Journal of Water Resource and Protection
Vol.08 No.05(2016), Article ID:66612,15 pages
10.4236/jwarp.2016.85047
Utilization of Infused Tea Leaves (Camellia sinensis) for the Removal of Pb2+, Fe2+ and Cd2+ Ions from Aqueous Solution: Equilibrium and Kinetic Studies
Chen Son Yue*, Kok How Chong, Cheah Cheng Eng, Ling Siang Loh
Faculty of Applied Sciences and Computing, Tunku Abdul Rahman University College, Kuala Lumpur, Malaysia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 April 2016; accepted 17 May 2016; published 20 May 2016
ABSTRACT
In this study, infused tea leaves as a low-cost adsorbent have been used in the removal of the Pb2+, Fe2+ and Cd2+ ions from aqueous solution. The adsorption study was carried out in a batch process and the effects of parameters such as initial pH, adsorbent dose, contact time and initial metal ion concentration were investigated. Experimental results showed that the maximum adsorption of metal ions occurred at pH 5 for Pb2+ and Fe2+ and at pH 6 for Cd2+. Adsorption of metal ions increased with increasing adsorbent concentration and contact time. The isothermal data for the adsorption of metal ions by infused tea leaves were found to fit well with the Langmuir equations. Based on the experimental data of the Langmuir isotherm model, the maximum adsorption capacities of the metal ions onto the infused tea leaves were found in the order of Pb2+ > Cd2+ > Fe2+ with the adsorption capacities of 26.32 mg∙g−1, 14.29 mg∙g−1 and 12.38 mg∙g−1 respectively. The adsorption process followed the pseudo-second order reaction and the corresponding rate constant were found to be 4.30 × 10−3 g∙mg−1∙min−1, 1.75 × 10−1 g∙mg−1∙min−1 and 1.45 × 10−2 g∙mg−1∙min−1 for Pb2+, Fe2+ and Cd2+ ions respectively.
Keywords:
Infused Tea Leaves, Isotherms, Kinetics, Lead, Cadmium, Iron

1. Introduction
The rapid growth of the manufacturing industries such as chemicals, foods, motor vehicles, paper, electronics, etc., have caused a lot of pollutions in the world especially in developing countries such as China, India, Brazil as well as Malaysia. The environmental pollution by heavy metals such as Cd, Zn, Fe, Ni, and Pb has become one of the many serious pollution issues. These heavy metals are often discharged into the environment by a number of industrial and domestic wastewater processes [1] - [3] . The release of large quantities of these heavy metals into the natural environment has resulted in a number of environmental problems because of their non-biodegradability and persistence characteristics [4] - [7] . The accumulation of heavy metals in living organisms is found to cause various diseases and disorders [8] - [11] . For example, cadmium causes serious renal damage, anemia, hypertension and itai-itai disease [11] . In view of this, various treatment methods, such as chemical precipitation and coagulation, ion exchange, reduction, osmosis and reverse osmosis, membrane filtration, and electrolytic technologies have been used for the removal of heavy metal contaminants in industrial effluents [12] - [14] . In recent years, however, special attention has been paid on the use of natural adsorbents as an alternative to replace the conventional methods mentioned above, mainly due to environmental and economic reasons [15] [16] .
Any natural products that are produced in bulk will be a potential biosorbent for the removal of heavy metals from the aqueous environment. This is because the main components of natural products are lignin and cellulose which contain high amount of electronegative functional groups such as amino, hydroxyl, carboxylic acid and ester. These electronegative functional groups can thus provide good chemical interactions between the biosorbent and the metal ions [17] .
Tea is a tropical plant and it belongs to the Theaceae family. The main tea producers in the world are the two most populous countries, that is, China and India, followed by Kenya and Sri Lanka [15] . Tea is the most widely-consumed beverage in the world after water. The infused tea leaves after consumption are usually disposed of into the environment or recycled as organic fertilizer. According to the Food and Agriculture Organization [18] , the annual production of tea in 2010 reached over 4.16 million∙tonnes, translating to approximately 3.98 million∙tonnes of infused tea waste as byproducts [19] . Although Malaysia produces only 0.45 percent of the world total tea output in 2011, as reported by the FAO, the consumption is higher, estimated at 23 million∙kgs as compared to the 3.8 million∙kgs produced annually [20] . Because of this, the use of infused tea leaves as a potential low cost adsorbent for the removal of heavy metals in the aqueous environment is a possibility that we can consider. In this study, the efficiency of infused tea leaves as a low cost adsorbent for the removal of heavy metal ions such as Pb2+, Fe2+ and Cd2+ ions from aqueous solution has been examined. The system variables being studied included contact time, adsorption capacity, kinetic and isotherm models. The structural and functional groups identification of the biosorbent were also performed using the FTIR spectrometer.
2. Materials and Methods
2.1. Chemicals and Materials
All the chemicals used were of analytical grade. The stock solutions (1000 mg∙L−1) of Pb2+, Fe2+ and Cd2+ were prepared in distilled water from their respective nitrate salts. All the standard solutions were prepared by diluting the stock solution with distilled water. Sample of tea leaves were obtained from packaged products of the BOH plantation Malaysia, the biggest tea producer in Malaysia, contributing to 70% of the total production in Malaysia.
2.2. Adsorbent Preparation
Consumed infused tea leaves were used in this study. They were first washed with hot distilled water (90˚C) to remove the remaining soluble and colored components. This process was done repeatedly until the discharging distilled water became colorless. The washed infused tea leaves were dried in an oven at 70 degree for 24 hours. It was then ground and sieved to obtain a powdered material with particle size ranging from 0.5 - 1.0 mm. This material was used for the metal adsorption studies without any physical or chemical treatment and will be called infused tea leaves.
2.3. Adsorption Experiments
The batch adsorption experiments for individual metal ions were carried out at a constant temperature (28˚C) on a rotary shaker (300 rpm) using 125 mL capped conical flasks. For each set of experiment, 0.2 g of infused tea waste was added to each 100 mL metal ion solutions of various concentrations (20 - 100 mg∙L−1) for isotherm study and 50 mg∙L−1 for kinetic study. The mixtures which contain the infused tea leaves and the respective metal ion were agitated on a rotary shaker at 300 rpm for 105 min for both the isotherm and kinetic studies. The mixtures were then filtered through Whatman no. 40 filter paper to remove particulates and the filtrate was analyzed with atomic absorption spectrometry (Shimadzu AA6200) to determine the concentration of metal ions. The same procedure was applied to the control samples with the same process and concentration but without infused tea leaves. The amounts of metal ion adsorbed on the biosorbent were determined from the difference of metal ion concentrations in the initial and final suspensions. The batch experiments were performed in triplicate and the mean value was used for each set of data. The amount of metal ions adsorbed at equilibrium, qe (mg∙g−1) and the percentage removal (%) of metal ions were calculated as follows [21] :
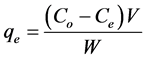 (1)
(1)
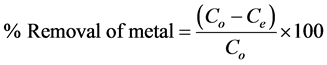 (2)
(2)
where Co and Ce are the initial and equilibrium concentrations of metal ions (mg∙L−1), V is the volume of solution (mL) and W is the weight of infused tea leaves used (g). The effect of pH on metal ions removal was examined by varying the pH from 2 to 7, with initial metal ion concentrations of 50 mg∙L−1, infused tea leaves of 0.20 g/100mL and agitation at 300 rpm for 105 min at 28˚C. The initial pH of the metal ions was adjusted by addition of 0.10 M HCl or NaOH.
2.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
The functional groups present in the infused tea leaves were characterized by a Perkin Elmer Spectrum 100 Fourier transform infrared (FT-IR) spectrometer fitted with Attenuated Total Reflection (ATR). In this analysis, the infused tea leaves with or without metal salts were analyzed directly without blending with KBr. The Spectra of the samples were recorded from 4000 to 650 cm−1.
3. Results and Discussion
3.1. Effect of pH
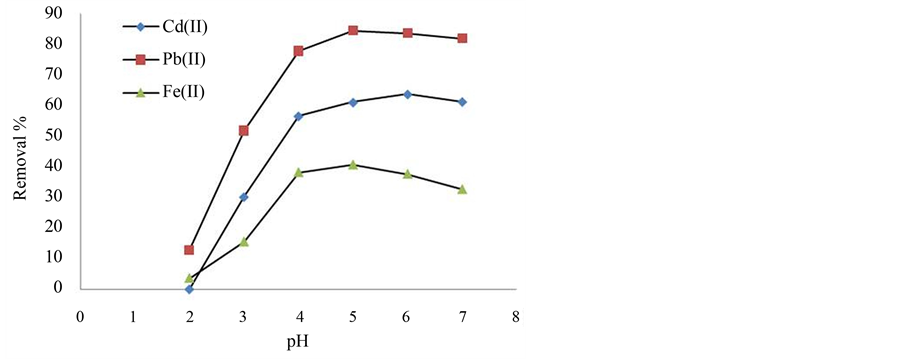
Figure 1 shows that the percentage removal of metal ions by the infused tea leaves is pH dependent. From the corresponding data for each metal, an increase in the pH resulted an increase in percentage removal of the metal ions. The maximum removal of metal ions was observed at pH 5 for Pb2+ and Fe2+ ions, and at pH 6 for Cd2+
Figure 1. The percentage removal of Cd2+, Pb2+, and Fe2+ ions from the standard solutions against different pH of the standard solutions.
ions. On higher pH values, a slight decrease in adsorption for Pb2+, Fe2+ and Cd2+ ions were observed. This is because at higher pH, metal precipitations such as Pb(OH)2, Fe(OH)2 and Cd(OH)2 were observed due to the existence of OH− ions in the adsorption medium. At lower pH such as pH 2, very little adsorption took place (0% Cd2+, 12.76% Pb2+, and 3.55% Fe2+). This could be due to the competition between the H+, produced from the protonation of the active sites of the adsorbent, and the M+ from the metal ions [12] . As the pH increased from pH 2 to the optimum values, the adsorption capacity of the adsorbent increased (63.68% for Cd2+, 84.31% for Pb2+, and 40.67% for Fe2+). This is due to the increase in the overall negative charge of the adsorbent. Then, the adsorption capacity decreased slightly when the pH values exceeded the optimum values. This is due to the existence of OH− ions which may form precipitates with the metal ions. Similar trend has also been observed in the adsorption studies such as the adsorption of Pb2+ and Cd2+ by mango peel waste [13] and the adsorption of Pb2+ by tree fern [22] . Since the optimum pH for the three metal ions (pH 5 for Pb2+ & Fe2+, and pH 6 for Cd2+) lie within the normal pH of distilled water (i.e. pH 5.2 - 5.6), all the experiments were conducted without further adjustment of the pH of the metal ion solutions.
3.2. Effect of Adsorbent Dosage
The removal of metal ions from aqueous solution was significantly increased with the increase in the amount of adsorbent dosage (Figure 2). The efficiencies of metal ions removal were increased with increasing amount of adsorbent used, i.e. from 54.76% to 87.62% for Cd2+, 80.64 to 99.72% for Pb2+, and 41.42 to 62.39% for Fe2+ when the adsorbent dosage (50 mg∙L−1, 100 mL) was increased from 0.2 to 1.0 g. The increase in metal ions removal is basically due to the available adsorption sites increased with the increase in adsorbent dose [23] [24] . However, the amount of metal ions adsorbed per unit of adsorbent decreases with the adsorbent dosage. This is
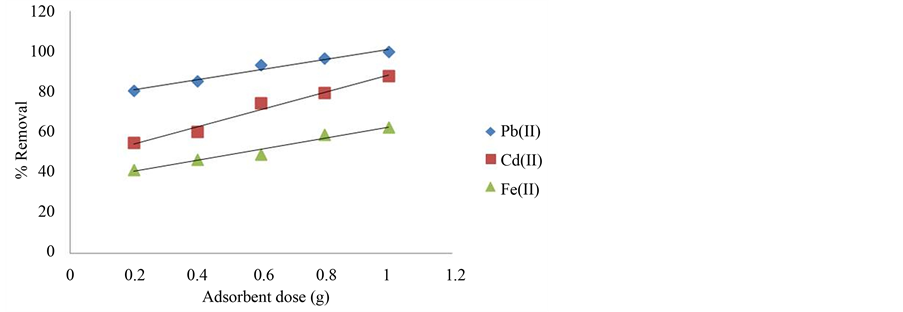
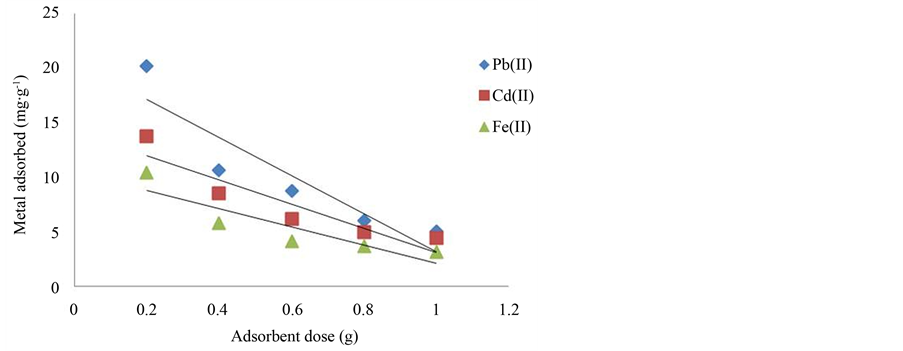
Figure 2. The effect of adsorbent dosage on metal ions removal.
due to the fact that at lower adsorbent dosage, the metal ions were competing for limited adsorption sites. There were excess of metal ions in the solution and gave a lower percentage removal of metal ions. At higher adsorbent dosage, there were more adsorption sites available for similar number of metal ions in the solution and caused the drop of the amount of metal ions adsorbed per unit mass of adsorbent. This explains why the percentage removal of metal ions increased at higher adsorbent dosage but the adsorption capacity (q) drops to a lower value. The similar trend has also been reported for other biomass materials such as cotton ball [23] , mungbean husk [25] , and sawdust [21] . Since the adsorbent dosage of 0.2 g gave the highest adsorption capacity among the rests, it was chosen for the rest of the studies.
3.3. Effect of Initial Metal Ions Concentration
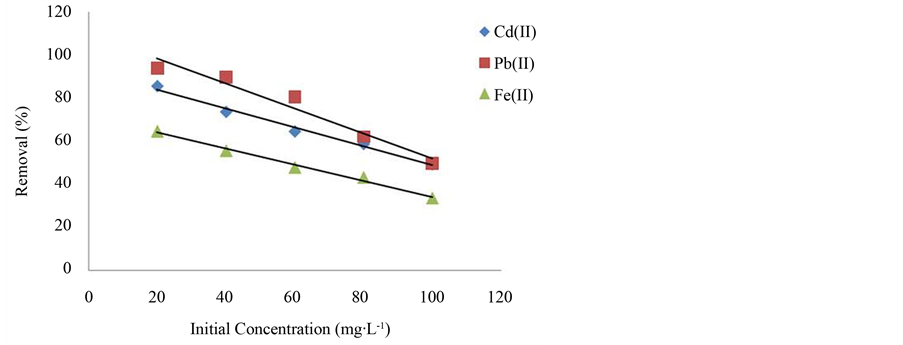
Figure 3 shows the percentage of ion removal as a function of initial metal ion concentration by infused tea leaves. The ion removal percentage decreased with increasing metal ion concentrations. At lower concentrations the ratio of surface active sites to the total metal ions in the solution is high and hence a greater driving force is created by the pressure gradient [26] [27] . This is because diffusion of metal ions through the solution to the surface of adsorbents is affected by the metal ion concentrations. Therefore, an increase of the metal ion concentrations accelerates the diffusion of metal ions from the solution to the surfaces of the absorbents. At higher concentrations, the decrease in the removal efficiency of metal ions could be due to the fact that all the adsorbents had a limited number of active sites, which could become saturated above a certain concentration [21] . For instance, the percentage removal of Cd2+, Pb2+, and Fe2+ were respectively decreased from 85.6% to 49.22%, 93.68% to 49.54% and 64.65% to 33.55%, when initial concentration of the metal ion was increased from 20 mg/L to 100 mg/L. However, the amount of Cd2+, Pb2+, and Fe2+ adsorbed per unit weight of adsorbent is higher at higher concentrations. The adsorption capacity of Cd2+, Pb2+, and Fe2+ was increased from 8.56 mg/g to 24.61 mg/g, 9.37 mg/g to 24.77 mg/g and 6.46 mg/g to 16.78 mg/g respectively due to the fact that more amount of metal ions were available for adsorption at higher concentrations. Similar behavior was also observed for the removal of Cd2+ by Nordmann fir leaves [17] and beech leaves [28] .
3.4. Adsorption Equilibrium Time Study
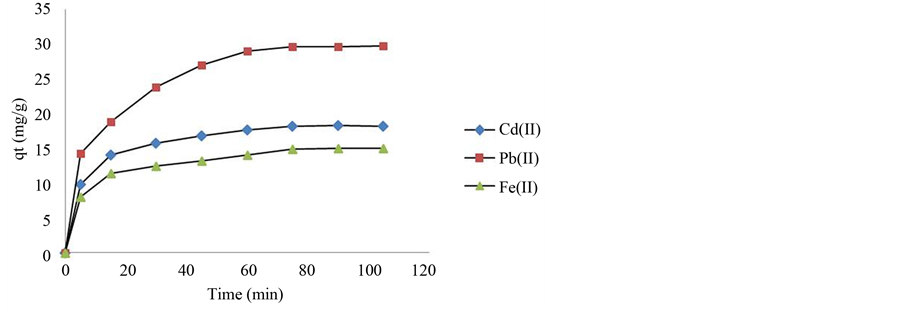
The adsorption of the three metal ions by the infused tea leaves versus contact time is illustrated in Figure 4. The results show that the adsorption process increased with the prolonging of contact time and it is a biphasic process. The initial stage is a fast process (from 0 - 10 min), whereby the adsorption uptake of metal ions increased rapidly and then gradually slow down [29] . In the following stage, the adsorption of metal ions increased slowly and attained a maximum at about 75 min when the equilibrium is reached. After that, a further increase in contact time has negligible effect on the adsorption of the metal ions. At the first stage (up to 10 min), a rapid increase in the adsorption could be attributed to a larger concentration gradient between the solutes and the solid surface of the absorbents. At the second stage (from 20 to 60 min), a slower uptake of metal ions was observed as a result of free binding sites becoming saturated gradually. This phenomenon was observed in other
Figure 3. The effect of initial concentration on metal ions removal.
Figure 4. The effect of contact time on metal ions removal (dose: 0.2 g/100 mL; agitation: 300 rpm; time: 0 - 105 min; initial concentration: 50 mg/L for each metal ion, pH: 5 - 6).
studies conducted by researches such as Gok and Aytas [30] , Yao et al. [31] and Örnek et al. [26] .
The amount of time required to reach equilibrium for the adsorption of the three metal ions is practically the same and is within 75 - 90 minutes. From Figure 4, it can be seen that the adsorption capacity for the three metal ions are in the sequence of Pb2+ > Cd2+ > Fe2+ with the maximum adsorption capacity of 29.68 mg∙g−1 for Pb2+, 18.21 mg∙g−1 for Cd2+ and 14.96 mg∙g−1 for Fe2+. The higher adsorption capacity of Pb2+ compared to Cd2+ and Fe2+ can be attributed to the ion electronegativity or/and ionic radius of these metal ions [32] . Pb2+ has the highest ion electronegativity (2.33) compared to Cd2+ (1.69) and Fe2+ (1.83) [33] . Furthermore, Pb2+ has the largest ionic radius (1.33 Å) compared to Cd2+ (0.92 Å) and Fe2+ (0.77 Å) [34] . According to the study conducted by Matos and Arruda [35] on the use of yermicompost as a natural adsorbent for the removal of metal ions from laboratory effluents, they found that the higher the electronegativity of the metal ion, the stronger the interactions between the metal ions and the biosorbent, therefore, the higher the adsorption capacity. However, in our case, the sequence of the adsorption capacity did not agree with the degree of electronegativity of the three metal ions, as the sequence of adsorption capacity should be in the order of Pb2+ > Fe2+ > Cd2+ based on their electronegativity values. But what we obtained here is Pb2+ > Cd2+ > Fe2+ which is in good agreement with the decreasing order of the metal ionic radius and not electronegativity. A similar phenomenon was observed in the study conducted by Chen et al. [36] on metal ions binding onto the calcium alginate-based ion-exchange resin where they found that Pb2+ has stronger binding compared to Cu2+ and Ca2+ which have smaller ionic radii. Hence, we may conclude that the ionic radius is the predominant factor in this study in determining the trend of adsorption capacity.
Usually the effects of electronegativity and ionic radius have been used by different researchers in explaining the trend of adsorption capacity separately. However, we strongly believe that a combination of both the electronegativity and ionic radius will be more appropriate in explaining the adsorption capacity for a particular metal ion onto a biosorbent. Hence, the covalent index of metal ions will be used in explaining the trend of the adsorption capacity in this study and it can be calculated using the following equation [37] :
 (3)
(3)
where Xm and r are the electronegativity and ionic radius of the metal ions respectively. The value of 0.85 is a constant assumed to reflect the radius of O and N donor atoms [37] . The calculated covalent indexes for the metal ions are 11.84, 5.06 and 5.42 for Pb2+, Cd2+ and Fe2+ respectively and the trend of adsorption capacity should be Pb2+ > Fe2+ > Cd2+. However, these values still do not agree with the trend of decreasing order of the adsorption capacity of the three metal ions in this study (i.e. Pb2+ > Cd2+ > Fe2+). The most likely reason for the lower adsorption capacity value of Fe2+ as compared to Cd2+ can be the instability of Fe2+ which may lead to the oxidation of Fe2+ to form Fe3+ which is precipitated out as a solid material such as Fe2O3∙xH2O [34] . This causes the drop of Fe2+ in the analysis and this explains why Fe2+ gave a smaller value of adsorption capacity as compared to Cd2+.
Nevertheless, what we can conclude in this study is that both the ionic radius and electronegativity do play important roles in determining the adsorption capacity of the infused tea leaves towards the three metal ions. Furthermore, it is also obvious that Pb2+ has a much higher adsorption capacity compared to the other two metal ions. The much higher value of covalent index of the Pb2+ indicates that Pb2+ has a higher degree of binding capacity towards the biosorbent. Our results show that Pb2+ is the most easily bonded component to the binding sites of the infused tea leaves, followed by Cd2+ and Fe2+. Similar results were found by other researches using different biosorbents. For examples, the use of Neurospora crassa for the removal of Pb(II) and Cu(II) from aqueous solutions [38] and the removal of Pb(II), Cu(II) and Zn(II) by loofasponge immobilized biomass of Phanerochaete chrysosporium [39] .
3.5. Adsorption Isotherms
The adsorption isotherm represents the relationship between the amounts of adsorbate adsorbed on the surface of a unit weight of solid adsorbent at a fixed temperature at equilibrium. It is usually described by certain constants whose values express the surface properties and affinity of the adsorbent. In the present work, Langmuir and Freundlich isotherms are used to describe the adsorption isotherm of the three metal ions. The Langmuir isotherm model assumes that there is a finite number of identical adsorption sites on the surface of the adsorbent and it is only valid for monolayer adsorption. Once a metal ion occupies a site, then there is no further adsorption taking place at that site [16] [21] . The linear form of the Langmuir monolayer isotherm equation can be represented as follows:
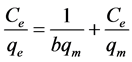 (4)
(4)
where Ce is the equilibrium concentration (mg∙dm−3) of metal ion, qe is the amount of adsorption at equilibrium (mg∙g−1), qm is qe for a complete monolayer (mg∙g−1), b is an equilibrium constant of Langmuir (dm3∙mg−1). A plot of Ce/qe versus Ce should indicate a straight line of slope 1/qm and an intercept of 1/bqm.
The essential features of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter RL, which is defined by the following equation [40] :
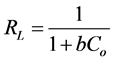 (5)
(5)
where Co is initial concentration (mg∙dm−3) and b is the Langmuir constant (dm3∙mg−1).
The value of RL indicates the pattern of the isotherm. If the RL > 1, the adsorption is unfavorable, and if RL = 1, there will be a linear adsorption. If 0 < RL < 1, the adsorption is favorable, and if RL = 0, the adsorption is irreversible.
The Freundlich isotherm is an empirical equation based on adsorption on a heterogeneous surface of an adsorbent [21] [23] [41] . This model provides no information on the monolayer adsorption, in contrast to the Langmuir model. It suggests that the binding sites are not equivalent and/or independent. The linear form of the Freundlich equation can be expressed as:
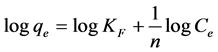 (6)
(6)
where KF and 1/n are the Freundlich constants of the systems, indicating the adsorption capacity and adsorption intensity, respectively. The adsorption constants of Freundlich isotherm Kf and 1/n can be determined from the intercept and slope of logqe versus logCe. The 1/n value is usually dependent on the nature and strength of the adsorbent as well as the distribution of active sites [30] . If the values of 1/n lie between 0.1 and 1, this indicates that adsorption of metal ions onto adsorbents is favorable at the examined condition [42] . The n value indicates the presence of a heterogeneous surface and binding sites with different adsorption energies. The higher the n value the higher the adsorption intensity [43] . However, this isotherm model does not predict any saturation of the adsorbent by the sorbate, and infinite surface coverage is predicted mathematically, indicating a multilayer adsorption on the surface of the adsorbent [25] [30] .
The Langmuir and Freundlich constants and their correlation coefficient values (R2) for the three metal ions calculated based on these isotherm models are summarized in Table 1. Very high correlation coefficient values were obtained for the three metal ions (0.996 for Cd2+, 0.995 for Pb2+ and 0.993 for Fe2+) from the Langmuir
Table 1. Langmuir and Freundlich constants for metal ions adsorption onto infused tea leaves.
isotherm, indicating that there is a good agreement between the experimental data and the Langmuir isotherm parameters. Whereas, for the Freundlich isotherm, the values of correlation coefficients for the three metal ions were found to be lower (0.952 for Cd2+, 0.830 for Pb2+ and 0.947 for Fe2+) than those of the Langmuir isotherm, indicating that the set of equilibrium data is not in as good agreement as in the case of the Langmuir isotherm. This confirms that the metal ions/infused tea leaves adsorption data follows the Langmuir isotherm.
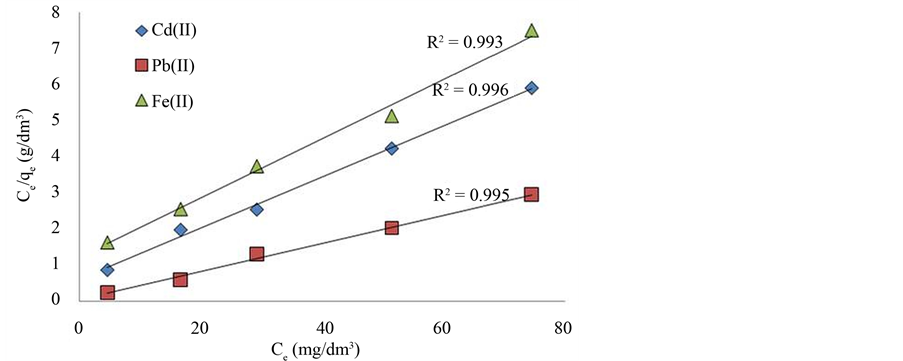
A plot of Ceq/qe versus the various concentrations of the three metal ions at equilibrium (Ceq) is shown in Figure 5. The monolayer adsorption capacities, qm, obtained from this model are 14.29, 26.32 and 12.38 mg∙g−1 for Cd2+, Pb2+ and Fe2+ respectively. This indicates that the adsorption capacity of infused tea leaves increased in the order of Pb2+ > Cd2+ > Fe2+. A greater equilibrium constant b indicates a stronger bond between metal ion and biosorbent [44] .
In this study (Table 1), the constants for the three metal ions was in the order of Pb2+ (0.5071 dm3∙mg−1) > Cd2+ (0.1077 dm3∙mg−1) > Fe2+ (0.0622 dm3∙mg−1), and this further suggests that the infused tea leaves were more efficient in adsorbing Pb2+ than the other two adsorbates. The dimensionless parameter, RL, which is a measure of adsorption favorability was found to be in the range of 0 and 1 (0.0193 for Pb2+, 0.0850 for Cd2+ and 0.1385 for Fe2+). This indicates that the adsorption of the three metal ions (Cd2+, Pb2+ and Fe2+) by the infused tea leaves is favorable. The fact that the Langmuir isotherm fits the experimental data very well suggest that there is a homogeneous distribution of active sites on the infused tea leaves’ surface. Similar finding was found in other studies such as the removal of Cu2+ from aqueous solutions by chemically modified sugar beet pulp [45] , FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste [13] , and removal of Cu2+ and Pb2+ by tartaric acid modified rice husk from aqueous solutions [46] .
The removal of heavy metal ions using tea waste as an adsorbent has been reported by several other researchers. The treatment conditions of the tea waste, the types of metal ions being removed, and the maximum adsorption capacities for each study are summarized in Table 2. It should be noted that the ability of the tea waste as an adsorbent for the metal ions removal may vary depending on many parameters such as particle size, pH of the solution, temperature, and the design of the experimental conditions [47] . Generally, Pb2+ gave better adsorption capacity compared to other metal ions. This agrees with the results of the present work. The results also show that the treated tea waste gave higher adsorption capacity than the untreated tea waste. However, this may incur additional cost.
3.6. Kinetics of Adsorption
The adsorption kinetics of the removal of metal ions by the infused tea leaves was described using the pseudo-first-order and pseudo-second-order models. The pseudo-first-order in the linear form is generally expressed as follows [31] [55] :
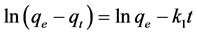 (7)
(7)
where qe and qt are the amount of metal ions adsorbed per unit weight on the adsorbent (mg/g) at equilibrium, and at time t, respectively, and k1 is the rate constant of the adsorption (min−1). Plot of 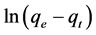 versus t gives a straight line for first order adsorption kinetics, which allows for the calculation of the adsorption rate constant k1. The linear form of the pseudo-second-order models can be expressed as [31] [56] :
versus t gives a straight line for first order adsorption kinetics, which allows for the calculation of the adsorption rate constant k1. The linear form of the pseudo-second-order models can be expressed as [31] [56] :
 (8)
(8)
Table 2. Maximum adsorption capacities, qo (mg/g) for the adsorption of metal ions onto tea waste materials reported in the literature.
aaverage value of different flow rates. bmaximum adsorption obtained at 60˚C.
Figure 5. The Langmuir isotherms for adsorption of Cd2+, Pb2+ and Fe2+ onto infused tea leaves (dose: 0.2 g/100 mL; agitation: 300 rpm for 105 min; initial concentration: 50 mg/L for each metal ion, pH: 5 - 6).
where k2 is the rate constant of adsorption (g∙mg−1∙min−1), and qe and qt are the amounts of metal ions adsorbed at equilibrium and at time t (mg∙g−1), respectively. The constant k2 can be determined experimentally by plotting t/qt against t. The initial adsorption rate, h (mg∙g−1∙min−1) can be calculated from the pseudo-second-order by the following equation [57] :
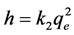
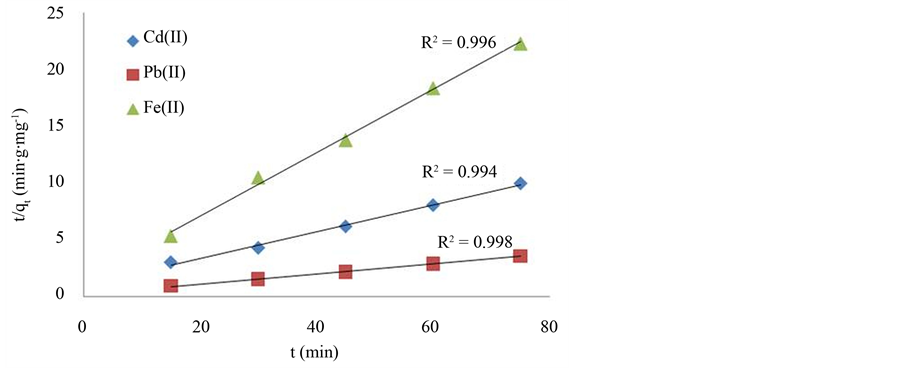
The corresponding results of the two kinetics models are summarized in Table 3. The adsorption kinetics for the three metal ions increased with time and attained saturation within 105 minutes (Figure 4). The experimental results (Table 3) indicated that the pseudo-second order kinetic model provided the best description of the data with an extremely high correlation coefficient, all R2 > 0.99 for the three metal ions as shown in Figure 6 in contrast to the pseudo-first order model (all R2 < 0.975, Table 3). These indicated that the adsorption system belonged to the pseudo-second order kinetic. The kinetic rate constant (k2) calculated from the equation shows that the Pb2+ had the lowest k2 value of 4.30 × 10−3 g∙mg−1∙min−1 which is lower than that of Cd2+ (1.45 × 10−2 g∙mg−1∙min−1) and Fe2+ (1.75 × 10−1 g∙mg−1∙min−1). This implies that the adsorption of Pb2+ onto the infused tea leaves from the aqueous solution is more rapid and favorable. This can be observed from the initial adsorption rate (h) which is higher for Pb2+ (2.498 mg∙g−1∙min−1) as compared to Cd2+ (1.055 mg∙g−1∙min−1) and Fe2+ (2.181 mg∙g−1∙min−1). Similar behavior was reported by Shahmohammadi-Kalalagh et al. [58] where Pb2+ adsorption on kaolinite was higher than Zn2+ and Cu2+. Moreover, the Pb2+ gave the highest maximum adsorption capacity
Table 3. Kinetic parameters for the adsorption of metal ions onto infused tea leaves.
Figure 6. The plots of pseudo-second order for the adsorption of Cd2+, Pb2+ and Fe2+ onto infused tea leaves (dose: 0.2 g/100mL; agitation: 300 rpm; time: 15 - 90 min; initial concentration: 50 mg/L for each metal ion, pH: 5 - 6).
(24.10 mg∙g−1), followed by Cd2+ (8.53 mg∙g−1) and Fe2+ (3.53 mg∙g−1). The reasoning is that Pb2+ which has a larger ion radius and electronegativity as compared to the other two metal ions is better accommodated by the infused tea leaves as discussed in the previous section. In addition, the calculated q2 values for the three metal ions were found relatively closer to the experimental qexp values compared to the q1 values for the three metal ions (Table 3). This further suggests that the adsorption of the three metal ions by the infused tea leaves follow the pseudo-second order kinetics; based on the assumption that the process controlling rate may be a chemical sorption which involves valence force through sharing or exchanging of electrons between the sorbent and sorbate [13] .
In order to understand the adsorption mechanisms and the rate limiting steps that may affect the kinetics of adsorption, the kinetic experimental results were fitted to the Weber’s intraparticle diffusion model. The rate parameters for intraparticle diffusion (kp) at different initial concentrations can be determined using the following equation [31] :
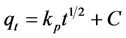
where C is the intercept and kp is the intraparticle diffusion rate constant (mg∙g−1∙min−1/2), which can be evaluated from the slope of a linear plot of qt versus t1/2. According to this model, the plot of qt versus t1/2 should be linear if intraparticle diffusion is involved in the adsorption process and if the plot passes through the origin then the intraparticle diffusion is the only rate-limiting step [57] . It was suggested that the adsorption process may involve three different steps. They are surface diffusion, a gradual adsorption stage where intraparticle diffusion is the rate limiting step, and the third is the equilibrium step [59] . Since the plot of the three metal ions did not pass through the origin (y = 3.888x ? 23.27 for Cd2+, y = 0.838x ? 9.615 for Pb2+ and y = 4.056x ? 18.93 for Fe2+), intraparticle diffusion may not be the only rate-limiting step. Thus, there were three processes controlling the adsorption rate but only one was rate-limiting in any particular time range. The rate constants for the three metal ions calculated from the slope of the intraparticle diffusion plot are listed in Table 3.
3.7. Fourier Transform Infrared Spectroscopy
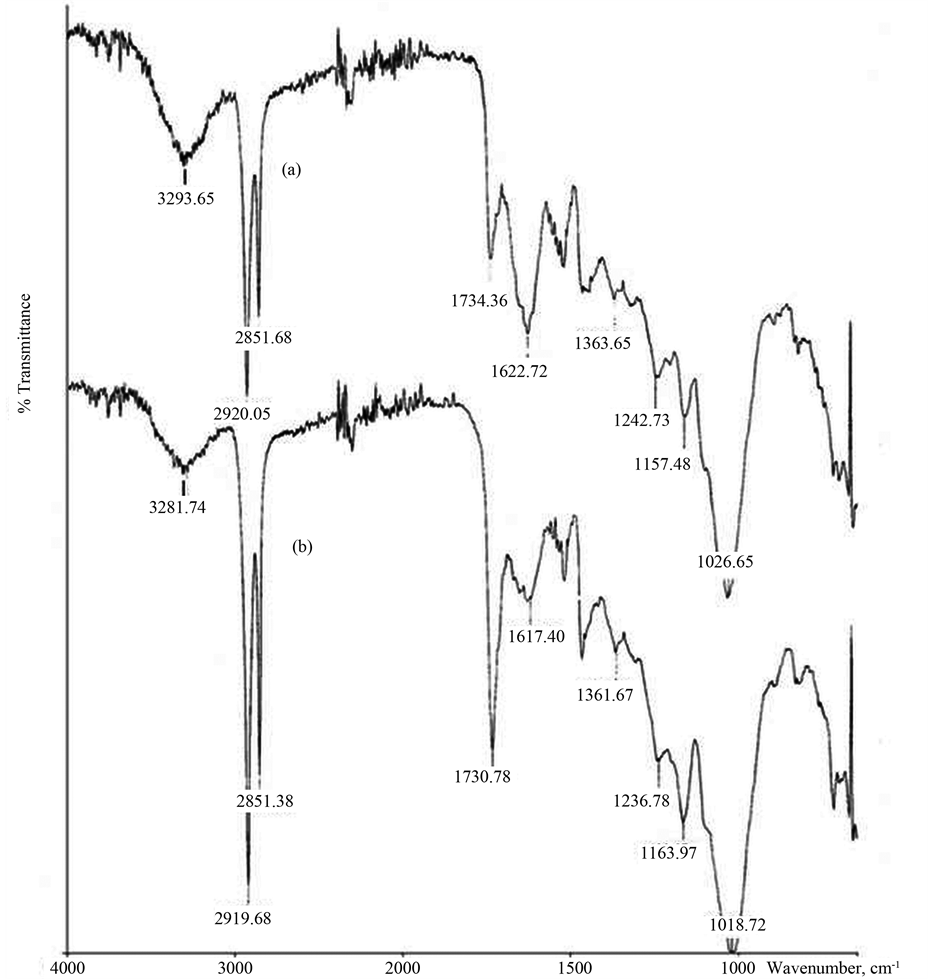
Like all other biomass, infused tea leaves are composed of cellulose, hemi-cellulose and lignin [32] . In order to understand the characteristic functional groups on the surface of infused tea leaves, FTIR spectra of infused tea leaves before and after treatment with metal ions were carried out in order to identify the functional groups involved in the adsorption process. The representative FTIR spectra of the infused tea leaves before and after treatment with the Pb2+ ions, indicated as (a) and (b) respectively are shown in Figure 7.
Before the adsorption of Pb2+ onto the infused tea leaves (Figure 7(a)), a broad band is observed at 3293.65 cm-1 indicating the presence of O-H stretching vibration in hydroxyl groups due to inter- and intramolecular hydrogen bonding of polymeric compounds in the infused tea leaves [60] . The peaks at 2920.05 and 2851.68 cm−1 are the indicator for the presence of symmetric and asymmetric sp3C-H stretching vibration of aliphatic compounds in the lignin structure of the infused tea leaves [60] . The band at 1734.36 cm−1 indicates the presence of
Figure 7. FTIR spectra of infused tea leaves (a) before (b) after Pb2+ adsorption.
C=O stretching vibration of non-ionic carboxyl groups (-COOH) or their esters (-COOCH3) and the symmetric and asymmetric stretching vibrations of ionic carboxyl groups (-COO−) are observed at 1622.72 cm−1 [13] . The peak appearing at 1026.65 cm−1 may be related to the deformation of C-H and C-O bonds of primary alcohols in the infused tea leaves [32] . After the adsorption of Pb2+ onto the infused tea leaves, all the peaks at 3293.65 cm−1, 1734.36 cm−1, 1622.72 cm−1, and 1026.65 cm−1 as mentioned above were significantly shifted to lower wavelength at 3281.74 cm−1 (−11.91 cm−1), 1730.78 cm−1 (−3.58 cm−1), 1617.40 cm−1 (−5.32 cm−1), and 1018.72 cm−1 (−7.93 cm−1) respectively (Figure 7(b)). All these peaks are shifted in the range of about 4 - 12 cm−1. The changes observed in the spectrum indicated the possible involvement of those functional groups on the surface of the infused tea leaves for the metal ions uptake in the adsorption process.
4. Conclusion
The present study shows that infused tea leaves like the most other natural absorbents are able to remove Cd2+, Pb2+ and Fe2+ ions from aqueous solutions. The equilibrium isotherms between metal ions and infused tea leaves have been developed and analyzed according to two isotherm equations, namely the Langmuir and Freundlich adsorption isotherm models. The results from this study are found to fit well to Langmuir adsorption isotherm model with the maximum adsorption capacity of 14.29 mg∙g−1, 26.32 mg∙g−1, and 12.38 mg∙g−1 respectively for Pb2+, Cd2+ and Fe2+ ions. A comparison of the kinetic models on the overall adsorption rate showed that the adsorption system is best described by the pseudo-second order kinetic model, and the rate constants are found to be 4.30 × 10−3 g∙mg−1∙min−1, 1.75 × 10−1 g∙mg−1∙min−1 and 1.45 × 10−2 g∙mg−1∙min−1 for Pb2+, Fe2+ and Cd2+ ions respectively. Kinetic results of the adsorption also indicate that chemical sorption is the basic mechanism involved in this system. The FTIR results show that OH, C=O, C-O, and C-H are the main functional groups of infused tea leaves that are involved in the metal ions adsorption. As a conclusion, the results show that infused tea leaves which are a natural waste can be used as an alternative low-cost and environment-friendly biosorbent for the removal of heavy metals from contaminated water.
Acknowledgements
We thank the Faculty of Applied Sciences and Computing of Tunku Abdul Rahman University College for financial support. We also wish to thank Dr. Teh Geok Bee, Dr. Lo Fook Loong and Ms. Selvi for their valuable comments and help in proof reading of the manuscript.
Cite this paper
Chen Son Yue,Kok How Chong,Cheah Cheng Eng,Ling Siang Loh, (2016) Utilization of Infused Tea Leaves (Camellia sinensis) for the Removal of Pb2+, Fe2+ and Cd2+ Ions from Aqueous Solution: Equilibrium and Kinetic Studies. Journal of Water Resource and Protection,08,568-582. doi: 10.4236/jwarp.2016.85047
References
- 1. Al-Othman, Z.A., Ali, R. and Naushad, M. (2012) Hexavalent Chromium Removal from Aqueous Medium by Activated Carbon Prepared from Peanut Shell: Adsorption Kinetics, Equilibrium and Thermodynamic Studies. Chemical Engineering Journal, 184, 238-247.
http://dx.doi.org/10.1016/j.cej.2012.01.048 - 2. Xing, S.T., Zhao, M.Q. and Ma, Z.C. (2011) Removal of Heavy Metal Ions from Aqueous Solution Using Red Loess as an Adsorbent. Journal of Environmental Sciences, 23, 1497-1502.
http://dx.doi.org/10.1016/S1001-0742(10)60581-5 - 3. Mathialagan, T. and Viraraghavan, T. (2001) Adsorption of Cadmium from Aqueous Solutions by Perlite. Journal of Hazardous Materials, B94, 291-303.
- 4. Yan, G. and Viraraghavan, T. (2001) Heavy Metal Removal in a Biosorption Column by Immobilized M. rouxii Biomass. Bioresource Technology, 78, 243-249.
http://dx.doi.org/10.1016/S0960-8524(01)00020-7 - 5. Kaewsarn, P. and Yu, Q. (2001) Cadmium(II) Removal from Aqueous Solutions by Pre-Treated Biomass of Marine Alga Padina sp. Environmental Pollution, 112, 209-213.
http://dx.doi.org/10.1016/S0269-7491(00)00114-7 - 6. Bakkaloglu, I., Butter, T.J., Evison, L.M., Holland, F.S. and Hancock, I.C. (1998) Screening of Various Types Biomass for Removal and Recovery of Heavy Metals (Zn, Cu, Ni) by Biosorption, Sedimentation and Desorptionl. Water Science Technology, 38, 269-277.
http://dx.doi.org/10.1016/S0273-1223(98)00587-3 - 7. Yetis, U., Ozcengiz, G., Filiz, B., Ergen, N., Erbay, A. and Dolek, A. (1998) Heavy Metal Biosorption by White-Rot Fungi. Water Science and Technology, 38, 323-330.
http://dx.doi.org/10.1016/S0273-1223(98)00515-0 - 8. Baik, W.Y., Bae, J.H., Cho, K.W. and Hartmeier, W.W. (2002) Biosorption of Heavy Metals Using Whole Mold Mycelia and Parts Thereof. Bioresource Technology, 81, 167-170.
http://dx.doi.org/10.1016/S0960-8524(01)00148-1 - 9. Matis, K.A., Zouboulis, A.I. and Lazaridis, N.K. (2003) Heavy Metals Removal by Biorsorption and Flotation. Water, Air, & Soil Pollution, 3, 143-151.
http://dx.doi.org/10.1016/S0960-8524(01)00148-1 - 10. Yu, M.H. (2005) Environmental Toxicology—Biological and Health Effects of Pollutants. 2nd Edition, CRC Press, Boca Raton.
- 11. Friberg, L., Piscator, M., Nordberg, G.F. and Kjellstrom, T. (1974) Cadmium in the Environmental. 2nd Edition, CRC Press, Boca Raton.
- 12. Saeed, A., Iqbal, M. and Akhtar, M.W. (2005) Removal and Recovery of Lead(II) from Single and Multimetal (Cd, Cu, Ni, Zn) Solution by Crop Milling Waste (Black Gram Husk). Journal of Hazardous Materials, 117, 64-67.
http://dx.doi.org/10.1016/j.jhazmat.2004.09.008 - 13. Iqbal, M., Saeed, A. and Zafar, S.I. (2009) FTIR Spectrophotometry, Kinetics and Adsorption Isotherms Modeling, Ion Exchange, and EDX Analysis for Understanding the Mechanism of Cd2+ and Pb2+ Removal by Mango Peel Waste. Journal of Hazardous Materials, 164, 161-171.
http://dx.doi.org/10.1016/j.jhazmat.2008.07.141 - 14. Gong, J.M., Wang, X.Q., Shao, X.L., Yuan, S., Yang, C.L. and Hu, X.L. (2012) Adsorption of Heavy Metal Ions by Hierarchically Structured Magnetite-Carbonaceous Spheres. Talanta, 101, 45-52.
http://dx.doi.org/10.1016/j.talanta.2012.08.035 - 15. Bailey, S.E., Olin, T.J., Bricka, R.M. and Adrian, D. (1999) A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Research, 33, 2469-2479.
http://dx.doi.org/10.1016/S0043-1354(98)00475-8 - 16. Babel, S. and Kurniawan, T.A. (2003) Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. Journal of Hazardous Materials, 97, 219-243.
http://dx.doi.org/10.1016/S0304-3894(02)00263-7 - 17. Serencam, H., Gundogdu, A., Uygur, Y., Kemer, B., Bulut, V.N., Duran, C., Soylak, M. and Tufekci, M. (2008) Removal of Cadmium from Aqueous Solution by Nordmann Fir (Abies nordmanniana (Stev.) Spach. Subsp. Nordmanniana) Leaves. Bioresource Technology, 99, 1992-2000.
http://dx.doi.org/10.1016/j.biortech.2007.03.021 - 18. Food and Agriculture Organization (2012) Tea. http://en.wikipedia.org/wiki/Tea
- 19. Basu, M.A., Bera, B. and Rajan, A. (2012) Tea Statistics: Global Scenario.
http://www.teaboard.gov.in/pdf/Tea%20Statistics%20Global%20Scenario.pdf - 20. World Tea News (2013) Discovering Tea in Malaysia.
http://www.worldteanews.com/profiles/discovering-tea-malaysia#sthash2KxXBNQo.dpbs - 21. Gupta, S. and Babu, B.V. (2009) Removal of Toxic Metal Cr(VI) from Aqueous Solutions Using Sawdust as Adsorbent: Equilibrium, Kinetics and Regeneration Studies. Chemical Engineering Journal, 150, 352-365.
http://dx.doi.org/10.1016/j.cej.2009.01.013 - 22. Ho, Y.S. (2005) Effect of pH on Lead Removal from Water Using Tree Fern as the Sorbent. Bioresource Technology, 96, 1292-1296.
http://dx.doi.org/10.1016/j.biortech.2004.10.011 - 23. Ozsoy, H.D. and Kumbur, H. (2006) Adsorption of Cu(II) Ions on Cotton Boll. Journal of Hazardous Materials, 136, 911-916.
http://dx.doi.org/10.1016/j.jhazmat.2006.01.035 - 24. Gercel, O. and Gercel, H.F. (2007) Adsorption of Lead(II) Ions from Aqueous Solutions by Activated Carbon Prepared from Biomass Plant Material of Euphorbia rigida. Chemical Engineering Journal, 132, 289-297.
http://dx.doi.org/10.1016/j.cej.2007.01.010 - 25. Saeed, A., Iqbal, M. and Holl, M.W. (2009) Kinetic, Equilibrium and Mechanism of Cd2+ Removal from Aqueous Solution by Mungbean Husk. Journal of Hazardous Materials, 168, 1467-1475.
http://dx.doi.org/10.1016/j.jhazmat.2009.03.062 - 26. Örnek, A., Özacar, M. and Sengil, I.A. (2007) Adsorption of Lead onto Formaldehyde or Sulphuric Acid Treated Acorn Waste: Equilibrium and Kinetic Studies. Biochemical Engineering Journal, 37, 192-200.
http://dx.doi.org/10.1016/j.bej.2007.04.011 - 27. Özacar, M. and Sengil, I.A. (2005) Adsorption of Metal Complex Dyes from Aqueous Solutions by Pine Sawdust. Bioresource Technology, 96, 791-795.
http://dx.doi.org/10.1016/j.biortech.2004.07.011 - 28. Salim, R., Al-Subu, M.M. and Sahrhage, E. (1992) Uptake of Cadmium from Water by Beech Leaves. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, 27, 603-627.
http://dx.doi.org/10.1080/10934529209375751 - 29. Lu, W.B., Shi, J.J., Wang, C.H. and Chang, J.S. (2006) Biosorption of Lead, Copper and Cadmium by an Indigenous Isolate Enterobacter sp. J1 Possessing High Heavy-Metal Resistance. Journal of Hazardous Materials, 134, 80-86.
http://dx.doi.org/10.1016/j.jhazmat.2005.10.036 - 30. Gok, C. and Aytas, S. (2009) Biosorption of Uranium(VI) from Aqueous Solution Using Calcium Alginate Beads. Journal of Hazardous Materials, 168, 369-375.
http://dx.doi.org/10.1016/j.jhazmat.2009.02.063 - 31. Yao, L., Ye, Z.F., Tong, M.P., Lai, P. and Ni, J.R. (2009) Removal of Cr3+ from Aqueous Solution by Biosorption with Aerobic Granules. Journal of Hazardous Materials, 165, 250-255.
http://dx.doi.org/10.1016/j.jhazmat.2008.09.110 - 32. Teixeira Tarley, C.R. and Zezzi Arruda, M.A. (2004) Biosorption of Heavy Metals Using Rice Milling by-Products. Characterisation and Application for Removal of Metals from Aqueous Effluents. Chemosphere, 54, 987-995.
http://dx.doi.org/10.1016/j.chemosphere.2003.09.001 - 33. Huheey, J.E. (1983) Electronegativity: Pauling, Allred-Rochow, and Mulliken-Jaffé Scales. Inorganic Chemistry, Harper & Row, New York.
- 34. Cotton, F.A., Wilkinson, G., Murillo, C.A. and Bochmann, M. (1999) Advanced Inorganic Chemistry. 6th Edition, Wiley & Sons Inc., New York.
- 35. Matos, G.D. and Arruda, M.A.Z. (2003) Vermicompost as Natural Adsorbent for Removing Metal Ions from Laboratory Effluents. Process Biochemistry, 39, 81-88.
http://dx.doi.org/10.1016/S0032-9592(02)00315-1 - 36. Chen, J.P., Hong, L., Wu, S. and Wang, L. (2002) Elucidation of Interactions between Metal Ions and Ca Alginate-Based on Ion-Exchange Resin by Spectroscopic Analysis and Modeling Simulation. Langmuir, 18, 9413-9421.
http://dx.doi.org/10.1021/la026060v - 37. Puranik, P.R. and Paknikar, K.M. (1999) Influence of Co-Cations on Biosorption of Lead and Zinc—A Comparative Evaluation in Binary and Multimetal Systems. Bioresource Technology, 70, 269-276.
http://dx.doi.org/10.1016/S0960-8524(99)00037-1 - 38. Kiran, I., Akar, T. and Tunali, S. (2005) Biosorption of Pb(II) and Cu(II) from Aqueous Solutions by Pretreated Biomass of Neurospora crassa. Process Biochemistry, 40, 3550-3558.
http://dx.doi.org/10.1016/j.procbio.2005.03.051 - 39. Iqbal, M. and Edyvean, R.G.J. (2004) Biosorption of Lead, Copper and Zinc Ions on Loofa Sponge Immobilized Biomass of Phanerochaete chrysosporium. Minerals Engineering, 17, 217-223.
http://dx.doi.org/10.1016/j.mineng.2003.08.014 - 40. Ho, Y.S., Huang, C.T. and Huang, H.W. (2002) Equilibrium Sorption Isotherm for Metal Ions on Tree Fern. Process Biochemistry, 37, 1421-1430.
http://dx.doi.org/10.1016/S0032-9592(02)00036-5 - 41. Ho, Y.S. (2004) Pseudo-Isotherms Using a Second Order Kinetic Expression Constant. Adsorption, 10, 151-158.
http://dx.doi.org/10.1023/B:ADSO.0000039870.28835.09 - 42. Amir, F.T., Tahereh, K. and Mansooreh, S. (2009) Adsorption of Cadmium from Aqueous Solutions on Sulfurized Activated Carbon Prepared from Nut Shells. Journal of Hazardous Materials, 165, 1159-1164.
http://dx.doi.org/10.1016/j.jhazmat.2008.10.131 - 43. Montanher, S.F., Oliveira, E.A. and Rollemberg, M.C. (2005) Removal of Metal Ions from Aqueous Solutions by Sorption onto Rice Bran. Journal of Hazardous Materials, 117, 207-211.
http://dx.doi.org/10.1016/j.jhazmat.2004.09.015 - 44. Blázquez, A., Mata, Y.N., Ballester, M.L., González, F. and Munoz, J.A. (2009) Sugar-Beet Pulp Pectin Gels as Biosorbent for Heavy Metals: Preparation and Determination of Biosorption and Desorption Characteristics, Chemical Engineering Journal, 150, 289-301.
http://dx.doi.org/10.1016/j.cej.2009.01.001 - 45. Tumen, F., Altundogan, H.S. and Arslan, N.E. (2007) Copper Removal from Aqueous Solutions by Sugar Beet Pulp Treated by NaOH and Citric Acid. Journal of Hazardous Materials, 149, 432-439.
http://dx.doi.org/10.1016/j.jhazmat.2007.04.008 - 46. Lee, C.K., Wong, K.K., Low, K.S. and Haron, M.J. (2003) Removal of Cu and Pb by Tartaric Acid Modified Rice Husk from Aqueous Solutions. Chemosphere, 50, 23-28.
http://dx.doi.org/10.1016/S0045-6535(02)00598-2 - 47. Amarasinghe, B.M.W.P.K. and Williams, R.A. (2007) Tea Waste as a Low Cost Adsorbent for the Removal of Cu and Pb from Wastewater. Chemical Engineering Journal, 132, 299-309.
http://dx.doi.org/10.1016/j.cej.2007.01.016 - 48. Malkoc, E. and Nuhoglu, Y. (2006a) Fixed Bed Studies for the Sorption of Chromium(VI) onto Tea Factory Waste. Chemical Engineering Science, 61, 4363-4372.
http://dx.doi.org/10.1016/j.ces.2006.02.005 - 49. Mondal, M.K. (2009) Removal of Pb(II) Ions from Aqueous Solution Using Activated Tea Waste: Adsorption on a Fixed-Bed Column. Journal of Environmental Management, 90, 3266-3271.
http://dx.doi.org/10.1016/j.jenvman.2009.05.025 - 50. Malkoc, E. and Nuhoglu, Y. (2006b) Removal of Ni(II) Ions from Aqueous Solutions Using Waste of Tea Factory: Adsorption on a Fixed-Bed Column. Journal of Hazardous Materials, 135, 328-336.
http://dx.doi.org/10.1016/j.jhazmat.2005.11.070 - 51. Malkoc, E. and Nuhoglu, Y. (2007) Potential of Tea Factory Waste for Chromium(VI) Removal from Aqueous Solutions: Thermodynamic and Kinetic Studies. Separation and Purification Technology, 54, 291-298.
http://dx.doi.org/10.1016/j.seppur.2006.09.017 - 52. Malkoc, E. and Nuhoglu, Y. (2005) Investigations of Nickel(II) Removal from Aqueous Solutions Using Tea Factory Waste. Journal of Hazardous Materials, 127, 120-128.
http://dx.doi.org/10.1016/j.jhazmat.2005.06.030 - 53. Çay, S., Uyanik, A. and Özasik, A. (2004) Single and Binary Component Adsorption of Copper(II) and Cadmium(II) from Aqueous Solutions Using Tea-Industry Waste. Separation and Purification Technology, 38, 273-280.
http://dx.doi.org/10.1016/j.seppur.2003.12.003 - 54. Tee, T.W. and Khan, A.R.M. (1988) Removal of Lead Cadmium, Zinc by Waste Tea Leaves. Environmental Technology Letters, 9, 1223-1232.
http://dx.doi.org/10.1080/09593338809384685 - 55. Ho, Y.S. and McKay, G. (1998) Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Safety and Environmental Protection, 76, 183-191.
http://dx.doi.org/10.1205/095758298529326 - 56. Ho, Y.S. and McKay, G. (1999) Pseudo-Second Order Model for Sorption Processes. Process Biochemistry, 34, 451-465.
http://dx.doi.org/10.1016/S0032-9592(98)00112-5 - 57. Hameed, B.H., Mahmoud, D.K. and Ahmad, A.L. (2008) Equilibrium Modeling and Kinetic Studies on the Adsorption of Basic Dye by a Low-Cost Adsorbent: Coconut (Cocos nucifera) Bunch Waste. Journal of Hazardous Materials, 158, 65-72.
http://dx.doi.org/10.1016/j.jhazmat.2008.01.034 - 58. Shahmohammadi-Kalalagh, S.H., Babazadeh, H., Nazemi, A.H. and Manshour, M. (2011) Isotherm and Kinetic Studies on Adsorption of Pb, Zn and Cu by Kaolinite. Caspian Journal of Environmental Sciences, 9, 243-255.
- 59. Boparai, H.K., Joseph, M. and O’Carroll, D.M. (2011) Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nanozerovalent Iron Particles. Journal of Hazardous Materials, 186, 458-465.
http://dx.doi.org/10.1016/j.jhazmat.2010.11.029 - 60. Rocha, C.G., Zaia, D.A.M., Alfaya, R.V.S. and Alfaya, A.A.S. (2009) Use of Rice Straw as Biosorbent for Removal of Cu(II), Zn(II), Cd(II) and Hg(II) Ions in Industrial Effluents. Journal of Hazardous Materials, 166, 383-388.
http://dx.doi.org/10.1016/j.jhazmat.2008.11.074
NOTES
*Corresponding author.