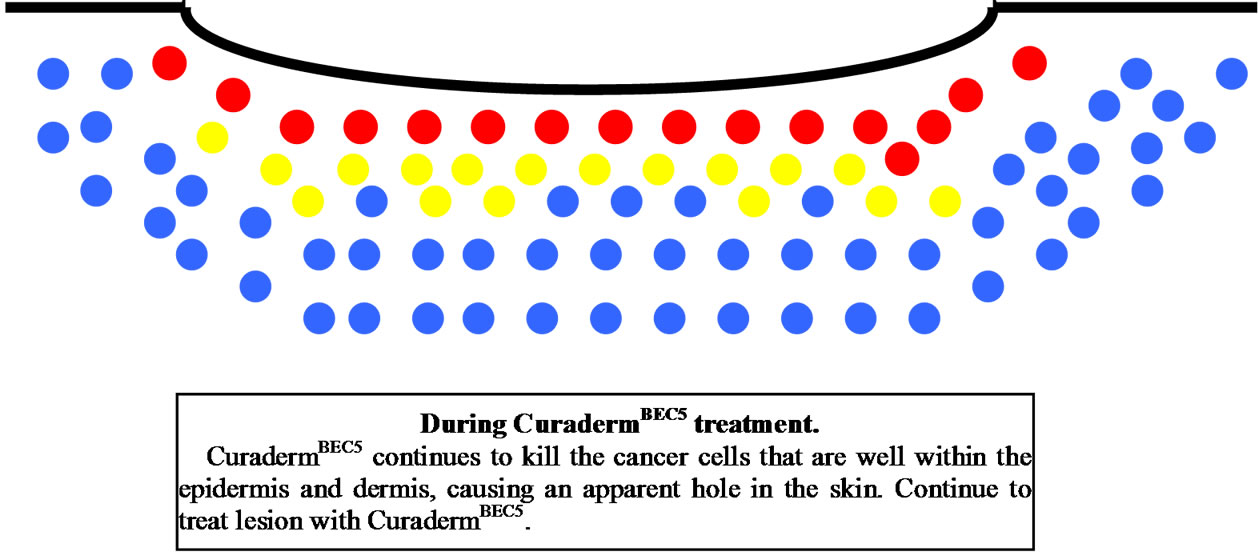
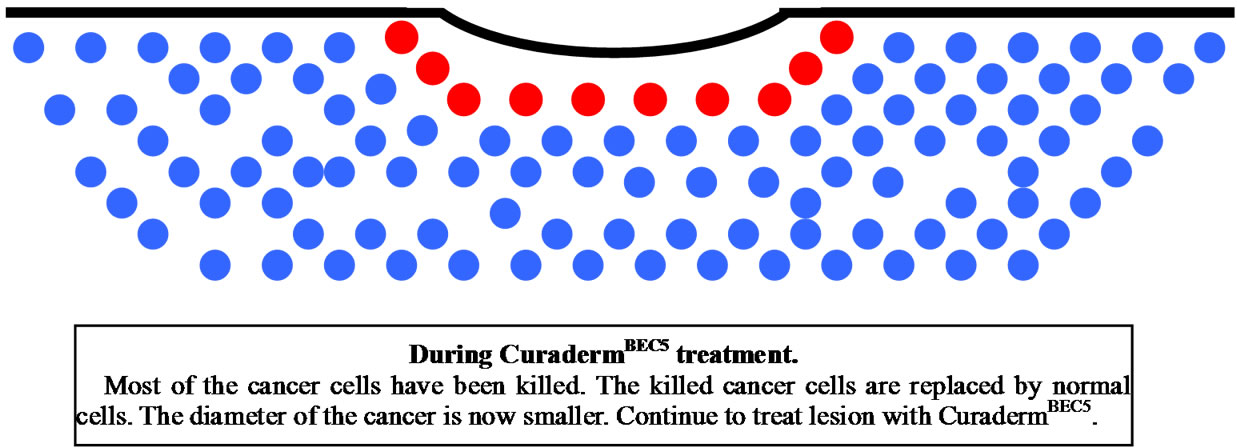
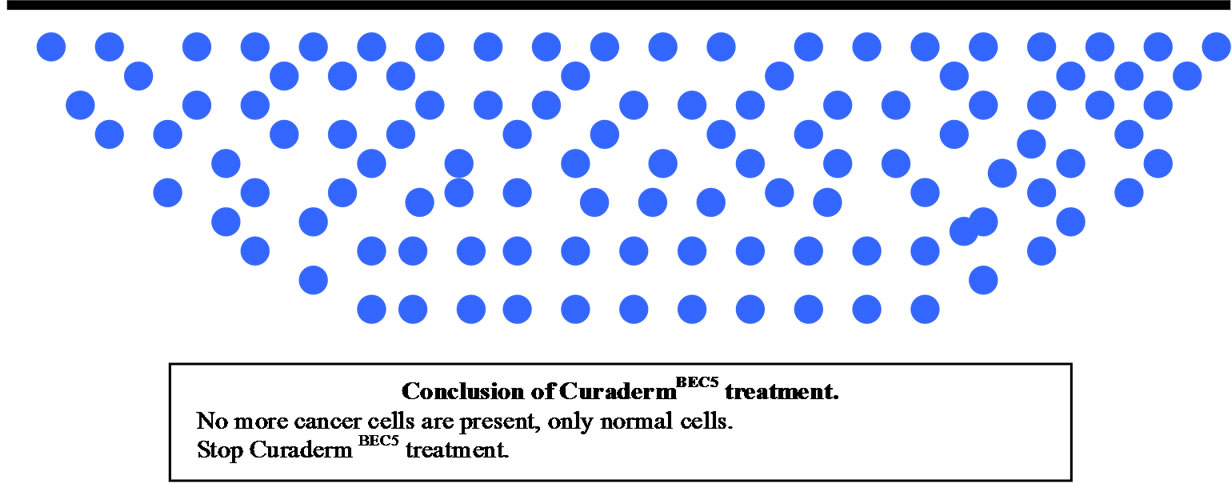
Figure 11. Schematic representation of the sequential events of skin cancer treatment with curadermBEC5.
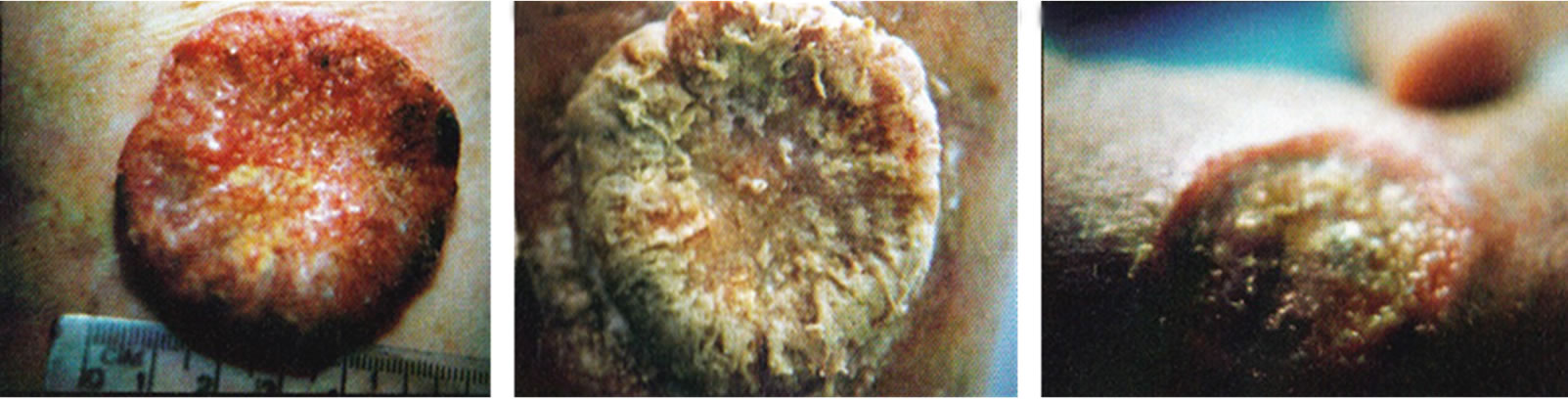
Figure 12 shows an actual clinical representation of the sequential events of a skin cancer (squamous cell carcinoma) treatment with CuradermBEC5 [38].
2.7. CuradermBEC5 Shows Superior Cosmetic Outcomes Compared with Other Available Treatments
One of the unfortunate after-effects of the most widely used skin cancer treatments is that they often leave scars after treatments. Because many skin cancers crop up on parts of the body that are most exposed to the sun, having a carcinoma on the face—and scar after treatment—is very common. Understanding that treating skin cancer by most procedures may result in scars or disfigurement can also be troubling.
The mode of action of BEC places CuradermBEC5 in an excellent enigmatic position. Consequently, treatment of skin cancer with CuradermBEC5 exhibits excellent cosmetic end results.
A unique case where a direct comparison could be made between the cosmetic effects of surgery and CuradermBEC5 treatment was previously reported [9,38]. Other studies have verified the non-comparable cosmetic outcomes of CuradermBEC5 therapy. Most recently studies were published whereby the huge benefit in terms of cosmetic outcomes of CuradermBEC5 therapy was shown [2,9,27,29,38,56,57].
Figures 13-21 illustrate some examples of cosmetic outcomes of various skin cancer treatments with CuradermBEC5 [2,9,24,26,27,29,30,34,37,38,41,42,46,54].
Health versus cosmetic outcomes with Curaderm BEC5 have additional benefits. The treatment procedure is easily performed as an outpatient treatment. The procedure is non-invasive with excellent cosmetic outcomes such as no, or very little scarring, improved colouring, texture and tone of the skin.
In most cases it is not possible to identify where the skin cancers were after CuradermBEC5 treatment. The results are permanent if proper instructions are followed as shown with follow-up studies for over a decade [2,9,27, 29,38,56].
3. Conclusions
Human skin cancers have been around perhaps since the existence of mankind. One of the earlier documentations describing skin cancer was done in 46 BC and it was stated “what drugs will not cure, the knife will, what the knife will not cure, the cautery will, what the cautery will not cure must be considered incurable” (Hippocrates’ book of Aphorisms) [42]. An early surgical removal followed by skin flap to cover the surgical wound was performed in 1846 [42].
 (a)
(a)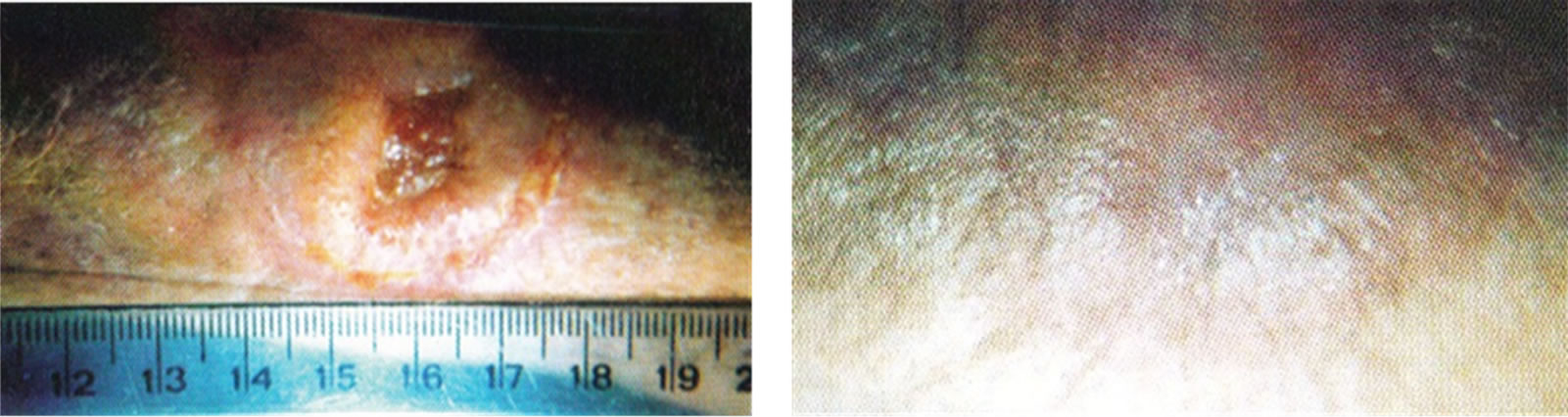 (b)
(b)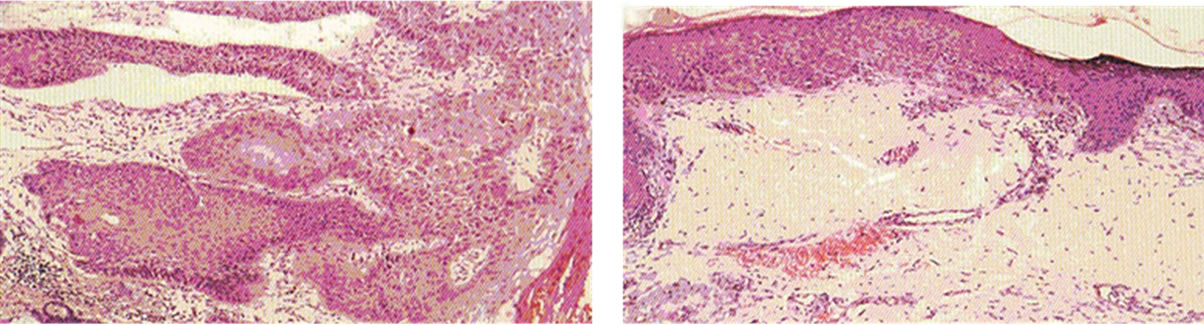 (c)
(c)
Figure 12. A very large SCC, 6 cm in diameter, before (a), during (b-d), and after (e) treatment with Curaderm. The treatment period was for 12 weeks. Note the specificity of Curaderm for the cancer cells and the regrowth of normal cells during Curaderm therapy. The clinical diagnosis was confirmed histologically by punch biopsy (f). After completion of the therapy histopathology determined that no residual cancer was present (g). Clinical assessment 5 years post treatment revealed that there was no recurrence.
 (a)
(a)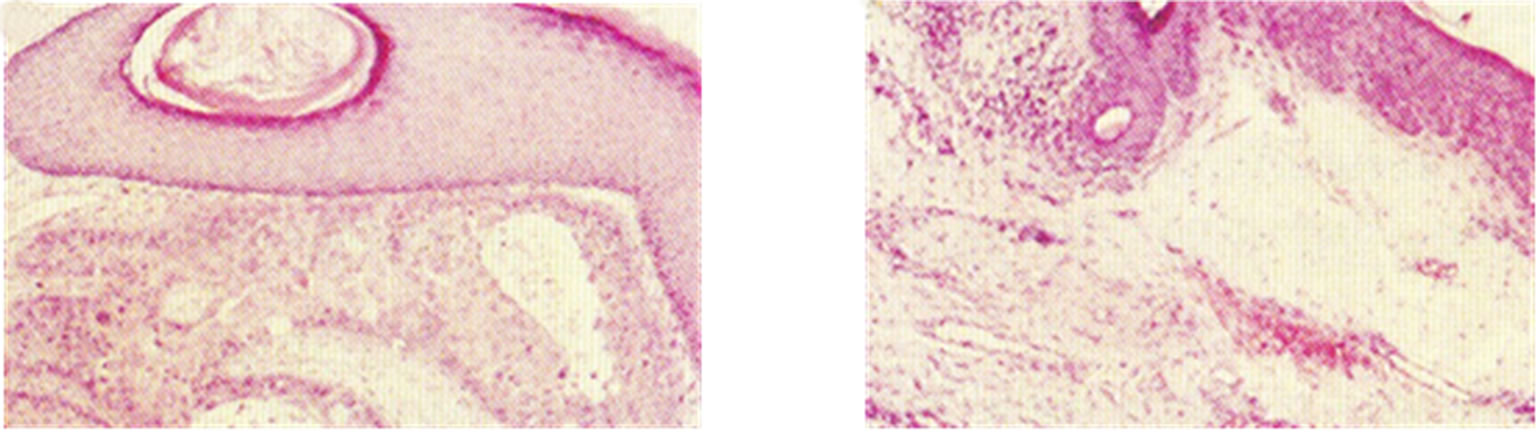 (b)
(b)
Figure 13. An intra-epithelial SCC on the penis of a patient before (a), during (b) and after Curaderm therapy (c). The prognosis of this patient before treatment with Curaderm was amputation. Treatment period was 6 weeks. The clinical diagnosis was confirmed histologically by biopsy (d). After completion of the therapy histopathology determined that no residual cancer was present (e). Clinical assessment 5 years post treatment revealed that there was no recurrence.
 (a)
(a)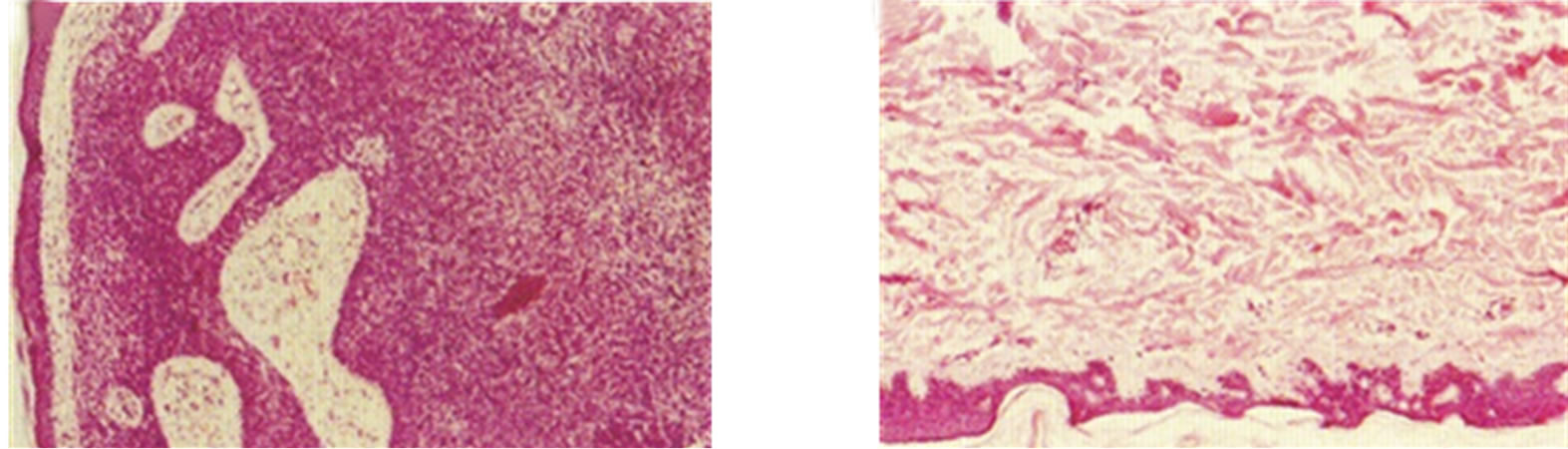 (b)
(b)
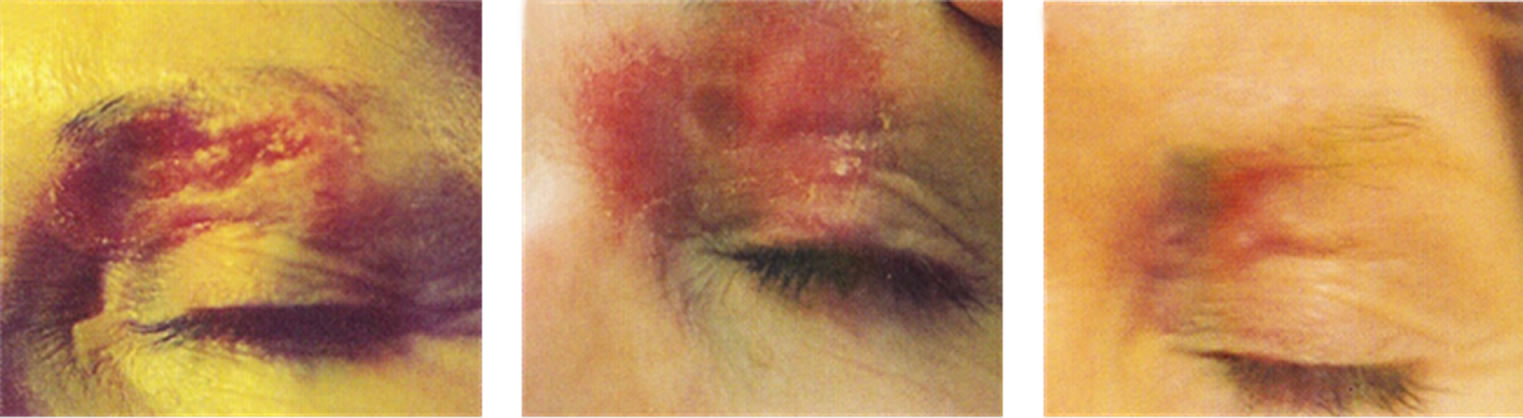
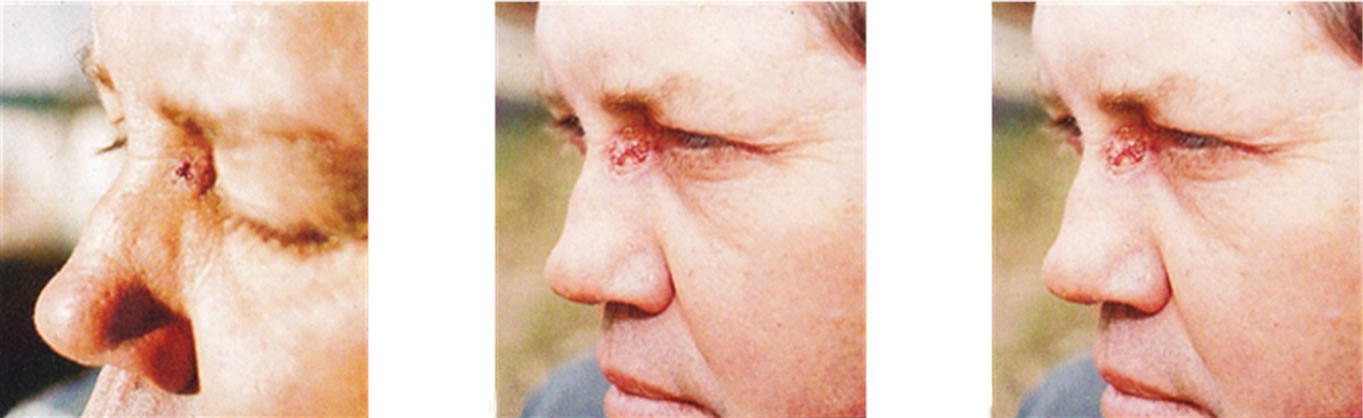
Figure 14. BCC over the left eye of a patient before (a), during (b) and after Curaderm treatment (c). Careful application of Curaderm was required to ensure that the cream did not enter the eye. During Curaderm therapy the distinct tumour can be seen surrounded by some inflammation. After treatment there was no trace of the BCC. Confirmation by histological analysis of the BCC before treatment (d) and after treatment (e) are shown. The total treatment period was 9 weeks. Clinical assessment 5 years post treatment revealed that there was no recurrence.
Subsequently, newer treatments such as Mohs micrographic surgery, curettage and electrodesiccation, cryotherapy, radiation therapy, laser therapy and topical cream treatments have evolved. Each such treatment has its benefits and its drawbacks. Some major drawbacks in all of these treatments are the nonspecifity of removing the cancer and the limited cosmetic outcomes.
More recently, a new class of antineoplastic agents, consisting of solamargine and solasonine, found naturally in Solanum plants such as S. melongena (aubergine or eggplant), have shown to be very promising candidates for internal cancers.
 (a) (b) (c)
(a) (b) (c)
Figure 15. Clinical diagnosis of a BCC on the nose of a patient before treatment with Curaderm (a), during therapy (b) and site of treated BCC after completion of therapy (c).
 (a) (b) (c)
(a) (b) (c)
Figure 16. Clinical progress of a BCC close to the eye of the patient before treatment (a), during therapy (b), and site of treated BCC after completion with Curaderm (c).
 (a)
(a)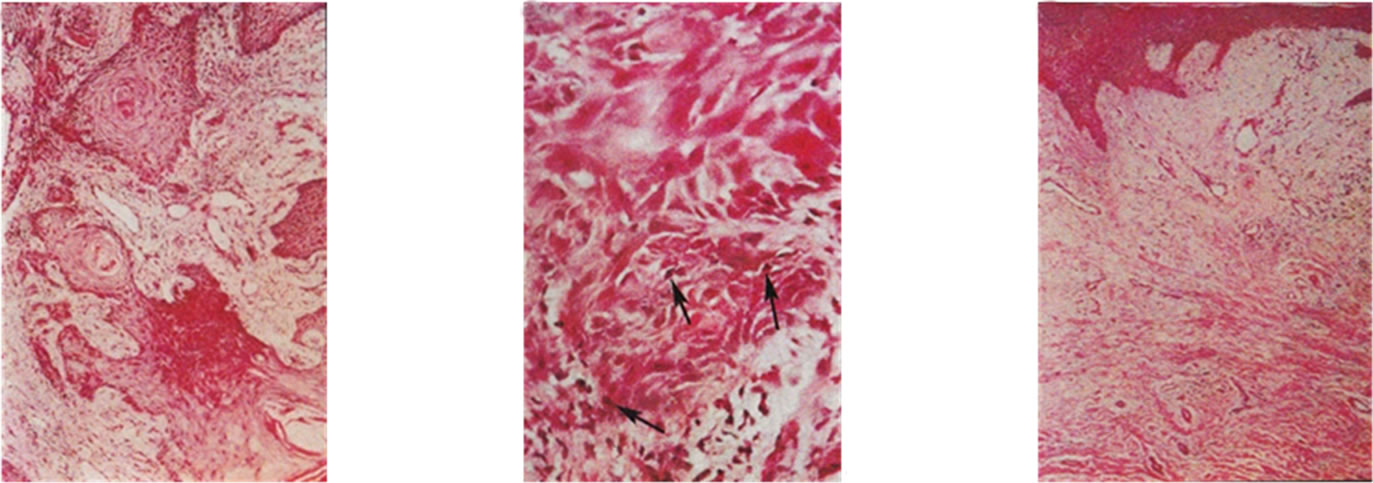 (b)
(b)
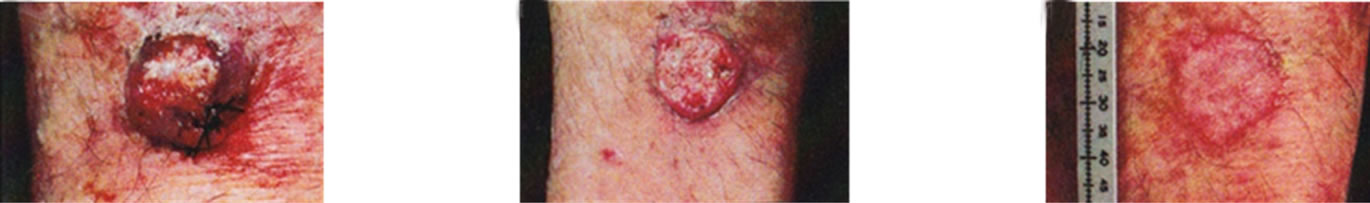
Figure 17. Clinical and histological diagnosis of an SCC on a leg of a patient before treatment (lane a), during therapy (lane b) and site of treated SCC after completion of therapy (lane c). 1. Clinical diagnosis; 2. Histological diagnosis. Arrows indicate cancer cells dying during Curaderm treatment (lane B2). The observation of this type of cell death caused by Curaderm is similar to those obtained in cell culture studies.
 (a)
(a)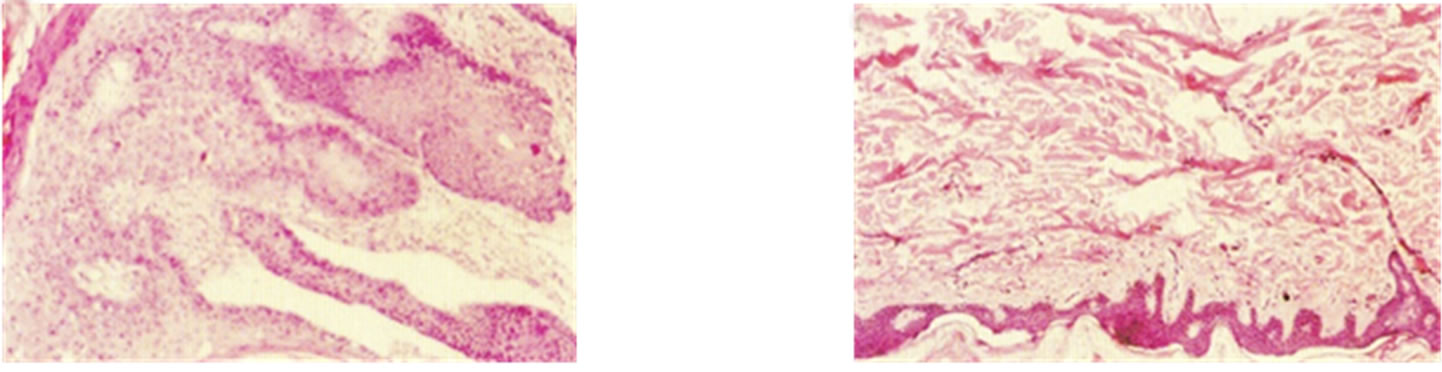 (b)
(b)
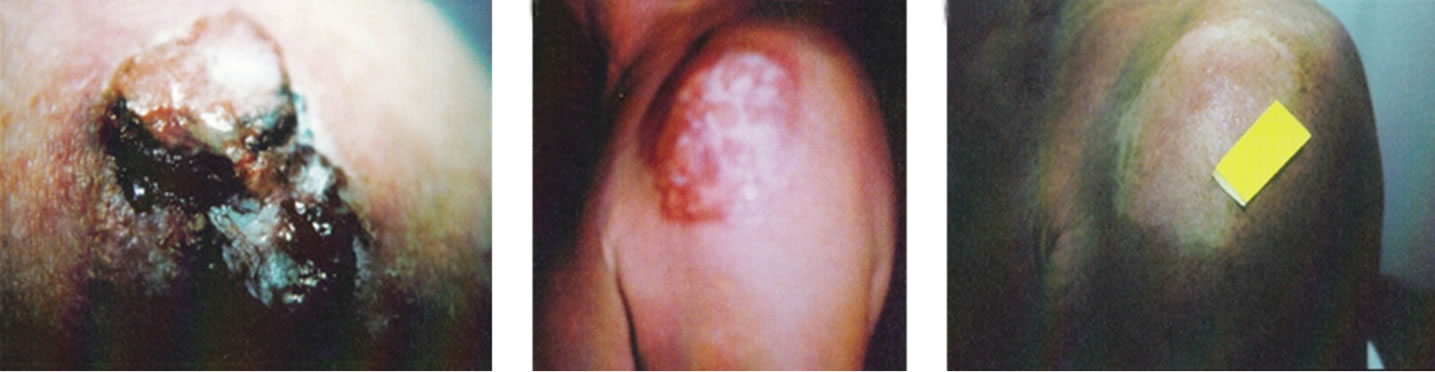
Figure 18. A large SCC (approximately 8 cm × 6 cm) on the shoulder of a patient before (a), during (b) and after (c) treatment with Curaderm. After 10 weeks the tumour was completely healed. The clinical diagnosis was confirmed histologically by punch biopsy (d). After completion of the therapy histopathology determined that no residual cancer was present (e). Clinical assessment 5 years post treatment revealed that there was no recurrence.
 (a)
(a)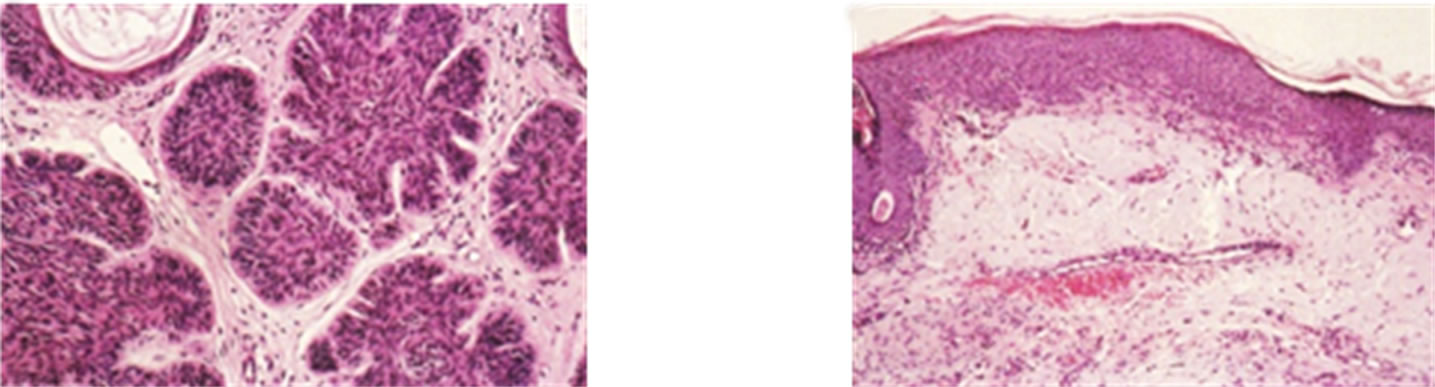 (b)
(b)
Figure 19. Large BCC on the temple of a woman (a). This BCC had been surgically removed and skin grafts applied on two previous occasions only to return. Four weeks treatment with Curaderm resulted in full regression (b). Note the cosmetic result. The clinical diagnosis was confirmed histologically by punch biopsy (c). After completion of the therapy histopathology determined that no residual cancer was present (d). Clinical assessment 5 years post treatment revealed that there was no recurrence.
 (a)
(a)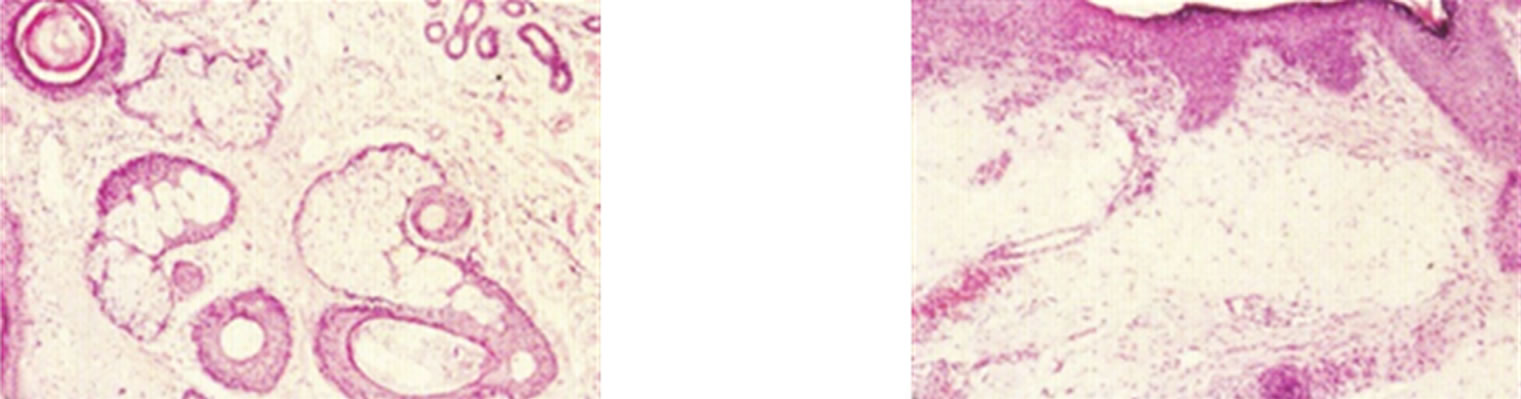 (b)
(b)
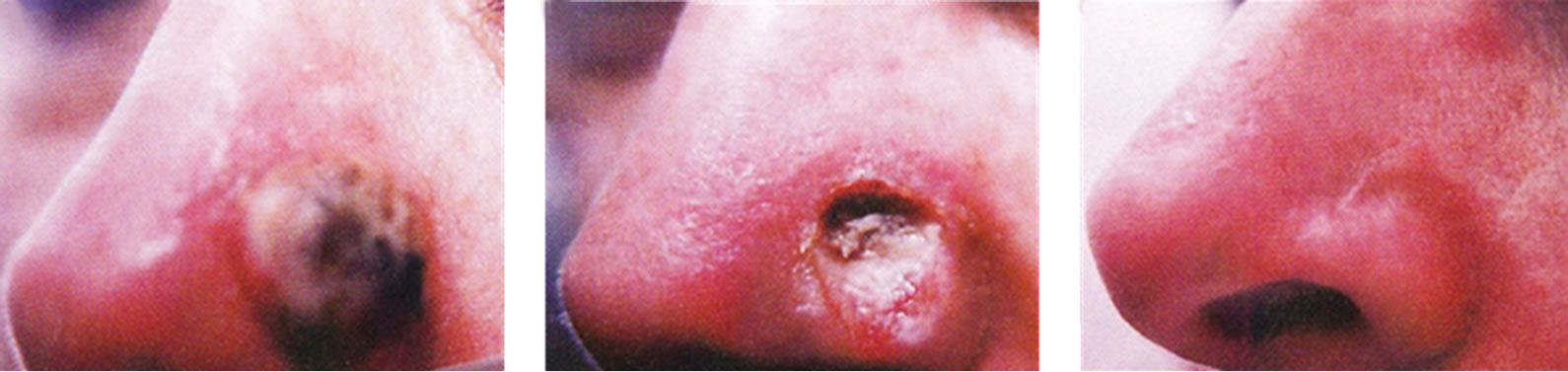
Figure 20. SCC on the nose of a patient before (a), during (b) and after Curaderm treatment (c). Curaderm was applied for 5 weeks. Note the depth of the cancer as cartilage was exposed during treatment. The clinical diagnosis was confirmed histologically by punch biopsy (d). After completion of the therapy histopathology determined that no residual cancer was present (e). Clinical assessment 5 years post treatment revealed that there was no recurrence.
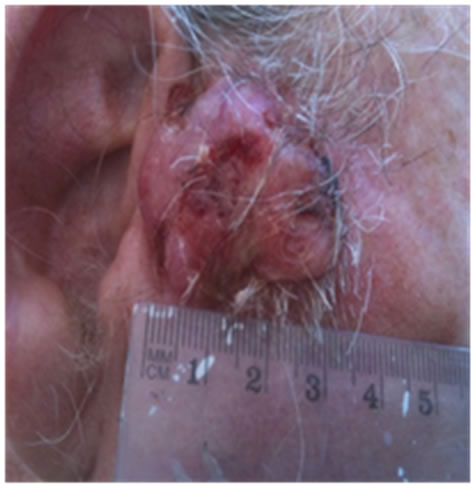
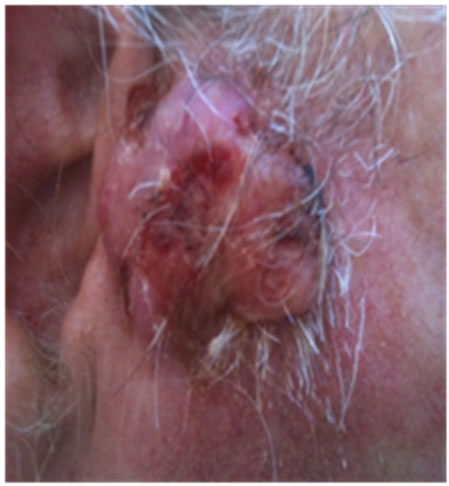
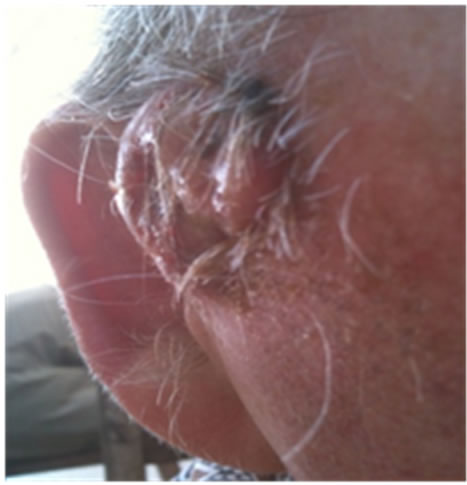
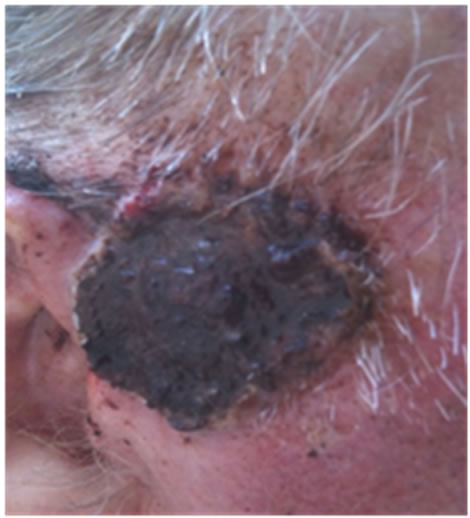
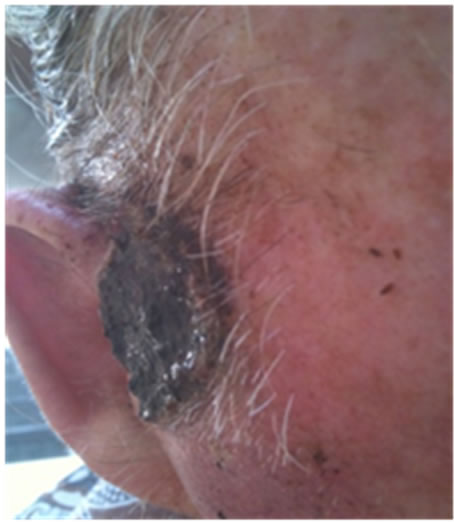
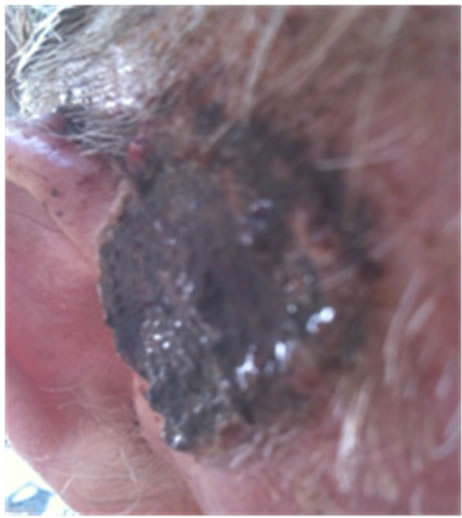

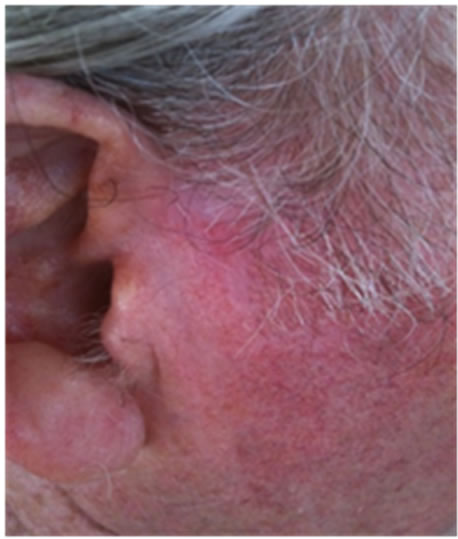
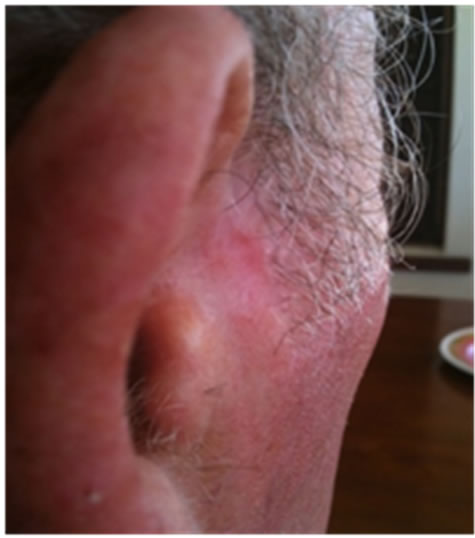
Figure 21. An extensive protruding (4 cm × 4 cm × 2 cm) BCC with central ulceration and raised curly borders on the right side of his face next to his ear is seen in this patient (top row). Treatment with Curaderm resulted in rapid break-down of the tumour and after 2 weeks of treatment the lesion was reduced to about a half of the original size. Minor bleeding had occurred during this treatment period (middle row). After 14 weeks of treatment the lesion was clinically eliminated. Normal skin cells had replaced the tumour and the cosmetic end result was excellent, with no scar tissue formation. Even the hairs had regrown where the tumour was originally (bottom row).
A topical cream, CuradermBEC5, containing these antineoplastic glycoalkaloids, is very effective and safe for the treatment of nonmelanoma skin cancers. The treatment of skin cancers with CuradermBEC5 overcomes the major drawbacks of other currently available therapies. CuradermBEC5 is specific and eliminates the cancer cells only, without harming normal cells and consequently the cosmetic end result is excellent.
The claims of CuradermBEC5 which were investigated appear to be justifiable.
Finally, CuradermBEC5 for skin cancers, it is!
REFERENCES
- H. W. Rogers, N. A. Weinstock, A. R. Harris, M. R. Hinckley, S. R. Feldman, A. B. Fleischer and B. M. Coldiron, “Incidence Estimate of Nonmelanoma Skin Cancer in the United States,” Archives of Dermatology, Vol. 146, No. 3, 2010, pp. 283-287. doi:10.1001/archdermatol.2010.19
- B. E. Cham, “Topical Solasodine Rhamnosyl Glycosides Derived from the Eggplant Treats Large Skin Cancers: Two Case Reports,” International Journal Clinical Medicine, Vol. 2, No. 4, 2011, pp. 473-477. doi:10.4236/ijcm.2011.24080
- M. B. Lens and M. Dawes, “Epidemiological Trends of Cutaneous Malignant Melanoma: Incidence of Cutaneous Malignant Melanoma,” British Journal of Dermatology, Vol. 150, 2004, pp. 179-185. doi:10.1111/j.1365-2133.2004.05708.x
- L. A. E. Sussman and D. F. Liggins, “Incompletely Excised Basal Cell Carcinoma a Management Dilemma?” Australian and New Zealand Journal of Surgery, Vol. 66, No. 5, 1996, pp. 276-278. doi:10.1111/j.1445-2197.1996.tb01184.x
- B. E. Cham, M. Gilliver and L. Wilson, “Antitumour Effects of Glycoalkaloids Isolated from Solanum sodomaeum L.,” Planta Medica, Vol. 53, No. 1, 1987, pp. 34-36. doi:10.1055/s-2006-962612
- B. E. Cham and B. Daunter, “Solasodine Glycosides. Selective Cytoxicity for Cancer Cells and Inhibition of Cytotoxicity by Rhamnose in Mice with Sarcoma 180,” Cancer Letters, Vol. 55, No. 3, 1990, pp. 221-225. doi:10.1016/0304-3835(90)90122-E
- B. E. Cham, “Monograph on the Compound BEC,” Drugs of the Future, Vol. 13, 1988, pp. 714-716.
- B. E. Cham and L. Wilson, “HPLC of Glycoalkaloids from Solanum sodomaeum,” Planta Medica, Vol. 53, No. 1, 1987, pp. 34-36. doi:10.1055/s-2006-962612
- B. E. Cham, “Eggplant Wins War against Skin Cancer. Conquest on the Horizon for Internal Cancers?” Book, in Preparation.
- P. G. Jones and G. R. Fenwick, “The Glycoalkaloid Content of Some Edible Solanaceous Fruits and Potato Products,” Journal Science Food Agriculture, Vol. 32, No. 4, 1981, pp. 419-421. doi:10.1002/jsfa.2740320418
- P. Vijayan, V. Kumar, S. Dhanaraj, S. A. Badami and B. Suresh, “In Vitro Cytotoxicity and Anti-Tumour Properties of the Total Alkaloid Fraction of Unripe Fruits of Solanum Pseudocapsicum,” Pharmaceutical Biology, Vol. 40, 2002, pp. 456-460. doi:10.1076/phbi.40.6.456.8444
- T. Nakamura, C. Komori, Y. Lee, F. Hashinoto, S. Yahara, T. Nohara and A. Ejina, “Cytotoxic Activities of Solanum Steroidal Glycosides,” Biological and Pharmaceutical Bulletin, Vol. 19, No. 4, 1996, pp. 564-566. doi:10.1248/bpb.19.564
- K. W. Kuo, S. H. Hsu, Y. P. Li, W. L. Lin, L. F. Liu, L. C. Chang, C. C. Lin, C. N. Lin and H. M. Sheu, “Anticancer Activity Evaluation of the Solanum Glycoalkaloid Solamargine. Triggering Apoptosis in Human Hepatoma Cells,” Biochemistry Pharmacology, Vol. 60, No. 1-2, 2000, pp. 1865-1873. doi:10.1016/S0006-2952(00)00506-2
- L. C. Chang, T. R. Tsai, J. J. Wang, C. N. Lin and K. W. Kuo, “The Rhamnose Moiety of Solamargine Plays a Crucial Role in Triggering Cell Death by Apoptosis,” Biochemical and Biophysical Research Communications, Vol. 242, No. 1, 1998, pp. 21-25.
- S. Badami, S. A. Manohara Reddy, E. P. Kumar, P. Vijayan and B. Suresh, “Antitumour Activity of Total Alkaloid Fraction of Solanum Pseudocapsicum Leaves,” Phytotherapy Research, Vol. 17, No. 9, 2003, pp. 1001-1004. doi:10.1002/ptr.1229
- A. Esteves-Souza, T. M. Silva and C. C. F. Alves, “Cytotoxic Activities against Ehrlich Carcinoma and Human K562 Leukaemia of Alkaloids and Flavonoid from Two Solanum Species,” Journal of Brazil Chemistry Society, Vol. 13, No. 6, 2002, pp. 838-842. doi:10.1590/S0103-50532002000600017
- L. A. Chami, R. Méndez, B. Chataing, J. O’Callaghan, A. Usubillaga and L. LaCruz, “Toxicological Effects of α- Solamargine in Experimental Animals,” Phytotherapy Research, Vol. 17, No. 3, 2003, pp. 254-258. doi:10.1002/ptr.1122
- C. A. Hall, T. Hobby and M. Cipollini, “Efficacy and Mechanisms of α-Solasonine-and α-Solamargine-Induced Cytolysis on Two Strains of Trypanosoma cruzi,” Journal of Chemical Ecology, Vol. 32, No. 11, 2006, pp. 2405- 2416. doi:10.1007/s10886-006-9153-5
- R. Verpoorte, “Exploration of Nature’s Chemodiversity: The Role of Secondary Metabolites as Leads in Drug Development,” Drug Discovery Today, Vol. 3, No. 5, 1998, pp. 232-238. doi:10.1016/S1359-6446(97)01167-7
- J. G. Roddick, A. L Rÿrenber and M. Weissenber, “Membrane-Disrupting Properties of the Steroidal Glycoalkaloids Solasonine and Solamargine,” Phytochemistry, Vol. 29, No. 5, 1990, pp. 1513-1518. doi:10.1016/0031-9422(90)80111-S
- K. W. Kuo and C. N. Lin, “Pharmacological Composition for Treating Cancer Cells,” United States Patent 6, 1999, pp. 214-803.
- Solbec Pty Ltd. hhtp://www.solbec.com.au
- C. Amalfi, “The Little Mouse Who Wouldn’t Say Die,” Cosmos Magazine, 2006, p. 471.
- B. E. Cham, “Plants Yield Skin Cancer Cure,” Austral Technology Review, Vol. 2, 1989, p. 53.
- R. Evans, B. E. Cham and B. Daunter, “Letter to the Editor,” Your Pharmacy, Vol. January, 1989, p. 1.
- B. E. Cham, “Curaderm (Antineoplastic) Launched in Australia,” Drug News and Perspectives, Vol. 2, No. 2, 1989, p. 112.
- B. E. Cham, “Solasodine Rhamnosyl Glycosides in a Cream Formulation Is Effective for Treating Large and Troublesome Skin Cancers,” Research Journal Biological Science, Vol. 2, No. 7, 2007, pp. 749-761.
- A. K. Taraphdar, M. Roy and R. K. Bhattacharya, “Natural Products as Inducers of Apoptosis: Implication for Cancer Therapy and Prevention,” Current Science, Vol. 80, No. 11, 2001, pp. 1387-1395.
- B. E. Cham, “Solasodine Rhamnosyl Glycosides Specifically Bind Cancer Cell Receptors and Induce Apoptosis and necrosis. Treatment for Skin Cancer and Hope for Internal Cancers,” Research Journal Biological Science, Vol. 2, No. 4, 2007, pp. 503-514.
- B. E. Cham, “Cancer Intralesion Chemotherapy with Solasodine Rhamnosyl Glycosides,” Research Journal Biological Sciences, Vol. 3, 2008, pp. 1008-1017.
- L. Y. Shiu, L. C. Chang, C. H. Liang, Y. S. Huang, H. M. Sheu and K. W. Kuo, “Solamargine Induces Apoptosis and Sensitizes Breast Cancer Cells to Cisplatin,” Food Chemistry Toxicology, Vol. 45, No. 11, 2007, pp. 2155- 2164. doi:10.1016/j.fct.2007.05.009
- B. Daunter and B. E. Cham, “Solasodine Glycosides. In Vitro Preferential Cytotoxicity for Human Cancer Cells,” Cancer Letters, Vol. 55, No. 3, 1990, pp. 209-220. doi:10.1016/0304-3835(90)90121-D
- X. Li, Y. Zhao, W. K. K. Wu, S. Liu, M. Cui and H. Lou, “Solamargine Induces Apoptosis Associated with p53 Transcription-Dependent and Transcription-Independent Pathways in Human Osteosarcoma U20S Cells,” Life Science, Vol. 88, No. 7-8, 2011, pp. 314-321. doi:10.1016/j.lfs.2010.12.006
- B. E. Cham, “Solasodine Glycosides as Anti-Cancer Agents: Pre-Clinical and Clinical Studies,” Asia Pacific Journal Pharmacology, Vol. 9, No. 2, 1994, pp. 113-118.
- L. Y. Shiu, C. H. Liang, Y. S. Huang, H. M. Sheu and K. W. Kuo, “Downregulation of HER2/neu Receptor by Solamargine Enhances Anticancer Drug-Mediated Cytotoxicity in Breast Cancer Cells with High-Expressing HER2/ neu,” Cell Biology and Toxicology, Vol. 24, No. 1, 2008, pp. 1-10. doi:10.1007/s10565-007-9010-5
- C. Paquet, A. T. Sané, M. Beauchemin and R. Bertrand, “Caspase-and Mitochondrial Dysfunction Dependent Mechanisms of Lysosomal Leakage and Cathepsin B Activation in DNA Damage-Induced Apoptosis,” Leukemia, Vol. 19, 2005, pp. 784-791. doi:10.1038/sj.leu.2403717
- B. E. Cham and T. R. Chase, “Solasodine Rhamnosyl Glycosides Cause Apoptosis in Cancer Cells, Do They Also Prime the Immune System Resulting in Long Term Protection Against Cancer?” Planta Medica, in Press.
- B. E. Cham, “The Eggplant Cancer Cure. A Treatment for Skin Cancer and New Hope for Other Cancers from Nature’s Pharmacy,” Smart Publications, Petaluma, 2007, p. 122.
- L. Sun, Y. Zhao, H. Yuan, X. Li, A. Cheng and H. Lou, “Solamargine, a Steroidal Alkaloid Glycoside, Induces Oncosis in Human K562 Leukemia and Squamous Cell Carcinoma KB Cells,” Cancer Chemotherapy and Pharmacology, Vol. 65, No. 4, 2010, pp. 1125-1130.
- I. Davis and A. Dowling, “Side Effects of Chemotherapy,” Cancer Council Victoria, 2010. www.cancervic.org.au
- B. E. Cham, “Solasodine Rhamnosyl Glycosides Specifically Bind Cancer Cell Receptors and Induce Apoptosis and Necrosis. Treatment for Skin Cancer and Hope for Internal Cancers,” Research Journal Biological Science. Vol. 2, No. 4, 2007, pp. 503-514.
- Basic Cell Cercinoma Information. www.basalcellcarcinoma.info
- B. E. Cham and B. Daunter, “Topical Treatment of PreMalignant and Malignant Skin Cancers with Curaderm,” Drugs of Today, Vol. 26, 1990, pp. 55-58.
- B. E. Cham, B. Daunter and R. Evans, “Topical Treatment of Malignant and Premalignant Skin Cancers by Very Low Concentration of a standard mixture of Solasodine Glycosides,” Cancer Letters, Vol. 59, No. 3, 1991, pp. 183-192.
- B. Schuell, T. Greenberger, G. V. Kornek, N. Durran, D. Depisch, F. Lang, B. Schneeweiss and W. Scheithauer, “Side Effects during Chemotherapy Predict Tumour Response in Advanced Colorectal Cancer,” British Journal Cancer, Vol. 93, 2005, pp. 744-748. doi:10.1038/sj.bjc.6602783
- B. E. Cham and H. M. Meares, “Glycoalkaloids from Solanum sodomaeum L. Are Effective in the Treatment of Skin Cancers in Man,” Cancer Letters, Vol. 36, No. 2, 1987, pp. 111-118. doi:10.1016/0304-3835(87)90081-4
- B. E. Cham, “Efficacy and Mode of Action of Solasodine Glycosides (BEC) on Cancer Cells,” Proceedings of the 4th Oceania Symposium on Complementary Medicine, Mark S. Walker, Ed., Bio Concepts Publishing, Brisbane, 1993, pp. 41-51.
- B. E. Cham, B. Daunter and R. Evans, “Topical Treatment of Malignant and Premalignant Skin Cancers by Very Low Concentrations of a Standard Mixture of Solasodine Glycosides,” Cancer Letters, Vol. 59, No. 3, 1991, pp. 183-192. doi:10.1016/0304-3835(91)90140-D
- B. E. Cham, “Solasodine Glycosides: A New Modality for Cancer,” Proceeding of 2nd Oceania Symposium, Mark S. Walker, Ed., Bio Concepts Publishing, Brisbane, 1991, pp. 30-36.
- M. Ono, K. Nishimura, K. Suzuki, T. Fukushima, K. Igoshi, H. Yoshimitsu, T. Ikeda, and T. Nohara, “Steroidal Glycosides from the underground Parts of Solanum Sodomaeum,” Chemical & Pharmaceutical Bulletin, Vol. 54, No. 2, 2006, pp. 230-233. doi:10.1248/cpb.54.230
- L. F. Liu, C. H. Liang, L. Y. Shiu, W. L. Lin, C. C. Lin and K. W. Kuo, “Action of Solamargine on Human Lung Cancer Cells-Enhancement of the Susceptibility of Cancer Cells to TNFs,” FEBS Letter, Vol. 577, No. 1-2, 2004, pp. 67-74. doi:10.1016/j.febslet.2004.09.064
- K. R. Lee, N. Kozukue, J. S. Han, J. H. Park, E. Y. Chang, E. J. Back, J. S. Chang and M. Friedman, “Glycoalkaloids and Metabolites Inhibit the Growth of Human Colon (HT29) and Liver (HepG2) Cancer Cells,” Journal Agriculture Food Chemistry, Vol. 52, No. 10, 2004, pp. 2832- 2839. doi:10.1021/jf030526d
- B. Chataing, N. B. Christancho and A. Usubillaga, “Topical Treatment of Herpes Simplex, Herpes Zoster and Genital Herpes with a Mixture of Solanaceous Glycoalkaloids,” MedULA. Universidad de Los Andes, Vol. 7. 1998, pp. 30-34.
- N. Walsh, “Promising Topical Therapy for Actinic Keratoses—Brief Article,” International Medical News Group, Morristown, 2005.
- S. Punjabi, I. Cook, P.Kersey, R. Marks, A. Finlay, G. Sharpe, et al., “A Double Blind, Multi-Centre Parallel Group Study of BEC-5 Cream in Basal Cell Carcinoma,” European Academy Dermatology Venereol, Vol. 14, 2000, pp. 47-60.
- S. Punjabi, L. J. Cook, P. Kersey, R. Marks and R. Cerio, “Solasodine Glycoalkaloids: A Novel Topical Therapy for Basal Cell Carcinoma. A Double-Blind, Randomized, Placebo-Controlled, Parallel Group, Multicentre Study,” International Journal Dermatology, Vol. 47, 2008, pp. 78-82.
- B. E. Cham, “Combination Therapies with Intralesion Injection and Topical Application of Solasodine Rhamnosyl Glycosides (BEC) Rapidly Remove Large Skin Cancers,” In Preparation.

