Journal of Modern Physics
Vol. 2 No. 9 (2011) , Article ID: 7150 , 5 pages DOI:10.4236/jmp.2011.29129
Structural Characterization of Borate Glasses Containing Zinc and Manganese Oxides
1Department of Physics, Sarojini Naidu College for Women, Kolkata, India
2Department of Physics, The University of Burdwan, West-Bengal, India
3CSIR-Central Mechanical Engineering Research Institute, Durgapur, India
4The University of Burdwan, Bardhaman, India
E-mail: m_pal@cmeri.res.in
Received January 31, 2011; revised April 22, 2011; accepted May 6, 2011
Keywords: Borate Glass, Differential Thermal Analysis (DTA), Infrared (IR) Spectroscopy, Optical Band Gap
ABSTRACT
We have investigated the effect of inclusion of two transition metal ions (TMI) on structure and optical properties of borate glass system having composition xMnO2 – yZnO – (100 – x – y) B2O3 (9 ≤ x ≤ 12, 36 ≤ y ≤ 48) prepared by melt quenched route. Thermal study by using a differential scanning calorimeter (DSC) reveals that the glass transition temperature (Tg) and crystallization temperature (Tc) of the glasses increases with the increase of borate content in the system. Fourier transform infrared (FTIR) spectra indicate that inclusion of TMI produces BO3 and BO4 structural units by breaking the boroxol (B3O6) ring. The optical band gap energy estimated from ultraviolet-visible spectra shows a decreasing tendency when TMI are incorporated in the borate structure.
1. Introduction
Glasses are receiving considerable attention due to their unique properties like hardness, good strength, transparency and excellent corrosion resistance. X-ray diffraction (XRD), infra-red spectroscopy (IR), differential scanning calorimetry (DSC) studies has been extensively employed over the years to investigate the structure of glasses [1-4]. Borate glasses, in particular, have been the subject of numerous infra-red studies due to their structural peculiarities [5-8]. In pure B2O3 glass structure most of the boron is involved in B3O6 (boroxol) ring. Addition of modifier breaks boroxol ring and thereby produced BO3 and BO4 units [6,8]. In addition, modifier also changes the physical properties along with structural modifications.
Recently, the study of oxide glasses doped with transition metal ions (TMI) has received considerable attention due to their attractive combination of physical and chemical properties. TMI doped borate glasses have application in microelectronics, optical glasses and solid state laser [9-11]. Continued effort for the development of new glassy materials either by doping or by adding TMI, and the study of their novel properties is highly relevant due to their potential applications in various technological fields [12,13]. Keeping in mind the very fact of creating novel functionalities we have chosen an uncommon glass system. We report here the preparation, structural characterization and optical properties of manganese and zinc oxide containing B2O3 glass with an intention to precipitate Mn-doped ZnO crystal in the borate glass matrix, which may lead to a new composite spintronics material.
2. Experimental Procedure
2.1. Preparation
Multicomponent transition metal oxide glasses containing MnO2-ZnO-B2O3 having different compositions, presented in Table 1, have been prepared from analytical grade precursors MnO2, ZnO and B2O3. Batches of 5 gm sample were prepared by taking weighted amounts of three oxides in an alumina crucible and melting mixture in a precisely controlled high temperature furnace (Thermolyne type 46100) at a temperature 1200˚C in ordinary air atmosphere. Melted mixtures were repeatedly swirled to ensure complete homogenization. The
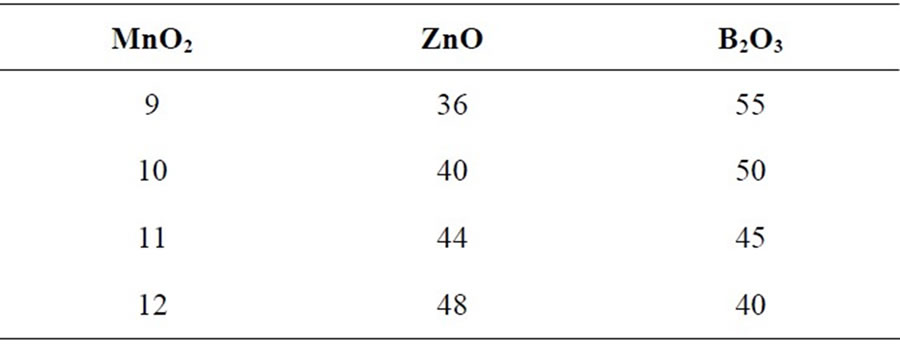
Table 1. Nominal chemical composition of the glasses (mol%).
glasses were prepared by quenching the melted materials between two brass plates.
2.2. Characterization
X-ray diffraction technique was used to check for possible crystallinity of the samples after quenching and annealing. We have utilized Philips (Philips PW 1050/51) x-ray powder diffractometer with CuKa radiation. Differential scanning calorimetry study was carried out on a Schimazdu DSC-60 in the temperature range 30˚C - 600˚C at a constant rate 10˚C/min. under ordinary air atmosphere using aluminum pan. The accuracy in determining the Tg and Tc were ± 3˚C. Scanning electron microscopy (SEM) study was performed in a Hitachi made instrument (S-3000N). Vibrational spectra of various asprepared glasses were obtained by KBr pellet technique in the range 400 - 4000 cm–1 using a Nicolet Magma-IR (750, Series II) spectrophotometer. The optical absorption spectra of as-prepared glass samples were recorded at room temperature in the range 200 - 800 nm using a double beam Hitachi spectrophotometer (model U3410).
3. Result and Discussions
3.1. XRD and SEM Study
Figure 1 presents the XRD pattern of the sample containing 45% B2O3 which is typical for other samples. XRD patterns of all the as-prepared samples show no sharp Bragg’s peak, but only a broad diffuse hump around low angle region. This is the clear indication of amorphous nature within the resolution limit of XRD instrument. Scanning electron microscopy studies of these as-prepared samples also exhibit a clear surface without the presence of any microstructure. Figure 2 shows a typical SEM micrograph of as-prepared sample having 55% B2O3. Absence of microstructure in SEM picture also indicates the amorphous as well as homogeneous nature of the samples.
3.2. DSC Analysis
Figure 3 shows the DSC curve of as-prepared sample
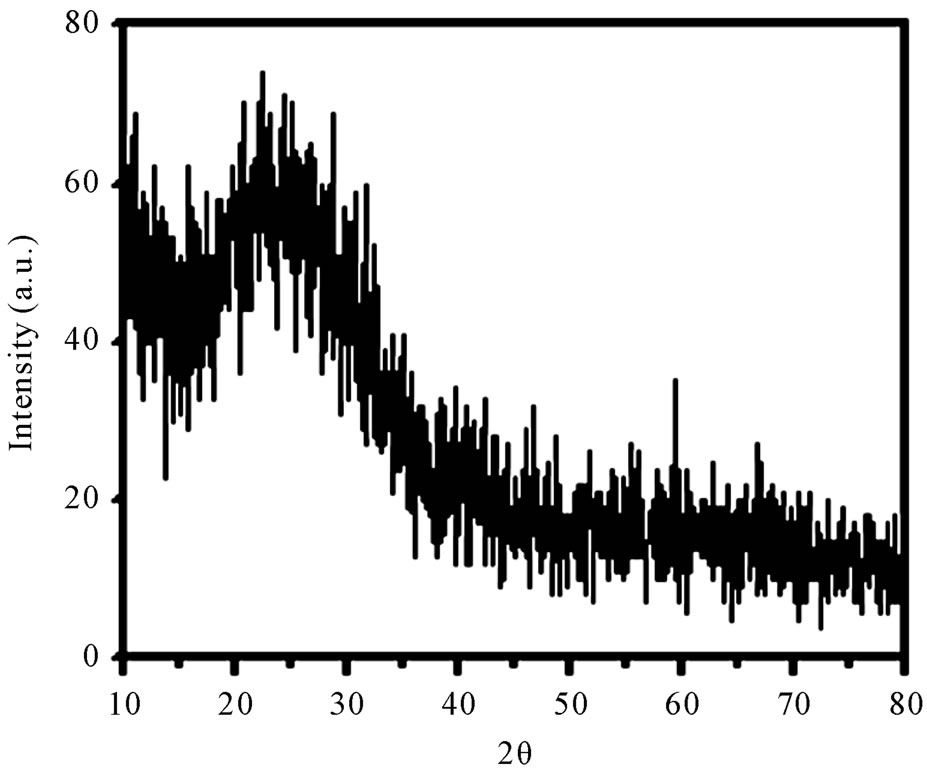
Figure 1. XRD patterns of the sample containing 45 mole% B2O3.
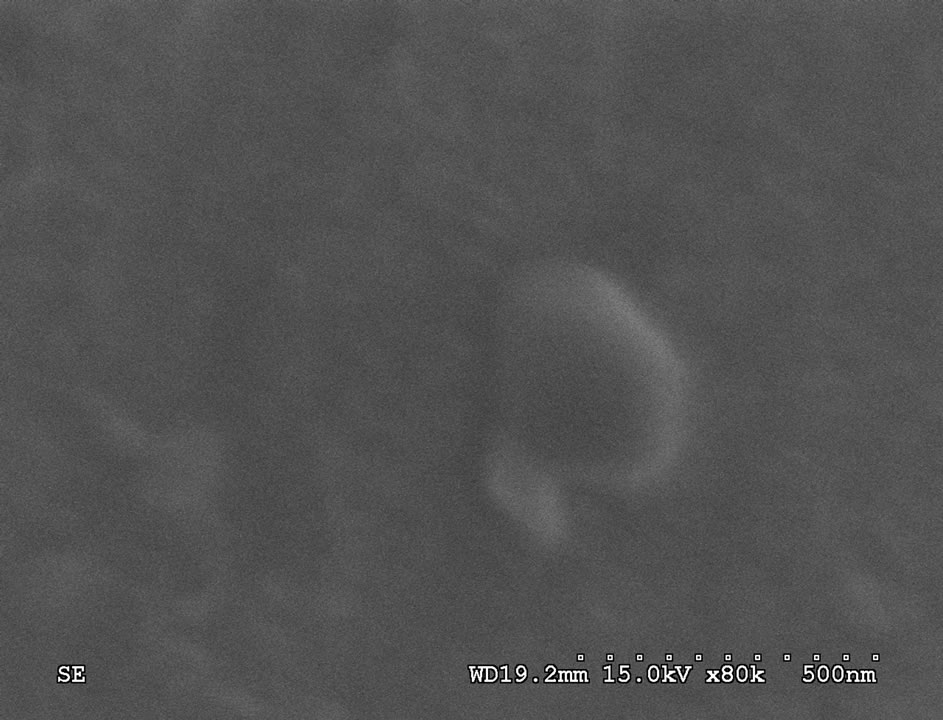
Figure 2. SEM micrograph of as-prepared sample having 55 mole% B2O3.
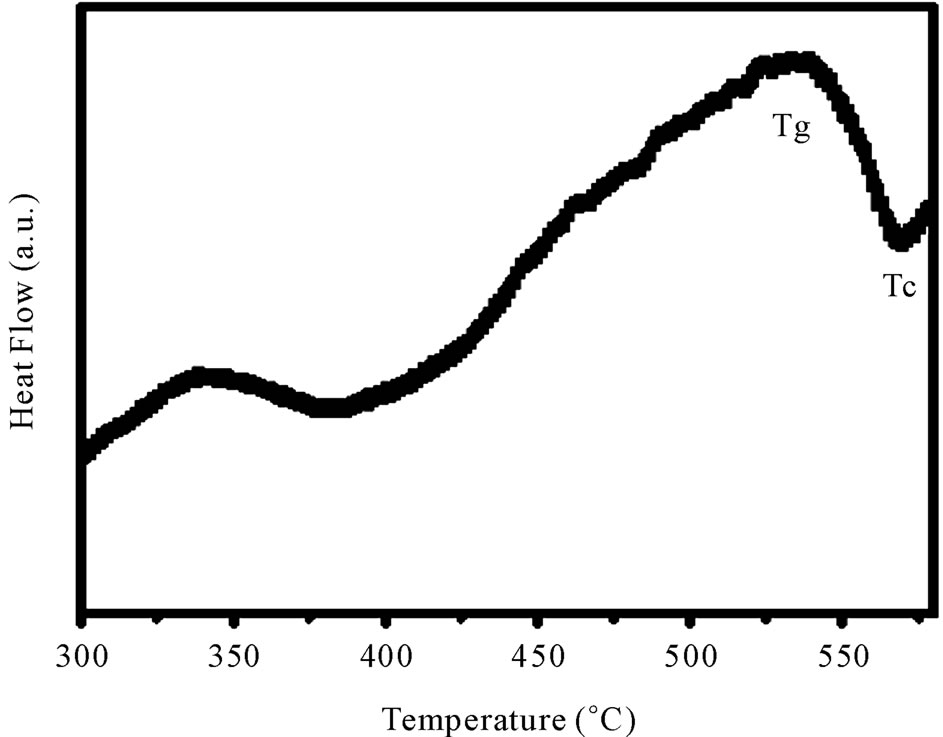
Figure 3. Differential scanning calorimetric curve of 40 mol% B2O3 glass.
having 40 mol% B2O3. This curve clearly shows one endothermic peak and one exothermic peak.
The endothermic peak corresponds to the glass transition while the exothermic peak indicates the crystallization point of the glass. The glass transition (Tg) as well as crystallization temperatures (Tc) are estimated by the slope intercept method. The nature of the DSC curves is typical for other glass compositions.
Thermal study of the glasses were performed because any change in the coordination number of network forming atoms, or the formation of nonbridging oxygen, is known to be reflected in the Tg. Figure 4 illustrates the variation of Tg and Tc with compositions. DSC study reveals that both Tg and Tc increase monotonically with the increase of B2O3 content, which is the network former here. It is reported that generally Tg and Tc increases with the increase of network former/glass former [14] which is observed in this present study also. However several reports of germanate anomaly are there which shows a decrease in Tg with the increase of GeO2 content in the glass system [15]. A maximum in the Tg vs. B2O3 content curve is also reported [16]. It is believed that Tg is depend on the strength of chemical bonds in the structure. TMI in general, plays the role of a network modifier and non-bridging oxygen increases with the increase of TMI content in the glass system. Increase of non-bridging oxygen indicates the breaking of chemical bonds, which in turn decrease the Tg. Increase of Tg with the increase of network former, in other words, decrease of TMI, indicates the increase of strength and connectivity of the glass structure in this case.
3.3. FTIR Study
Infrared spectroscopy has proved to be an important tool for the investigation of structure and dynamics of disorder materials. IR spectra of materials may help to get the idea of the nature of vibration in a disorder system [16]. The room temperature vibration spectra of the glasses were obtained using KBr pellet technique in the range 400 - 4000 cm–1. A typical FTIR spectrum of the as-prepared glass containing 50% B2O3 is shown in Figure 5. As expected, these spectra exhibit broad absorption bands as a consequence of the general disorder in the network, mainly due to a wide distribution of structural units occurring in these glasses.
The band marked as A attributed to the presence of transition metal ions in bi-valent state (Zn2+, Mn2+). The absorption bands marked as B, C and D are due to borate matrix. Details of the appeared peaks are presented in Table 2. The peak assignment is consistent with other published work [17-19]. Absence of peak around 806 cm–1, which is clear from the inset of Figure 5, indicates
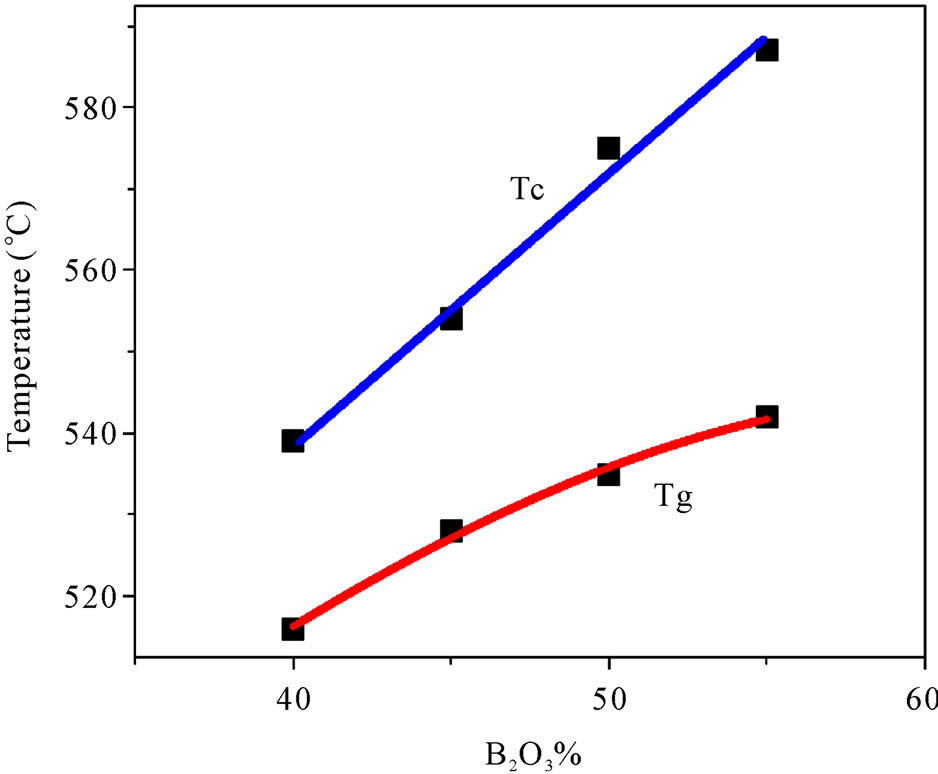
Figure 4. Compositional dependency of glass transition and crystallization temperature (Line connecting the data points is a guide for the eye).
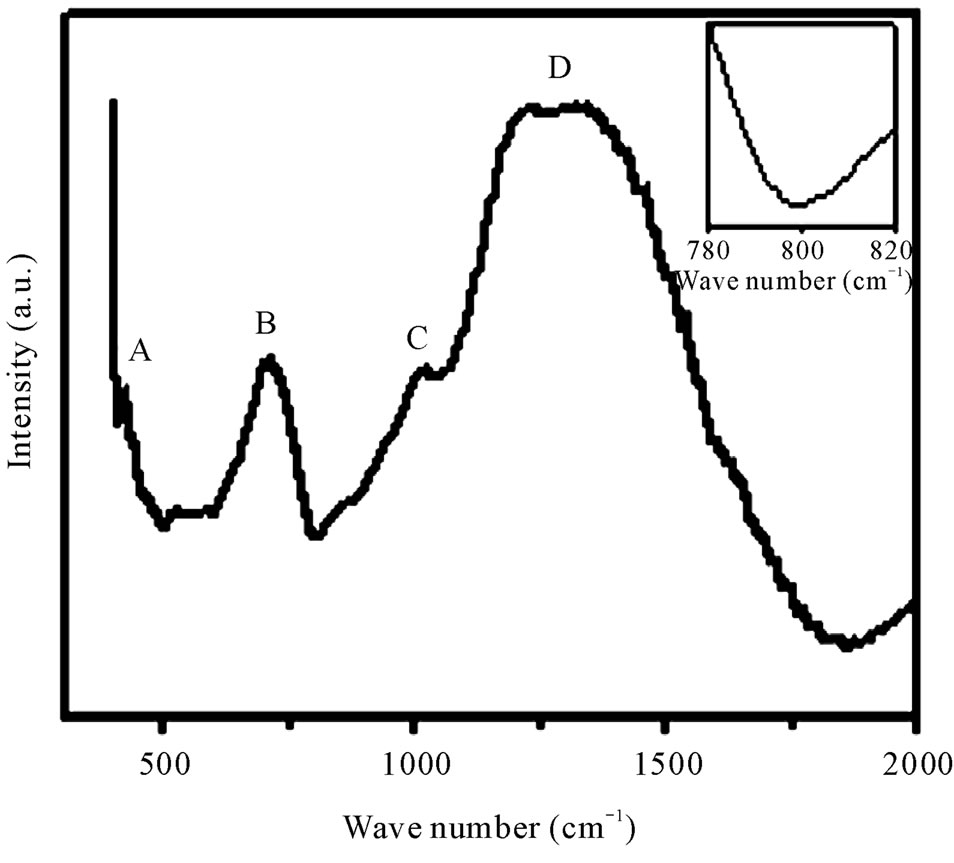
Figure 5. Room temperature FTIR spectra of zinc manganese borate glasses for 50 mol% B2O3. Inset: Magnified version of FTIR curves to prove the absence of boroxol rings.
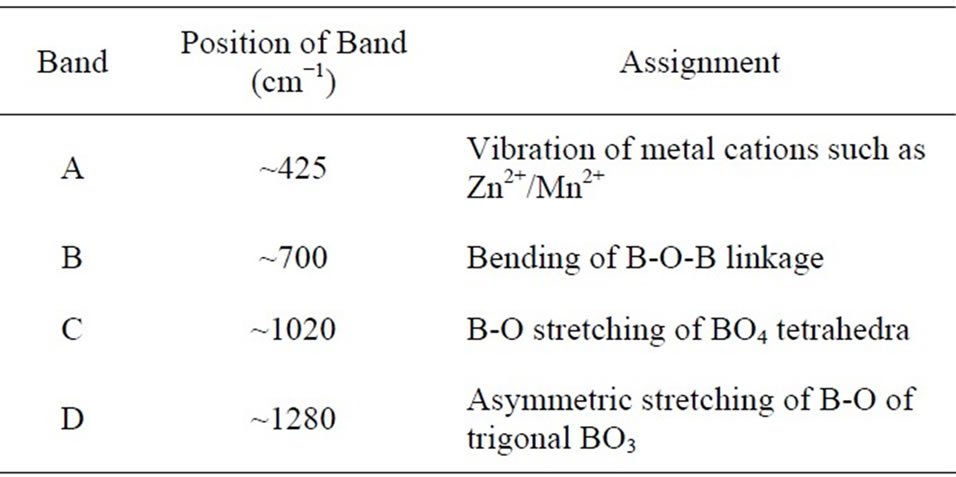
Table 2. Various absorption peak positions obtained from FTIR spectra.
that borate network does not contain any boroxol ring [17]. The broadness of D band is due to the presence of Zn2+ in the system. Generally, in pure B2O3 glass most of the boron is involved in B3O6 boroxol rings [18]. The addition of TMI breaks these rings and increasingly BO3 and BO4 units are formed which is, reflected in our samples also [20]. No detectable variation in peak positions and band shape are observed with the change of composition.
3.4. Optical Absorption Study
The fundamental optical band gap of the glasses has been computed based on their UV-Vis absorption spectra, for understanding their optically induced transitions. There are two types of optical transition, which can occur at the fundamental absorption edge of crystalline and noncrystalline materials. They are direct and indirect transitions. In both the cases, electromagnetic waves interact with the electrons in the valence band, which are raised across the fundamental band gap to the conduction band. For photon energies hn just above the fundamental edge, the absorption a follows the standard relation,
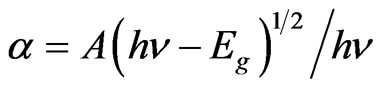
where A is a constant and Eg is defined as the energy band gap. A typical plot of (αhn)2 versus hn for the glass having 55% B2O3 is presented in Figure 6. Extrapolation of this plot to a2 = 0 gives the optical band gap Eg for direct transition. We have estimated the optical band gap for all the glasses, which varies from 3.45 - 4.05 eV for direct transition while B2O3 content changes from 40% - 55%. The value of Eg for indirect transition is obtained by extrapolation of (ahn)1/2 versus hn plot to a1/2 = 0 [21]. The optical band for indirect transition varies from 3.39 - 3.60 eV while B2O3 content changes from 40% - 55%. Figure 7 reveals the variation of optical bandgap with the change of composition. It can be observed from Figure 7 that both the direct and indirect band gap increases with the increase of B2O3. In other words, the optical bandgap decreases with the increase of TMI concentration. This can be attributed to the structural changes that are taking place with the introduction of TMI. Inclusion of TMI in borate structure may create some defect states in the midgap, which is responsible for the decrease of bandgap. The broadness of the absorption edge may be due to the presence of localized state in the band tails.
This paper is a part of more general study concerning the synthesis, structural characterization and optical study of borate glass system. It is noteworthy to mention here that we have annealed one glass sample at 500˚C for 60 min. XRD pattern of annealed sample confirms the

Figure 6. (ahg)2 versus hg plot for the glass containing 55 mol% B2O3.
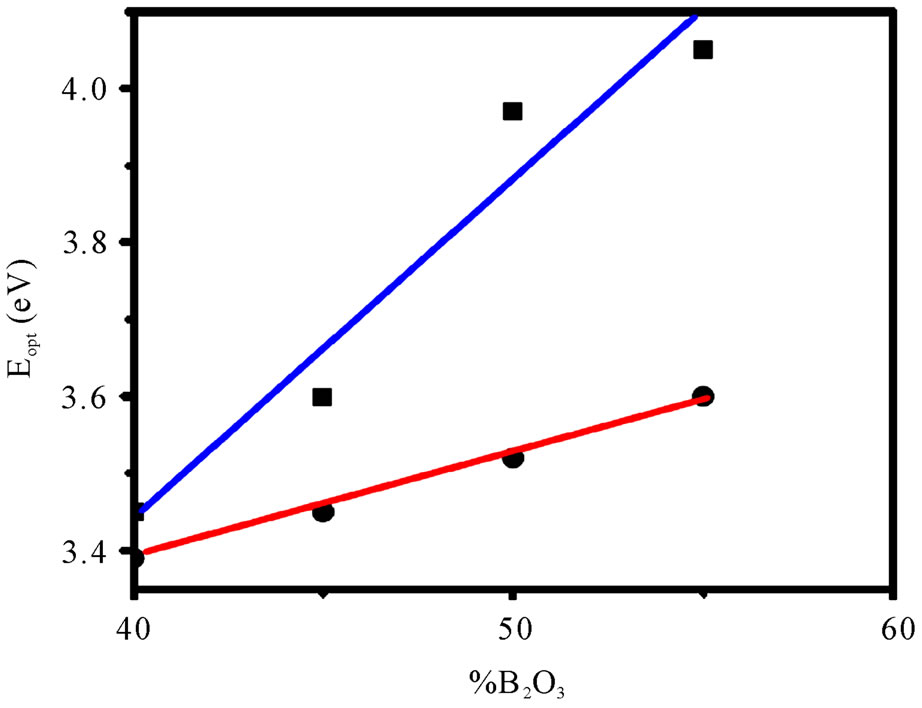
Figure 7. Compositional dependency of optical bandgap Blue line (■): Direct transition, Red line (●): Indirect transition (Line connecting the data points is a guide for the eye).
precipitation of nanocrystalline Mn-doped ZnO, which also shows magnetization at room temperature. This result indicates the possibility towards the development of borate based spintronic materials. Details of the magnetization are reported elsewhere [22].
4. Conclusions
In conclusion, we are able to prepare series of uncommon manganese zinc oxide containing B2O3 glasses through melt-quenched technique and report the experimental investigation of thermal stability and spectroscopic studies. Both the x-ray diffraction and SEM studies confirm the amorphous nature of the as-prepared glasses. The effect of TMI dopping on structure and optical property is discussed. In addition, we have demonstrate that magnetic Mn-doped ZnO nanoparticles can be precipitated in the borate matrix if the glasses annealed at particular temperature.
5. Acknowledgements
Financial support from the project (No.: CSR/AO/MUM/ CRS-M-144/08/423) of UGC-DAE Consortium for Scientific Research, Govt. of India, is gratefully acknowledged. The authors also thank the DST Unit on Nanoscience of Indian Association for the Cultivation of Science, Kolkata, India for extending their DSC facility.
REFERENCES
- A. Pan and A. Ghosh, “A New Family of Lead-Bismuthate Glass with a Large Transmitting Window,” Journal of Non-Crystalline Solids, Vol. 271, No. 1-2, 2000, pp. 157-161. doi:10.1016/S0022-3093(00)00111-3
- Š. Jiri, K. Ladislav, M. Petr, M. Lionel, R. Bertrand and G. Ivan, “Structure and Properties of MoO3-Containing Zinc Borophosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 355, No. 16-17, 2009, pp. 970-975. doi:10.1016/j.jnoncrysol.2009.04.017
- C. W. Adrian, “Borate Structures: Crystalline and Vitreous,” Physics and Chemistry of Glasses—European Journal of Glass Science and Technology Part B, Vol. 51, No. 1, 2010, pp. 1-39.
- M. Pal, “Structure and Physical Properties of Sodium Antimony Germinate Glasses,” Journal of Materials Research, Vol. 11, No. 7, 1996, pp. 1831-1835. doi:10.1557/JMR.1996.0231
- W. L. Konijnendijk and J. M. Stevels, “Structure of Borate and Borosilicate Glasses,” In: L. D. Pye, V. D. Fréchette and N. J. Kreidl, Eds., Borate Glasses: Structure, Properties, Applications, Plenum Press, New York, 1978, p. 259.
- I. Kashif, H. Farouk, A. S. Aly and A. M. Sanad, “Differential Scanning Calorimetry and Infrared Study of Barium Borate Glass Containing Transition Elements,” Physics and Chemistry of Glasses, Vol. 32, No. 2, 1991, pp. 77-78.
- A. C. Hannon, D. I. Grimley, R. A. Hulme, A. C. Wright and R. N. Sinclair, “Boroxol Groups in Vitreous Boron Oxide: New Evidence from Neutron Diffraction and Inelastic Neutron Scattering Studies,” Journal of Non-Crystalline Solids, Vol. 177, No. 1, 1994, pp. 299-316. doi:10.1016/0022-3093(94)90544-4
- D. L. Griscom, “Borate Glass Structrure,” In: L. D. Pye, V. D. Fréchette and N. J. Kreidl, Eds., Borate Glasses: Structure, Properties, Applications, Plenum Press, New York, 1978, p. 11.
- C. Li and Q. Su, “Action of Co-Dopant in ElectronTrapping Materials: The Case of Sm3+ in Mn2+ Activated Zinc Borosilicate Glasses,” Applied Physics Letters, Vol. 85, No. 12, 2003, pp. 2190-2192. doi:10.1063/1.1797562
- J.-M. Wu and H.-L. Huang, “Microwave Properties of Zinc, Barium, and Lead Borosilicate Glasses,” Journal of Non-Crystalline Solids, Vol. 260, No. 1-2, 1999, pp. 116- 124. doi:10.1016/S0022-3093(99)00513-X
- L. D. Bogomolova and M. P. Glassova, “The Impurity Effects in Vanadate Semiconducting Glasses,” Journal of Non-Crystalline Solids, Vol. 37, No. 3, 1980, pp. 423-426. doi:10.1016/0022-3093(80)90079-4
- M. Pal, D. Chakravorty and A. Bhowmik, “Structural Study of Iron Borate Glasses Containing NiO and ZnO,” Journal of Materials Research, Vol. 13, No. 11, 1998, pp. 3287-3292. doi:10.1557/JMR.1998.0447
- L. Aleksandrov, R. Iordanova and Y. Dimitriev, “Glass Formation in the MoO3-La2O3-B2O3 System,” Physics and Chemistry of Glasses, Vol. 48, 2007, p. 242.
- R. M. Almedia and J. D. Mackenzie, “Vibrational Spectra and Structure of Fluorozirconate Glasses,” Journal of Chemical Physics, Vol. 74, No. 11, 1981, pp. 5954-6537. doi:10.1063/1.441033
- S. Sakka and K. Kamiya, “Structure of Alkali Germanate Glasses Studied by Spectroscopic Techniques,” Journal of Non-Crystalline Solids, Vol. 49, 1982, p. 103. doi:10.1016/0022-3093(82)90110-7
- P. Becker, “Thermal and Optical Properties of Glasses of the System Bi2O3 - B2O3,” Crystal Research and Technology, Vol. 38, No. 1, 2003, pp. 74-82. doi:10.1002/crat.200310009
- A. H. Verhoef and H. W. den Hartog, “A Molecular Dynamics Study of B2O3 Glass Using Different Interaction Potentials,” Journal of Non-Crystalline Solids, Vol. 146, 1992, pp. 267-278. doi:10.1016/S0022-3093(05)80501-0
- S. Ram, “Infrared Study of the Dynamics of Boroxol Rings in the Crystallization of BaFe12O19 Microcrystal in the Borate Glass,” Physical Review B, Vol. 51, No. 10, 1995, pp. 6280-6286. doi:10.1103/PhysRevB.51.6280
- N. M. Bobkova1 and S. A. Khot’ko1, “Zinc Oxide in Borate Glass-Forming Systems,” Glass and Ceramics, Vol. 62, No. 5-6, 2005, pp. 167-170. doi:10.1007/s10717-005-0064-7
- W. Soppe, J. Kleerebezem and H. W. den Hartog, “Raman Spectroscopy Study of (B2O3)1−x−y(Li2O)x(Li2Cl2)y and (B2O3)1−x−y(Li2O)x(Cs2O)y,” Journal of Non-Crystalline Solids, Vol. 93, 1987, p. 142. doi:10.1016/S0022-3093(87)80034-0
- L. Edwards, M. Gouterman and X. V. Porphyrins, “Vapor Absorption Spectra and Stability: Phthalocyanines,” Journal of Molecular Spectroscopy, Vol. 33, No. 2, 1970, pp. 292-310. doi:10.1016/0022-2852(70)90040-8
- M. Pal, “Borate Based Spintronic Materials in Bulk Form above Room Temperature,” Journal of Surface Science and Technology, Vol. 21, No. 1-2, 2005, pp. 91-96.

