Journal of Water Resource and Protection
Vol.06 No.18(2014), Article ID:52397,12 pages
10.4236/jwarp.2014.618148
A Survey of Experience Gained from the Treatment of Coal Mine Wastewater
Estêvão A. Pondja Jr.1,2, Kenneth M. Persson1, Nelson P. Matsinhe2
1Department of Building and Environmental Technology, Lund University, Lund, Sweden
2Department of Chemical Engineering, Eduardo Mondlane University, Maputo, Mozambique
Email: estevao.pondja@tvrl.lth.se, tindjombo@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 October 2014; revised 5 November 2014; accepted 1 December 2014
ABSTRACT
During coal mining, water resources may be polluted by acid mine drainage (AMD) if appropriate measures are not taken. AMD releases metals to the environment, which can be harmful to aquatic species and reduce biodiversity. There is a great deal of information available in the literature on the generation and treatment of AMD and this paper tries to summarize some of them in order to facilitate the choice of the most appropriate method for AMD treatment at a specific mining site. The objective of this study was to identify and describe different methods of treating polluted water from coal mining, and to discuss the choice of suitable methods at specific mining sites. Both active and passive methods of AMD treatment are discussed in order to provide a general picture of the measures that have been taken around the world by coal mining companies. From this study, we were able to conclude that in order to choose the appropriate method for a specific mining site it is necessary to analyze the chemistry of the acid water and the flow rate from that site. The cost, implementability and effectiveness of the method should also be considered. Minimizing the amount of drainage water generated is naturally the first choice of management strategy and the containment of the AMD is the second choice. The third alternative is the treatment of the wastewater.
Keywords:
Coal Mining, Acid Mine Drainage, Treatment of Mining Water, Passive and Active Treatment

1. Introduction
Coal mining plays an important role worldwide in both the energy and metallurgical industries. Thermal coal for the production of electricity and coking coal for steel production are the main products of the coal mining industry. Coal mining activities also produce solid waste, and air and water pollutants. Acid mine drainage (AMD) is the main environmental problem caused by mining activities.
Acidic leachate can occur naturally, due to the weathering of minerals containing sulfides, leading to the oxidation of elemental sulfur, but the greatest sources of acidic wastewater arise from anthropogenic activities such as mining [1] . Mining accelerates the process of weathering of reactive sulfide by increasing the available surface area of reactive components allowing enormous amounts of material containing sulfides to be exposed to air and water [1] . The most dominant sulfide mineral in many ore deposits is pyrite, and this plays a key role in the generation of AMD [2] . However, other sulfide minerals are also present, and their oxidation also affects mine water chemistry. Pyrite, pyrrhotite, marcasite and mackinaw wite are the most reactive sulfides, and their oxidation results in water with a low pH [2] . Sulfide minerals are formed in the absence of oxygen in ore mineral deposits, i.e., they are formed under reducing conditions and will become unstable when exposed to oxygen, for example, in mining water, and during excavation, mineral processing and other activities that involve the removal of mineral-containing material [1] .
The generation of AMD can be explained by Equations (1)-(5). Pyrite can react directly with oxygen forming an acidic solution Equation (1), and this reaction can take place in the presence or absence of microorganisms. Ferric iron (Fe3+) dissolved in water can oxidize pyrite, Equation (2), and the ferric iron is replenished by the oxidation of ferrous iron in the presence of aerobic bacteria, which catalyze the reaction in Equation (3). Oxidation and hydrolysis of ferrous iron (Fe2+) under slightly acidic to alkaline conditions lead to the formation of an insoluble hydroxide, Equation (4). When reactions (1) and (4) take place at a pH above 4.5, Equation (5) results, and the acidity is doubled compared to reaction 1.
For example:
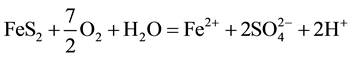 (1)
(1)
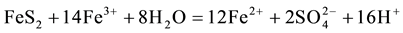 (2)
(2)
 (3)
(3)
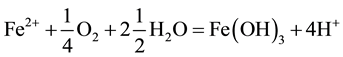 (4)
(4)
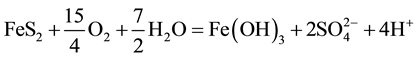 (5)
(5)
1.1. Sources of AMD
The main sources of AMD are ore and coal stockpiles, tailing storage facilities, waste rock piles, leach piles, mine adits, and pit walls, shafts and floors [2] . Rocks containing sulfides are considered to be one of the major sources of AMD, and their management is thus very important [3] . The composition of AMD depends on the mineralogy of the local rocks, and water and oxygen availability, and thus every mine is unique with regard to its potential to generate AMD [3] .
1.2. Acid-Buffering Reactions
Rocks normally contain alkaline materials such as carbonates (calcite and dolomite), silicates and hydroxide, which can neutralize AMD [4] . Silicates constitute the largest reservoir of buffering capacity on Earth, but a wide range of calcites also occur, and these are considered to be the most important neutralizing agent due to their rapid reaction rate compared with silicates [2] . When AMD interacts with alkaline material in the rocks, some of the acidity is neutralized, which means that not all the leachate from waste or stock piles at a mining site will generate an acid solution. To determine whether a certain waste containing sulfide can generate acid water it is necessary to perform static and kinetic tests. Static test determines balance between neutralizing potential and acid potential of mine waste while kinetic test provide information about leachate quality and rate [5] .
1.3. Impact of AMD on the Environment
AMD can have several effects on the environment, the main ones being the release of metals into waterways causing the death of fish and other aquatic species. Fish may also become contaminated by eating contaminated sediment and food, due to the high content of metals in the water [6] . One of the main products of pyrite oxidation is iron hydroxide (Fe(OH)3), which precipitates in streams giving them a red/orange color (Figure 1). It can also cover the surface of sediments and stream beds, contributing to the destruction of habitats [6] .
1.4. Wastewater Generation from Coal Mining
Solid, liquid and gaseous effluents are produced during mining, and mining companies should take measures to minimize or eliminate these effluents in order to achieve sustainable production. The subject of this study is the acid water produced by mining. Wastewater resulting from coal mining can be divided into mine water, process wastewater, domestic wastewater and storm water [7] . Mine water can be defined as the ground or surface water at a mining site [2] . Process water can be divided into liquid effluent and tailings. Wastewater resulting from machinery, the washing of trucks and working areas, and pipe leakage are considered to be liquid effluent. This type of wastewater contains a high level of non-filterable residue Waste resulting from the coal washing process is called slurry tailings and is a potential source of acid water [7] . AMD can be generated when storm water comes into contact with the surface of sulfide-containing minerals (e.g. pits walls) or overburden. Precipitation can seep through waste piles resulting in groundwater contamination. Domestic wastewater arises from offices around the mining area. If fine particles of coal are not removed from employees’ clothing and bodies after mining, they may find their way into domestic wastewater as a result of washing.
1.5 Control of AMD
AMD can be controlled using 3 different techniques: prevention, containment and remediation (treatment).The aim of prevention is to completely avoid the generation of acid water by avoiding contact between sulfide-con- taining minerals and water/oxygen. The common methods used are the isolation of metallic sulfide, oxygen exclusion using wet and dry covers, and alkaline additives [8] . The aim of containment is to avoid flows of AMD to the environment. Some of the methods used are impoundment of AMD, alkaline-permeable barriers, and the disposal of tailings in impermeable cells [8] . The aim of remediation is to increase the pH and reduce the concentrations of pollutants such as metals, solids and salts present in AMD, to avoid contamination of surface water and groundwater [8] . Remediation methods can be divided into active and passive treatment.
Other strategies can be used to reduce the amount of water requiring treatment, such as the construction of upstream dams to intercept and divert surface water, the avoidance of seepage of rain water to contaminated areas, maximization of the reuse or recycling of water, separation of water with different qualities, the avoidance of infiltration of contaminated water into the groundwater, and appropriate management of waste containing sulfides [3] .
2. Methods of Treating Coal Mining Wastewater
In cases where AMD is unavoidable, it is necessary to treat it using an appropriate technique. Treatment technologies can be divided into passive and active treatment, both of which include biological, physical and chemical approaches. Active treatment requires continuous operation with regular addition of reactants and labor, while passive treatment requires only occasional maintenance [9] .
Figure 1. Examples of effects of AMD in South Africa, showing the typical red/orange color due to iron hydroxide (pictures taken by the author).
2.1. Active Treatment
There are many active methods for the treatment of AMD, but the most common are: aeration, neutralization (including chemical precipitation), metal removal, chemical precipitation, membrane filtration, ion-exchange processes and biological sulfate removal [1] .
2.1.1. Aeration
The objective of aeration is to oxidize dissolved Fe2+ as it is one of the main pollutants in AMD. If the wastewater contains more than 50 mg/l Fe2+ then it must be aerated. Aeration increases the level of dissolved oxygen (DO), promoting the oxidation of iron and manganese, which increases the efficiency of chemical treatment and thus reduces costs. During aeration, dissolved carbon dioxide from underground mine water will be released, resulting in an increase in the pH and a reduction in the cost of reagents [1] .
2.1.2. Neutralization
AMD can be neutralized by chemicals such as sodium and calcium hydroxide and their carbonates in order to precipitate metals. Neutralization and precipitation are used quite often due to the feasibility of treating large volumes of contaminated water, the low cost and the simplicity of the process [10] .
Quicklime (CaO) and hydrated lime (Ca(OH)2) are used for the neutralization of AMD due to their abundance and high reactivity. During neutralization metals such as Fe2+, Fe3+, Al, Cu, Zn and Pb are precipitated in the form of metal hydroxides. The sludge resulting from this process is a mixture of metal hydroxides and gypsum (CaSO4). Equation (6) below gives the main neutralization reaction when using hydrated lime [10] .
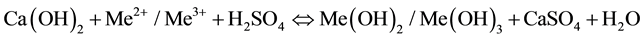 (6)
(6)
Sludge containing Fe3+ is more stable than sludge containing Fe2+, and air is therefore used during neutralization to oxidize Fe2+ to Fe3+. Clarifiers or thickeners are used to settle the sludge produced, and if the solids content is less than 1 mg/l, sand filters can be used to polish the treated water. The solids content of sludge is strongly affected by the concentration of metals in the water and the type of treatment process applied, and can vary from 1% to 30%. The process is optimized by adjusting the process parameters (neutralization rate, oxidation rate, ratio of Fe2+/Fe3+, ion concentration, temperature, sludge age, crystal formation and sludge recycling) in order to obtain a denser sludge, thus reducing the volume [10] .
This process is called high sludge density (HSD) and it is a modification of conventional neutralization process and it aims to produce a higher sludge density [11] . Neutralization reactors are used to oxidize iron from Fe2+ to Fe3+ at certain pH. Treated water from the reactors is flocculated with a polymer, and the solids are separated from the liquid in a thickener or clarifier. The sludge produced in the thickener is routed back to the process [12] . The illustration of HSD process can be seen in Figure 2.
Figure 2. Basic configuration of the HDS [1] .
The configuration illustrated in Figure 2 is the standard commercial HDS process used for the treatment of AMD, and has the following advantages: the low cost of lime and its efficient use, only a small site is required for sludge disposal due to high density of the sludge, the water/solid separation is good, and it is a very robust process, with the ability to treat AMD with different properties (flow, metal loading and acidity) [1] .
Limestone has been used to treat AMD for many years in the coal mining industry because it is the cheapest material available, it is easy to handle, and is the safest chemical for treating AMD. The contaminants of greatest concern are iron and aluminum, and limestone is very effective in neutralizing these. However, the application of limestone is limited because it has a low solubility and has tendency to form an external coating of Fe(OH)3 during the treatment of AMD [10] .
Under certain conditions, HDS can be achieved using limestone to neutralize AMD instead of lime [10] . Limestone reacts with acid water, leading to the dissociation and release of carbon dioxide, as in Equations (7) and (8) below.
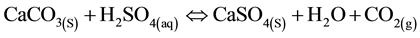 (7)
(7)
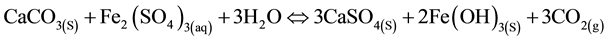 (8)
(8)
The carbon dioxide released forms carbonate ions, which buffer the pH to an upper limit of 6.5. As a consequence of this, some metals cannot be removed as they require a pH above 6.5 for precipitation. To overcome this problem, a combination of limestone and lime can be used, as shown in Figure 3.
This process has three different steps: 1) pre-neutralization with limestone, which is a little cheaper than lime; 2) neutralization with lime in order to reach a certain pH that is determined by the metal to be removed; and 3) adjustment of the pH and re-carbonation using carbon dioxide produced in the limestone neutralization reactor [1] . When choosing the appropriate neutralization agent for the treatment of acid water from a particular mining site, the following parameters must be take into account: the type of material (including transportability, storability and dosing), the hazardous properties of the material, the reliability and availability of suppliers of reactants, the efficiency of neutralization, problems such as coating, clogging and scaling of the equipment, and the cost of the process [10] . Neutralization and hydrolysis are key aspects in the treatment of AMD. Table 1 lists different types of alkalis and materials used to treat AMD [1] .
To determine the amount of alkali required to treat a certain AMD, it is necessary to consider the cost of the alkali, the objective of the treatment (in this case, the removal of metals), and the effects of the residue produced (INAP, 2013). The data given in Table 1 can be used to determine the amount of alkali required to neutralize a certain amount of acid, and to estimate the cost of the alkali required to perform the task. The flowchart shown in Figure 4 and the data in Table 1 can be used to design an appropriate neutralization system for any coal mining wastewater, providing the flow rate and water chemistry of the AMD are known. In order to select the
Figure 3. AMD treatment using a combination of limestone and lime [1] .
Figure 4. Flowchart that can be used to design a site-specific AMD treatment system [13] .
Table 1. Materials and alkali applied for AMD treatment [1] .
*Acidity is expressed as CaCO3; **Market prices in January 2009.
appropriate neutralization agent, it is important to known the concentrations of iron and manganese. Manganese is very soluble in the pH interval 4.5 to 8 making its removal difficult. The best way to remove Mn is by raising the pH to a value above 9 in order to oxidize Mn2+ to Mn3+ or Mn4+, allowing the insoluble manganese carbonate or manganese oxide to be removed [13] .
2.2. Passive Treatment
Various kinds of passive treatment can be used; the most common being aerobic wetlands, anaerobic wetlands, anoxic limestone drains, open limestone drains, and reducing and alkalinity-producing systems [1] . The critical parameters in the design of passive treatment systems for AMD are the flow, the properties of the AMD and land availability (Zipper, et al., 2011).
2.2.1. Aerobic Wetlands
The simplest type of passive treatment is the aerobic wetland, but it cannot be used to treat all types of acid water efficiently. Its capacity to neutralize acidity is limited, but it can be used to treat net alkaline water that has a high content of iron. Mine water is aerated while it flows slowly through the vegetation, and dissolved iron will thus be oxidized, and the oxidation product precipitated. The pH will fall as a result of the precipitation of iron due to the generation of H+ ions, and the treated water will thus have a lower pH than the influent water, despite the fact that the iron concentration is higher in the influent water. Aerobic wetlands can also be used to remove Mn, but the oxidation of Mn only starts when the oxidation of Fe is completed. To remove Mn using aerobic wetlands it is necessary to have large areas to allow the complete oxidation of Fe and thus the oxidation of Mn. Alternatively, another wetland cell can be added. Figure 5 shows a typical aerobic wetland where aquatic plants transport oxygen through the roots to the subsurface to help the oxidation process. Composted organic matter or natural soil can be used as substrate, and water levels between 10 - 30 cm are used to maintain aerobic conditions, and to allow the cattails to grow in order to improve wetland performance [14] .
2.2.2. Anaerobic Wetlands
Anaerobic wetlands are a modification kind of aerobic wetlands, where a bed of limestone and a layer of biodegradable organic matter are added in order to allow the treatment of acid water.
The limestone is located below the substrate to enhance the generation of alkalinity in the form of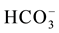 . Under anoxic conditions (low oxygen levels) sulfate can be reduced in the presence of biodegradable organic matter. Sulfate-reducing bacteria use the oxygen in
. Under anoxic conditions (low oxygen levels) sulfate can be reduced in the presence of biodegradable organic matter. Sulfate-reducing bacteria use the oxygen in 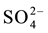 that enters the system under anoxic conditions to reduce sulfate to H2S gas or to a solid sulfide by the biodegradation of organic matter in a metabolic process [14] .
that enters the system under anoxic conditions to reduce sulfate to H2S gas or to a solid sulfide by the biodegradation of organic matter in a metabolic process [14] .
This process is illustrated by Equation (9).

If metals (M) are present in the solution, the reduction process leads to metal sulfides, as can be seen from Equation (10). These metal sulfides are deposited in the substrate.

Alkalinity can also be generated by the reaction between acid water and the limestone below the substrate, as in Equation (11).
Figure 5. Cross section of aerobic wetland [14] .

These three equations illustrate the production of bicarbonate ions, which are the source of alkalinity, and they can raise the pH by the neutralization of H+ (Equation (12)), thus contributing to the precipitation of soluble metals present in acid water.

Figure 6 illustrates anaerobic wetlands also known as composted wetlands and the curved arrows in Figure 6 indicate diffusion of water.
2.2.3. Anoxic Limestone Drains
Anoxic limestone drains (ALDs) (Figure 7) is an engineered method where limestone is used to intercept AMD in anoxic conditions. Limestone in contact with AMD dissolves and generates alkalinity. To avoid contact between oxygen and AMD, limestone is crushed and buried with compacted soil or clay and the effluent water is led to a settling pond where the pH is adjusted to bring about the precipitation of metals [15] . When ALDs are working properly, they are more cost-effective than wetlands, but they cannot be used to treat AMD with significant amounts of Fe3+, Al and DO, due to clogging resulting from the precipitation of metal hydroxides when the pH reaches or exceeds 4.5. To avoid clogging, the influent concentrations of Fe3+, Al, and dissolved oxygen must all be below 1 mg/l. Under anoxic condition armoring by iron hydroxide cannot take place because Fe2+ cannot precipitate as Fe(OH)2 at a pH below 6 [14] .
2.2.4. Vertical Flow Systems
Most of AMD can be treated by the passive methods described above, but if mine water contains DO, Fe3+ and Al in great quantity, successive alkalinity-producing systems (SAPS) or vertical flow system which is a combination of ALDs and anaerobic wetlands with the objective of compensating for the limitations of each method can be used [16] . When AMD enters the system it flows vertically downwards through the organic layer where dissolved oxygen is removed by aerobic bacteria using biodegradable organic matter as their energy source, while other bacteria generate alkalinity by reducing sulfate to sulfide (Figure 8). The organic matter layer must
Figure 6. Cross section of an anaerobic wetland [14].
Figure 7. Cross section of an ALD system [14] .
Figure 8. Cross section of a vertical flow system [14] .
be able to reduce the level of dissolved oxygen to less than 1 mg/l to avoid limestone armoring and to allow the reduction of sulfate. The limestone layer allows the dissolution of CaCO3 by acid water, and under anoxic conditions more alkalinity will be produced. Finally, the water is discharged to a settling pond where the acid is neutralized and the metals precipitated. When the influent AMD contains a significant amount of Fe3+ and sediments, pretreatment in an aerobic wetland or a settling pond is necessary to avoid the accumulation of solids. When the influent is highly acidic, it is necessary to divide the system into several vertical flows that can be separated by different settling ponds [14] .
The flowchart in Figure 9 can be of use when choosing the appropriate kind of passive treatment of AMD. As with active methods, it is important to know the flow rate and the chemical composition of the AMD. Samples should be collected from tailing seepage or mine discharge, and the levels of Fe, Mn, alkalinity, pH and acidity measured (Hedin et al., 1994). The composition of AMD can change considerable with the seasons, and it is thus important to collect and analyze samples at different times of the year [17] .
3. Summary of Treatment Technologies
Summaries of passive and active treatments are presented in Table 2 and Table 3, including the advantages and disadvantages of each method. These tables can be used to help decide which active or passive treatment is most suitable for a certain AMD.
To ensure successful treatment of the particular acid water, parameters such as acidity, flow rate, dissolved oxygen and pH should be analyzed. Table 4 can be used to decide which method can be used successfully in a particular case.
According to Skousen & Ziemkiewicz [20] , it is necessary to take into account flow rate, water chemistry, topography and the characteristics of the area in order to select and design a suitable passive treatment system. Table 5 lists some aspects that should be considered during the selection and design of a passive treatment system for AMD.
Comparing Table 4 and Table 5 it can be seen that they give almost similar information. Combining these tables provides very useful information for the selection and design of a suitable passive treatment method.
4. Selection of Remedial Technique
To select the appropriate remedial technique it is necessary to perform a feasibility study in each specific case. This process starts with the specification of the problem (e.g. chemicals, risks, etc.), followed by the identification of potential techniques and, finally, evaluation of the feasibility of the selected techniques. According to [21] , the steps in evaluating the feasibility are, first: Effectiveness―“the potential for the alternative to achieve remedial goals established for the site”, second: Implementability―“the ability to comply with technical and administrative issues and constraints involved in implementing a technique at a specific site and third: Cost― “typically an estimate of net present cost for each technique”. In practice, mining companies first identify the techniques that can meet their water quality goals (effectiveness), then they eliminate those that cannot be applied for practical reasons (implementability), and finally, the least expensive method is implemented (cost) [23] .
To select a suitable technique to treat a certain AMD, it is necessary to analyze the AMD and asses the available options based on the information given in Figure 4 and Figure 9. Table 4 and Table 5 can also be used for construction and successfully treatment. Figure 10 summarizes what should be done to choose appropriate technique to treat a site specific AMD.
Figure 9. Flowchart to aid the selection of passive treatment method for AMD [18] .
Table 2. Summary of passive methods of treating AMD.
Figure 10. Method employed for the selection of the best technique for the treatment of AMD in specific cases.
Table 3. Summary of active methods of treating AMD.
Table 4. Influent characteristics of AMD required for successful treatment [9] .
Table 5. Influent AMD characteristics and design factors for successful passive treatment of AMD [20] .
5. Discussion and Conclusions
The generation of AMD and its treatment are complex issues requiring careful analysis in each individual case. Prevention and containment of AMD are the best management strategies, as treatment is often costly. However, if prevention and containment are not possible, treatment must be applied to avoid contamination of the water resources surrounding mining site. The most appropriate technique for the treatment of AMD is site-specific, as it depends on the flow rate and chemistry of the acid water. Costs, implementability and effectiveness must also be taken into consideration.
Many active and passive methods of treating AMD have been discussed to provide a general picture of the strategies applied by coal mining companies. Only the basic methods were described, whereas in reality there are many variations in use around the world. In general, active treatment methods are suitable in cases where no land is available and in cases where it is necessary to control the process. These methods can be used to remove pollutants from AMD efficiently, but the investment, maintenance and operating costs are high. Passive methods, on the other hand, are in general suitable for mines no longer in operation as they need less maintenance and operate naturally. However, they require large areas of land and long retention time to operate efficiently.
Methods of active treatment have several advantages, for example high removal efficiency, large volumes of AMD with different characteristics can be treated, the systems can be controlled automatically, and they occupy a relatively small area. However, they are associated with high costs and they generate sludge. The advantages of passive treatment systems are: low costs, they last for many years, and they do not require any power, but large areas and long retention times are required for them to operate efficiently.
In conclusion, active methods are more suitable for operating mines, while passive methods are suitable for closed mines.
Acknowledgements
First, I thank God for giving me strength, health and wisdom to write this paper. I also would like to express my gratitude to my supervisors, Lund University and Eduardo Mondlane University for all support that they gave me.
References
- INAP (2013) The Global Acid Rock Drainage Guide. International Network for Acid Prevention (INAP), 2013.
- Lottermoser, B.G. (2010) Mine Wates: Characterization, Treatment and Environmental Impacts. 3rd Edition Edition, Queensland: Springer, 2010.
- Akcil, A. and Koldas, S. (2005) Acid Mine Drainage: Causes, Treatment and Case Studies. Cleaner Production, 2005.
- Skousen, J., Geidel, G., Foreman, R., Evans, R. and Hellier, W. (1998) A Handbook of Technologies for Avoidance and Remediation of Acid Mine Drainage. National Mine Land reclamation Center, Virginia.
- MEND (2008) Acid Rock Drainage Prediction Manual. Electronic Revision Edition, Mend, Pacoima.
- Jennings, S., Neuman, D. and Blicker, P. (2008) Acid Mine Drainage and Effects on Fish Health and Ecology: A Review. Reclamation Research Group, Alaska.
- Dharmappa, H., Wingrove, K., Sivakumar, M. and Singh, R. (1999) Wastewater and Storwater Minimisation in a Coal Mine. Journal of Cleaner Production, 8, 24-34.
- EPA (2008) Coal Mining Detailed Study. United States Environmental Protection Agency, Washington DC.
- Taylor, J., Pape, S. and Murphy, N. (2005) A Summary of Passive and Active Treatment Technology for Acid and Metalliferous Drainage. Earth Systems, Fremantle.
- Kuyucak, N. (2006) Selecting Suitable Methods for Treating Mining Effluent. Golder Association.
- Maree, J.P., Strydom, W.F., Adlem, C.J.L., de Beer, M., van Tonder, G.J. and van Dijk, B.J. (2004) Neutralization of Acid Mine Water and Sludge Disposal. CSIR.
- DWA (2013) Feasibility Study for a Long Term Solution to Address the Acid Mine Drainage Associated with the East, Central and West Rand Underground Mining Basins. Department of Water Affairs (DWA), Pretoria.
- Trumm, D. (2010) Selection of Active and Passive Treatment System for AMD―Flow Chart for New Zealand Conditions. Journal of Geology and Geophysics, 53, 195-210.
- Zipper, C., Skousen, J. and Jage, C. (2011) Passive Treatment of Acid Mine Drainage. Virginia Tech, Blacksburg.
- Watzlaf, G.R., Schroeder, K.T. and Kairies, C.L. (2000) Long Term Performance of Anoxic Limestone Drain. Mine Water and the Environment, 19, 98-110.
- Ordóñez, A., Loredo, J. and Pendás, F. (2012) A Successive Alkalinity Producing System (SAPS) as Operational Unit in a Hybrid Passive Treatment System for Acid Mine Drainage. Mine Water and Environment, 575-580.
- Hedin, R., Narin, R. and Kleinmann, R. (1994) Passive Treatment of Coal mine Drainage. Bureau of Mines.
- Ford, K. (2003) Passive Treatment Systems for Acid Mine Drainage. Technical Note 409. Bureau of Land Management, Colorado.
- Magdziorz, A. and Sewerynsky, J. (2000) The Use of Membrane Technique in Mineralize Water Treatment for Drinking and Domestic Purposes at Pakoj Coal Mine District Under Liquidation. Central Mining Institute, Department of Water Protection.
- Skousen, J. and Ziemkiewicz, P. (2005) Performance of 116 Passive Treatment Systems for Acid Mine Drainage. Proceedings of the 2005 National Meeting of the American Society of Mining and Reclamation, Breckenridge, 19-23 June 2005, 1103.
- EPA (2006) Management and Treatment of Water from Hard Rock Mine. United States Environmental Protection Agency, Washington DC.
- Kirby, C., Dennis, A. and Kahler, A. (2009) Aeration to Degas CO2, Increase pH and Increase Iron Oxidation Rates for Efficient Treatment of Alkaline Mine Drainage. Applied Geochemistry, 24, 1175-1184.
- Geldenhuys, A., Maree, J., Beer, M. and Hlabela, P. (2003) An Integrated Limestone/Lime Process for Partial Sulphate Removal. The Journal of the South African Institute of Mining and Metallurgy, 345-354.













