Open Journal of Obstetrics and Gynecology
Vol.3 No.1(2013), Article ID:27153,6 pages DOI:10.4236/ojog.2013.31020
In vivo effect of 17-β-estradiol, progesterone, hCG and expression of P53 and P21 in endometrial Ishikawa cells
![]()
1Department of Obstetrics and Gynecology, Florence Nightingale Kadikoy Hospital, Istanbul, Turkey
2Istanbul Bilim University, Institute of Health Sciences, Department of Histology & Embryology, Istanbul, Turkey
3Division of Gynecology, Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston, USA
Email: *farukabike@gmail.com
Received 3 October 2012; revised 5 November 2012; accepted 14 November 2012
ABSTRACT
Purpose: The study examined the effect of 17-β-estradiol, progesterone and hCG on cell proliferation and the effect of cell cycles regulating P53 and P21 protein on expression levels of Ishikawa endometrium epithelium cells. Methods: Ishikawa cells was growed in flasks including DMEM-F12 medium. It was addicted 17-β-estradiol (0.4 μM) in mediums for shows the estrogen effects to cells. Besides, hydroksiprogesteron caproate (1 μg/ml) and hCG (20 ng/ml) were addicted to cells for shows to effect of progesterone and hCG to cells. Cell culture groups were incubated at 24 hours for assesment of cell proliferation. Although it was incubated at 48 hours for determination of P53 and P21 in cell groups. Cells in the G1, S, G2 and M phases of cellular cycle were marked with immunohistochemical marking of proliferated cell nuclear antigen (PCNA). S phase cell rates were also assessed using 5- bromo 2-deoxi-uridine (BrdU) marking method. Results: No difference was determined between the PCNA marked cells and control group subject to 17-β-estradiol however, a significant increase was recorded in the rate of S-phase proliferation. No relation was indicated in the comparison of increase in proliferation rate and P53 and P21 protein expression levels. The proliferation rates of cells subject to progesterone and hCG and P53 and P21 protein expression levels were identified to have very close values to control group. Conclusions: It has been concluded that the 17-β-estradiol, progesterone and hCG hormones at concentrations and durations of experiment, do not effect the P53 and P21 protein expression levels during the proliferation regulation of Ishikawa cells.
Keywords: Ishikawa Cells; 17-β-Estradiol; Progesterone; hCG; P53; P21; Endometrium
1. INTRODUCTION
Steroid hormones hold a major role in female reproductive system. Estrogens act through specific receptors located in the nuclei of epithelial and stromal cells of the endometrium. Estrogens stimulate the synthesis of these receptors and the progesterone inhibits their synthesis. Estrogens induce the proliferation of the mucosa during the proliferative phase. They also stimulate the synthesis of receptors for the progesterone, which is a prerequisite for progesterone activity. Endometrial cancers are either associated with a hyperestro-genic or atrophic background [1]. Human chorionic gonadotropin (hCG) may exert direct effects on the endometrium. These effects, not mediated by ovarian hormones, are probably a consequence of stimulation of endometrial hCG/LH receptors. Effects of hCG on the endometrium could alter endometrial receptivity. Evidence has been reported of decidualization of stromal cells of the human endometrium in vitro as a result of exposure to gonadotropins, including hCG. hCG administration as commonly used in some treatments may exert direct effects in vivo on endometrial histology and partake in endometrial transformations of the luteal phase [2].
PCNA is a DNA replication protein which is showed cellular phase of G1 and S. PCNA is used to show cellular proliferation rate [3-5]. BrdU is a timidine analogue which is marker of cellular phase S [3,6,7]. P21 is a protein that makes control of cell cycle after the bounding of cyclin-Cdk1 complex. P21 has negative regulation on cell cycle and it makes as mainly inactivation of Cdk system. P21 plays role in apopytosis as inhibiting of Cdk-Cyclin complex. When the losing control of Cdk activity, cell proliferation could be increased and genomic instability and the finally apopytosis lose and cancer could be developed [8-10]. DNA damage causes to increasing of P53 protein. Activating of P53 with P21 is bounding Cdk complex and make to arrest of cell cycle in G1 phase. Besides, P21 bounds with PCNA could be arrested of DNA replication and repaired of DNA damage [10-13]. P53 plays role in control of cell proliferation. It makes to effect on DNA synthesis and repairing, control of apopytosis and inhibits of cell cycle. When the DNA damage is done, P53 levels is increasing in the cell which is arrest to cell cycle on G1 phase. That arresting of cell cycle could be allowed to time for repairing of DNA damage. This process is made to activate of Cdk1 complex with P53 and P21. When the damaged DNA is repaired, cell cycle is continued on resume place. If the DNA damage is not able to repaired, cell cycle is arrested and developed to apopytosis. It can not be repaired of DNA damage that could be caused to mutations of developing cancer [10,12,13].
Ishikawa cells are a sequence of human uterin endometrial cells and it could be alternative model for human embryo transplantation [14]. Ishikawa cells are well differentiated endometrial adenocarcinoma cells and it has carried out on estrogen and progesterone receptors. Thus, ishikawa cells could be an appropriate model for researchs of implantation biology [15].
In our study, it s estabilished that relationship between progesterone, estrogen and hCG effects on endometrium and P53 and P21 expressions were determined by using anti-BrdU as timidine analogue for specipic to phase of S in cell cycle and anti-PCNA markering methods. Short term in vivo tests might be helpful for the evaluation of proliferative effects of estrogen progestogene.
2. MATERIAL-METHOD
2.1. Cell Culture
Ishikawa cells was growed in flasks including DMEMF12 (Dulbecco’s modified Eagle’s medium) medium which is containing 10% fetal bovine serum and antibiotics(100 U/ml penisilin G, 100 ug/ml streptomycin) under condition of 37˚C and 5% CO2 and 95% air. Ishikawa cells of control group were put in this medium. It was addicted 17-β-estradiol (0.4 μM) in mediums for shows the estrogen effects to cells. Besides, hydroksiprogesteron caproate(1 μg/ml) and hCG (20 ng/ml) were addicted to cells for shows to effect of progesterone and hCG to cells. Cell culture groups were incubated at 24 hours for assesment of cell proliferation. Although it was incubated at 48 hours for determination of P53 and P21 in cell groups.
2.2. PCNA Immunocytochemistry
Growed cells in cell culture were fed in lamells then fixed with methanol under condition of −20˚C and pathologic preparate was done with non immune serum for immunocytochemistrical assesment of PCNA. Then, 1/300 dilution of anti-PCNA antibodies (Zymed) were applied at 1 hour and under condition of room temperature. Seconder antibodies(Histostain Plus Kit, Zymed) were applied with biotin-streptoavidine peroxidase at 20 minutes after the primer antibody application. It was implemented chromogen as aminoetilkarbazol (AEC) for description of specific reaxion after the washing of phosphate buffer solution (PBS).
2.3. BrdU Immunocytochemistry
BrdU markering method is specific for cell phase of S. Cells were incubated with BrdU (1 mM) at 1 hour and 37˚C before the fixation. It was neutralized with borate buffer (pH = 8) after the fixation was done with methanol then cells’ DNA was denatured by using 2 N HCL and 37˚C at 30 minutes. Because of the preventing non specific reaxions, it was applied non immune serum at 20 minutes after the washing of PBS. It was incubated with 0.5/100 dilution of Anti-BrdU primer antibody (Mouse monoclonal-NeoMarkers) at 1 hour and room temperature. Biotin-streptoavidine peroxidase seconder antibodies (Histostain Plus Kit, Zymed) were applied at 20 minutes for each group. AEC cromogen was used for visualization to specific colour reaxion.
2.4. P53 and P21 Protein Immunocytochemistry
Cells of fed in lamells were fixed with methanol under condition of −20˚C and 5 minutes for assesment of P53 and P21 expressions. Non immune serum(Histostain Plus Kit, Zymed) was applied for the preventing of non specific reaxions. It was incubated with anti-P53 and anti-P21 monoclonal primer antibodies at dilution of 3 µl/ml and room temperature. Biotin marked antibodies was used at 20 minutes after the incubation. It was incubated with streptoavidine enzyme conjugate at 20 minutes after the PBS washing. Then, AEC chromogen was applied. In the phase of chromogen applied, reactions were stopped after the visualization of specific colour reaxion with invert microscope. Samples were closed by using water based closing method.
2.5. Microscobic Assesment
In this study, microscopic assesment was made by using Olympus X70 invert microscope. Immunocytochemical evaluation was done by using Olympus BX 50 light microscope. It was calculated cells of S phase with BrdU and PCNA pozitive cells in experiments were done three times. Proliferation index was determined dividing pozitive marked cells to total cells.
2.6. Statistics
Statistical determination was done by using SPSS 10.0 version. Krusker Wallis test was used for multipl group comparison and Mann Whitney U test was used for double group comparison. Statistical meaning value was accepted lower than as p < 0.05.
3. RESULTS
3.1. Assesment of PCNA Marked Cells
PCNA marked Ishikawa cells ratio were determined 90.33 ± 6.40 in control group, 92.00 ± 7.12 in 17-β-estradiol, 89.08 ± 7.36 in progestrone group and 89.41 ± 6.81 in hCG group. It was not found any statistically correlation between PCNA reactivity and groups (p > 0.05) (Figure 1).
3.2. Assesment of BrdU Marked Cells
BrdU marked Ishikawa cells in S fraction of cell cycle were estabilished 54.00 ± 6.43 in control group, 63.91 ± 7.24 in 17-β-estradiol group and there was found a significantly different (p < 0.05) In contrast, BrDU marked Ishikawa cells in progesterone group (52.58 ± 6.88) and hCG group (53.25 ± 7.39) were not found significantly statiscally different (p > 0.05) (Figure 2).
3.3. Expression of P53 Protein
Expression and localization of P53 proteins were not
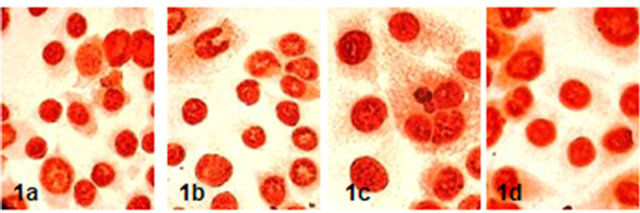
Figure 1. PCNA reactivity of Ishikawa cells in groups (x600) (1a: control, 1b: 17-β-estradiol, 1c: Progesterone, 1d: hCG).

Figure 2. BrDU marked Ishikawa cells on S fraction of cell cycle in groups (x600) (2a: Control, 2b: 17-β-estradiol, 2c: progesterone, 2d:hCG).
determined any difference in 17-β-estradiol, progestorene and hCG after the 48 hours incubation compared to control group (Figure 3).
3.4. Expression of P21 Protein
Expression and localization of P21 protien in 17-β-estradiol, progesterone and hCG group were not found difference than control group (Figure 4).
4. DISCUSSION
Ishikawa cells have estrogen and progesterone receptors and which is well-differentiated adenocarcinoma cells. Ishikawa cells are a good model for research of hormonal effect on endometrium [16]. Estradiol has increasing effect on endometrium after the 24 and 96 hours incuba-
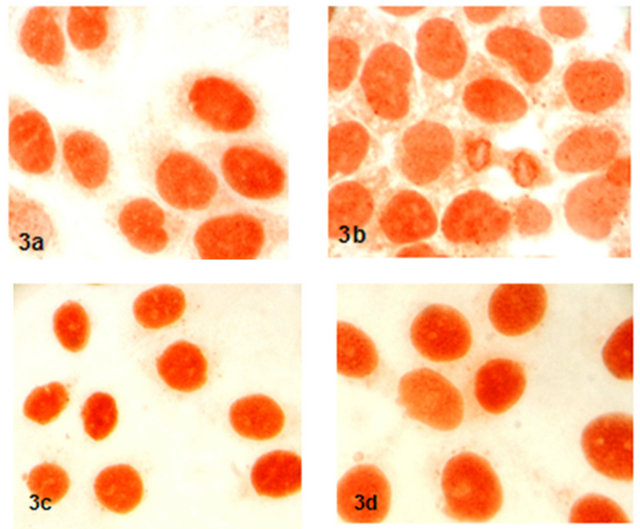
Figure 3. Expression and localization of P53 proteins in groups (x600) (3a: control, 3b: 17-β-estradiol, 3c: progestorene, 3d: hCG).
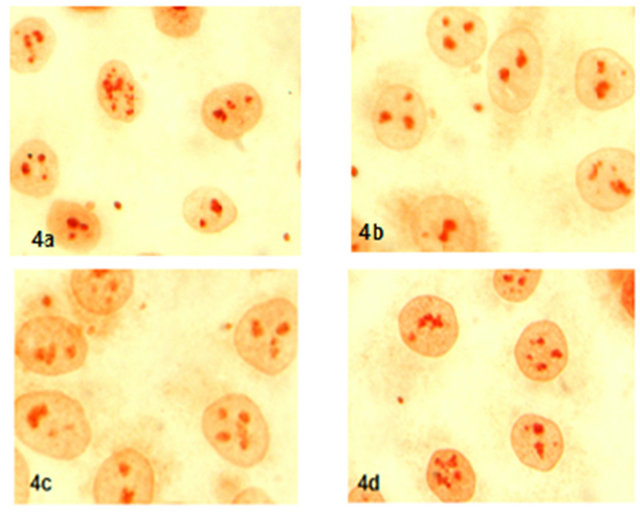
Figure 4. Expression of P21 protein (x600) (4a: control, 4b: 17-β-estradiol, 4c: progestorene, 4d: hCG).
tion, but it was found more proliferation on endometrium at over the 96 hours [17]. In our study, compared to PCNA expression of control and 17-β-estradiol were not found different with 24 hours incubation. In contrast, BrdU expression of 17-β-estradiol group was significantly higher than control.
In a previous study with 3H thymidine marked Ishikawa cells was showed progesterone is inhibited to proliferative effect of estrogen on endometrium at 48 and 72 hours incubation [18]. Intrauterine levonorgestrol releasing devices are used for inhibition to proliferative effect of estrogen in postmenopausal women [19]. In our study, we studied that endometrial cell proliferation and P53, P21 proteins expression were indicated with incubation of progesterone by using anti-PCNA antibodies and BrdU as S cell phase specific thymidine analogue. Rate of proliferation on cells in progesterone group with using PCNA and BrdU marked methods was found similar to control group at 24 hours incubation. It needs to more widely new studies for shows to inhibit effect of progesterone on endometrium.
It was estabilished that hCG is released to endometrial cells on secretory phase of menstrual cycle and trophoblasts after the implantation [20]. hCG has proliferative effect on myometrium and endothel of blood vessels in endometrium [21,22]. But mechanism of hCG effect on cell proliferation has been unclear. In a previous study indicated low dose hCG effect on endometrium. Sibling oocytes from the same donor were shared at random among two different recipients. In group I oocyte recipients received 750 IU of hCG every three days concomitant to endometrial preparation with estradiol until hCG injection to the donor, whereas in group II recipients received no hCG during endometrial priming with estradiol. Endometrial thickness was significantly lower in group I compared with group II. Pregnancy rates were significantly lower in group I than in group II (13.6% vs. 45.4%, p < 0.05). Implantation rates were also significantly lower in group I (1.7% vs. 22.4%, p < 0.01). It was estabilished that hCG administration in the proliferative phase might directly affect endometrial proliferation and receptivity [23]. Although, in our study we found that proliferation rates of cells with marked PCNA and BrdU in hCG group was not estabilished significantly difference compared to control group. It was studied that the steroidogenic capability of granulose cells in vitro was estimated by radioimmunoassay (RIA): estradiol, progesterone secreted into the culture medium were measured. hCG generally stimulated progesterone and estradiol secretion [24].
P53 protein plays important role in cell proliferation and apopytosis. P53 protein could be apopytosis and repairing of base or nucleotide as activated P21, p14 ARF, MDM2, bax and cell cycle is arrested on G1 and G2 in case of cells are exposed to stres as hypoxia, ultraviolet, radiation and some drugs [8,25]. It was showed that P53 is increased on late proliferative phase of menstruel cycle under effect of estrogen. P53 expression is some decreased to progesterone effect on endometrium but decreased levels of P53 expression was not found significantly [26,27]. A previous study was performed to investigate whether this process might be influenced by the hCG dependent expression of different tumor suppressor genes and hypoxia dependent transcription factors. The influence of hCG on the expression of VHL, P53, and HIF-2alpha were investigated. This study was showed that hCG regulates the expression of P53, VHL, and HIF-2alpha and hCG may determine the growth and development of the corpus luteum by mediating hypoxic and apoptotic pathways in human granulosa lutein cells [28]. In a other previous study that evaluate the expression of P53 in the mouse ovary during an artificially induced ovulatory cycle. Ovulation induction was performed using pregnant mares’ serum gonadotropin/human chorionic gonadotropin (PMSG/ hCG). It was indicated that maximal P53 expression occurs around the time of ovulation, beginning 48 hours after PMSG and peaking 6 - 12 hours after hCG administration. It was indicate that the temporal expression of P53 in the ovary during a PMSG/hCG artificially induced ovulatory cycle may indicate a role for P53 in processes of differentiation of granulosa cells into luteal cells [29]. We didn’t found any correlation P53 and P21 expression with incubation of HCG.
Recently, prospective experimental a study was done. Rabbits were primed with pregnant mare serum gonadotropin and hCG. Endometrial cells were cultured with E(2) and P(4) of different concentrations. The expression patterns of estrogen receptor and P receptor of rabbit endometrium were different before and after treatment with pregnant mare serum gonadotropin-hCG. One hundred nanomolar E(2) with 10 nmol/L P(4) facilitated the proliferation of epithelial cells whereas 100 nmol/L P(4) facilitated that of stromal cells. It was indicate that rabbit endometrial cells could be cultured with a longstanding proliferation capability by sex steroids and applied in uterine tissue engineering. Reconstructed endometrium with proliferated endometrial cells was akin to native endometrium in structure and function [30]. Basal expression studies indicated that ERalpha mRNA levels remain unchanged, whereas ERbeta mRNA levels increased with time in culture in vitro, suggesting that ERbeta is likely to play a dynamic role in mediating estrogen action in human granulosa-luteal cells (hGLCs). hCG treatment (10 IU/mL) significantly attenuated the ERalpha (45%; p < 0.01) and ERbeta (40%; p < 0.01) mRNA levels. Additional studies using a specific protein kinase A (PKA) inhibitor and an adenylate cyclase inhibitor further implicated the involvement of the cAMP/ PKA signaling pathway in hCG action in these cells. The hCG-induced decrease in ERalpha and ERbeta mRNA levels was prevented in the presence of these inhibitors. It demonstrated an inhibition of progesterone production in hGLCs in vitro by 17 beta-estradiol, and this inhibitory effect was eliminated by pretreatment of 10 IU/mL hCG. The demonstration of hCG induced down-regulation of ERalpha and ERbeta gene expression suggests that hCG may contribute to the control of granulosaluteal cell function. This data suggest that the effects of hCG on ERalpha and ERbeta expression in hGLCs are mediated in part by activation of PKA and PKC signaling pathways [31]. Uterine proliferation was assessed using markers for the proliferating cell nuclear antigen (PCNA) and by the bromodeoxyuridine (BrdU) method. The antiestrogens either reduced or prevented changes of myometrial and stromal proliferation indices (PI). In the luminal columnar epithelium, the antiestrogens depressed PCNA PI but enhanced BrdU PI, indicating a low continuous DNA synthesis in otherwise quiescent cells. The antiestrogens induced focal hyperplastic multilayered epithelia with PCNA-positive basal cells along segments of the luminal uterine epithelium [32].
In our study, P53 protein expression was not found different in all study groups compared to control group. It was determined that P53 expression mechanism didn’t affect at short time in vitro incubation with estrogen and progesterone. P53 effects has been unclear on endometrial cycle. It wasn’t found more study about relation of P53 and hCG in databases.
Cdk1 is a P21 protein and WAF1/CIP1 gene product which plays role in regulation of cell cycle [33]. Cdk1 stops cell cycle in G1 phase and prevent progression to S cell phase [12]. P21 protein is more expressed in case of increased P53 protein expression under condition of hypoxia, stres, ultraviolet exposition. Although P21 expression independent increases to P53 expression such as condition of tissue growth, serum stimulation and cell differantion [34]. P21, cyclin, Cdk and PCNA constitute a complex which play role in regulation of DNA replication [35]. P21 composes fourth complex and stimulates cell stimulation at low concentration but high concentration of P21 may play role in cell cycle as active inhibitory factor [34]. In our study, P21 expression didn’t increase with incubation of 17-β-estradiol, progesterone and hCG in Ishikawa endometrial cells.
In our study, cell proliferation was determined with stimulation of 17-β-estradiol, progesterone and HCG in Ishikawa cells as model of endometrium. PCNA expression was found similar with incubation of 17-β-estradiol, progesterone, hCG and control group. Cell proliferation was found to increase with BrdU marked method as S cell phase specific in incubation of 17-β-estradiol. In contrast, cell proliferation was not estabilished in hCG and progesterone group with BrdU marked method. P53 and P21 protein levels were not altered with stimulation of 17-β-estradiol, progesterone and hCG at short time. It needs to more widely, at different concentrations and more long timely studies for relation of cell proliferation and 17-β-estradiol, progesterone and hCG.
![]()
![]()
REFERENCES
- Bergeron, C. (2002) Effect of estrogens and antiestrogens on the endometrium. Gynécologie Obstétrique & Fertilité, 30, 933-937. doi:10.1016/S1297-9589(02)00486-1
- Fanchin, R., Peltier, E., Frydman, R. and de Ziegler, D. (2001) Human chorionic gonadotropin: Does it affect human endometrial morphology in vivo? Seminars in Reproductive Medicine, 19, 31-35. doi:10.1055/s-2001-13908
- Matsumoto, K., Moriuchi, T., Koji, T. and Nakane, P.K. (1987) Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. The EMBO Journal, 6, 637-642.
- Baserga, R. (1991) Growth regulation of the PCNA gene. Journal of Cell Science, 98, 433-436.
- Celis, J.E. and Madsen, P. (1986) Increased nuclear cyclin/ PCNA antigen staining of non S-phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Letters, 209, 277-283. doi:10.1016/0014-5793(86)81127-9
- Hegele-Hartung, C., Mootz, U. and Beier, H.M. (1992) Luteal control of endometriyal receptivity and its modification by progesterone antagonists. Endocrinology, 131, 2446-2460. doi:10.1210/en.131.5.2446
- Coskun, M. and Coskun, M. (2003) Biological dosimeter and related developments. Cerrahpaşa Journal of Medicine, 34, 207-218.
- Durmaz, R. and Vural, M. (2007) Genetics in primary and secondary glioblastoma. Turk Nörosirurji Dergisi, 17, 80-90.
- Sherr, C.J. (1996) Cancer cell cycles. Science, 274, 1672- 1677. doi:10.1126/science.274.5293.1672
- Ay, M.E., Terzioğlu, O., Terzi, C. and İzci Ay, Ö. (2006) Kolorektal kanserlerde, P21, p27, p57 siklin bağımlı kinaz inhibitör geni (CDKI) ekspresyonlarının değerlendirilmesi. Akademik Gastroenteroloji Dergisi, 5, 20-25.
- LaBaer, J., Garrett, M.D., Stevenson, L.F., Slingerland, J.M., Sandhu, C., Chou, H.S. et al. (1997) New functional activities for the P21 family of CDK inhibitors. Genes & Development, 11, 847-862. doi:10.1101/gad.11.7.847
- Dotto, G.P. (2000) P21(WAF1/Cip1): More than a break to the cell cycle? Biochim Biophys Acta, 1471, 43-56.
- Tsihlias, J., Kapusta, L. and Slingerland, J. (1999) The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annual Review of Medicine, 50, 401-423. doi:10.1146/annurev.med.50.1.401
- Heneweer, C., Schmidt, M., Denker, H.W. and Thie, M. (2005) Molecular mechanisms in uterine epithelium during trophoblast binding: The role of small GTPase RhoA in human uterine Ishikawa cells. Journal of Experimental & Clinical Assisted Reproduction, 2, 4. doi:10.1186/1743-1050-2-4
- Nishida, M. (2002) The Ishikawa cells from birth to the present. Human Cell, 15, 104-117. doi:10.1111/j.1749-0774.2002.tb00105.x
- Croxtall, J.D., Elder, M.G. and White, J.O. (1990) Hormonal control of proliferation in the Ishikawa endometriyal adenocarcinoma cell line. Journal of Steroid Biochemistry, 35, 665-669. doi:10.1016/0022-4731(90)90306-D
- Kayisli, U.A., Aksu, C.A.H., Berkkanoglu, M. and Arici, A. (2002) Estrogenicity of isoflavones on human endometriyal stromal and glandular Cells. The Journal of Clinical Endocrinology & Metabolism, 87, 5539-5544.
- Shiozawa, T., Horiuchi, A., Kato, K., Obinata, M., Konishi, I., Fujii, S. and Nikaido, T. (2001) Up-regulation of p27Kip1 by progestins is involved in the growth suppression of the normal and malignant human endometriyal glandular cells. Endocrinology, 142, 4182-4188. doi:10.1210/en.142.10.4182
- Taşkın, S., Özmen, B. and Ünlü, C. (2006) Therapeutic use of the levonorgestrel releasing intrauterine system. Journal of the Turkish German Gynecological Association, 7, 63-67.
- Wolkersdörfer, G.W., Bornstein, S.R., Hilbers, U., Zimmermann, G., Biesold, C., Lehmann, M., et al. (1998) The presence of chorionic gonadotrophin beta subunit in normal cyclic human endometriyum. Molecular Human Reproduction, 4, 179-184. doi:10.1093/molehr/4.2.179
- Lei, Z.M., Reshef, E. and Rao, V. (1992) The expression of human chorionic gonadotropin/luteinizing hormone receptors in human endometriyal and myometrial blood vessels. The Journal of Clinical Endocrinology & Metabolism, 75, 651-659. doi:10.1210/jc.75.2.651
- Környei, J.L., Lei, Z.M. and Rao, C.V. (1993) Human myometrial smooth muscle cells are novel targets of direct regulation by human chorionic gonadotropin. Biology of Reproduction, 49, 1149-1157. doi:10.1095/biolreprod49.6.1149
- Prapas, N., Tavaniotou, A., Panagiotidis, Y., Prapa, S., Kasapi, E., et al. (2009) Low-dose human chorionic gonadotropin during the proliferative phase may adversely affect endometrial receptivity in oocyte recipients. Gynecological Endocrinology, 25, 53-59. doi:10.1080/09513590802360769
- Stevenson, A.F. (2000) Human granulosa cells in vitro: Characteristics of growth, morphology and influence of some cytokines on steroidogenesis. Indian Journal of Experimental Biology, 38, 1183-1191.
- Smith, M.L. and Seo, Y.R. (2002) P53 regulation of DNA excision repair pathways. Mutagenesis, 17, 149-156. doi:10.1093/mutage/17.2.149
- Maia Jr., H., Maltez, A., Studart, E., Athayde, C. and Coutinho, E.M. (2004) Ki-67, Bcl-2 and P53 expression in endometriyal polyps and in the normal endometriyum during the menstrual cycle. British Journal of Obstetrics and Gynaecology, 111, 1242-1247. doi:10.1111/j.1471-0528.2004.00406.x
- Isaksson, E., Cline, J.M., Skoog, L., Söderqvist, G., Wilking, N., von Schoultz, E., et al. (1999) P53 expression in breast and endometriyum during estrogen and tamoxifen treatment of surgically postmenopausal cynomolgus macaques. Breast Cancer Research and Treatment, 53, 61- 67. doi:10.1023/A:1006172025349
- Herr, D., Keck, C., Tempfer, C. and Pietrowski, D. (2004) Chorionic gonadotropin regulates the transcript level of VHL, P53, and HIF-2alpha in human granulosa lutein cells. Molecular Reproduction and Development, 69, 397- 401.
- Yaron, Y., Schwartz, D., Evans, M.I., Aloni, R., Kapon, A. and Rotter, V. (1999) P53 tumor suppressor gene expression in the mouse ovary during an artificially induced ovulatory cycle. Journal of Reproductive Medicine, 44, 107-114.
- Wang, H.B., Lu, S.H., Lin, Q.X., Feng, L.X., Li, D.X., Duan, C.M., et al. (2010) Reconstruction of endometrium in vitro via rabbit uterine endometrial cells expanded by sex steroid. Fertility and Sterility, 93, 2385-2395. doi:10.1016/j.fertnstert.2009.01.091
- Chiang, C.H., Cheng, K.W., Igarashi, S., Nathwani, P.S. and Leung, P.C. (2000) Hormonal regulation of estrogen receptor alpha and beta gene expression in human granulosa-luteal cells in vitro. The Journal of Clinical Endocrinology & Metabolism, 85, 3828-3839. doi:10.1210/jc.85.10.3828
- Karlsson, S., Iatropoulos, M.J., Williams, G.M., Kangas, L. and Nieminen, L. (1998) The proliferation in uterine compartments of intact rats of two different strains exposed to high doses of tamoxifen or toremifene. Toxicologic Pathology, 26, 759-768. doi:10.1177/019262339802600608
- El-Deiry, W.S., Tokino, T., Velculescu, V.E., Levy, D.B., Parsons, R., Trent, J.M., et al. (1993) WAF1, a potential mediator of P53 tumor suppression. Cell, 75, 817-825. doi:10.1016/0092-8674(93)90500-P
- Zhang, H., Hannon, G.J. and Beach, D. (1994) P21-containing cyclin kinases exist in both active and inactive states. Genes & Development, 8, 1750-1758. doi:10.1101/gad.8.15.1750
- Macleod, K.F., Sherry, N., Hannon, G., Beach, D., Tokino, T., Kinzler, K., et al. (1995) P53-dependent and independent expression of P21 during cell growth, differentiation, and DNA damage. Genes & Development, 9, 935- 944. doi:10.1101/gad.9.8.935
NOTES
*Corresponding author.

