Journal of Biomedical Science and Engineering
Vol.09 No.10(2016), Article ID:70684,18 pages
10.4236/jbise.2016.910042
Quantification of the Erythropoietic Response to Anemia and Determination of the Hb × log(Epo) Component as an Independent Parameter, Indicating the Response to Hypoxic Stress
Argiris Symeonidis1, Alexandra Kouraklis-Symeonidis1, Vassiliki Labropoulou1, Vasileios Lazaris1, Paraskevi Katsaouni1, Evgenia Verigou1, Trifon Spiridonidis2, Evangelia Tzouvara1, Maria Tiniakou1, Dimitris Apostolopoulos2, Michalis Leotsinidis3
1Department of Internal Medicine, Hematology Division, University of Patras Medical School, Patras, Greece
2Laboratory of Nuclear Medicine, Hematology Division, University of Patras Medical School, Patras, Greece
3Department of Biostatistics and Public Health, Hematology Division, University of Patras Medical School, Patras, Greece

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: May 21, 2016; Accepted: September 17, 2016; Published: September 20, 2016
ABSTRACT
Serum erythropoietin (Epo) levels are influenced by the integrity of renal function, the severity and etiology of anemia, the percentage of hemoglobin F and other factors. We aimed to create a parametric expression for the response to anemic/hypoxic stress and we evaluated serum Epo levels in 1096 subjects with normal renal function, of whom 837 were anemic (Hb < 12 g/dl), 204 were non anemic (Hb 12 - 16.5 g/dl), and 55 were erythremic (Hb > 16.5 g/dl). Hb ranged between 3.6 and 23.8 g/dl and the corresponding Epo between <3.4 and 2670 mIU/ml. Between log-Epo and Hb the correlation coefficient r was −0.706. The function linking Hb and log-Epo was determined as log(Epo) = 3.05 - 0.131 × Hb. Investigating various mathematic models, which could parametrically express the “response to anemia/hypoxia”, we found that the Hb × log(Epo) product was a stable parameter and fitted a normal distribution. Arbitrarily defining as normal range about one SD between the mean (12 - 21) we found that only 224 patients (20.8%) exhibited a Response to Anemia Index (RAI) beyond these limits. Below the lower limit there were 106 patients, diagnosed with polycythemia vera (12/38, 31.6%) anemia of chronic disease (10/34, 29.4%), megaloblastic anemia (17/56, 30.4%) and β-thalassemia trait (11/41, 26.8%). Forty-eight patients (45.3%) were diabetic. Above the defined upper normal RAI limit there were 118 patients, mainly diagnosed with secondary erythrocytosis (24/34, 70.6%), aplastic anemia (8/20, 40%), hemolytic anemia (3/14, 21.4%) and myelodysplastic syndromes (46/326, 14.1%). RAI was a rather constant parameter for each individual, with minimal variation in different evaluations, when Epo was estimated in the absence of inflammatory conditions. We have validated the superiority of RAI for the prediction of response to Epo in a cohort of 669 patients, who received Epo treatment. It is concluded that RAI is a reliable parameter describing the response to hypoxic/anemic stress.
Keywords:
Erythropoietin, Hemoglobin, Anemia, Hypoxia, Response to Anemia

1. Introduction
Under normal renal function, for any hemoglobin (Hb) value, there is a short range of corresponding circulating serum erythropoietin (Epo) levels, and these two parameters are inversely associated [1] . Serum Epo levels reflect the individual’s response to hypoxic/anemic stress. This response is determined by various factors, such as the adequacy of renal and liver function [2] , the etiology, severity and probably the chronicity of the anemia, the presence and percentage of HbF [3] [4] , the circulation of inflammatory cytokines antagonizing erythropoietin [5] , the percentage of bone marrow red blood cell precursors, the metabolic rate of Epo, the presence of diabetes mellitus [6] [7] , and plasma viscosity. There are conflicting data regarding the effect of age on serum Epo levels [8] [9] . Therefore, “response to anemia” is created by a substantial number of contributing factors, but among them, the most representative parameter is clearly serum Epo. Serum Epo levels, in the absence of inflammation, renal and hepatic failure, are mainly determined by the etiology and the severity of the anemia, i.e. by the corresponding Hb levels.
In many publications, a “blunted” erythropoietin response to anemia in various diseases and conditions has been described [5] - [19] . This characterization is relied on a lower than expected serum Epo level; however a defined “normal” range of serum Epo values for any corresponding Hb level has not been established, and as a result, there is no definition or measure of a suboptimal response, which is defined rather arbitrarily. In some instances, other indeces, such as the Hb × Epo component, or the slope of the regression curve, which associates Hb and log-Epo (Hb/log-Epo) have been used as markers of a suboptimal response to anemia [20] . We have previously reported an inappropriately low erythropoietin response for the degree of anemia in a large cohort of anemic diabetic patients and a lower slope of the regression curve in diabetic patients, as compared to non-diabetics [7] .
Estimating the strength of the response to anemia and quantifying this parameter might be of particular interest, since it would represent the effective or ineffective activation of the compensatory mechanisms, aiming to correct anemia, and restore normal hemoglobin. It might also determine the indication for possible supportive exogenous administration of recombinant erythropoietin, and could, in some degree predict the response to treatment with it. For the last option, different cut-off values of serum Epo levels (200 or 500 mIU/ml) have been proposed as predictors of a favorable response in some diseases, such as the myelodysplastic syndromes [21] . Particularly for this group of diseases, erythropoietin response to anemic stress appears to predict more than simply the response to treatment with rh-Epo [22] .
Aim of the Study
We aimed to quantify and parametrically express the response to hypoxic/anemic stress, based on the serum Epo levels, corresponding to a specific Hb value, obtained at the same time-point, in adults with normal renal and hepatic function, and in the absence of any active inflammatory condition.
2. Patients and Methods
To determine the strength of the reaction to anemic/hypoxic stress we measured serum Epo levels, totally in 1096 adult subjects, 433 female and 663 male, with normal renal and hepatic function, as defined by a serum creatinine ≤1.5 mg/dl, or by a creatinine clearance ≥60 ml/min, and with normal liver function tests. Of these subjects, 1060 had a well-defined diagnosis of a systemic disease, possibly associated with an abnormal hemoglobin value and 36 were asymptomatic non-anemic healthy controls. Among the 1060 patients, 837 (79.0%) had anemia (Hb < 12 g/dl) of variable severity and etiology, 204 (19.2%) were not anemic (Hb 12 - 16.5 g/dl) and 55 (5.2%) had erythrocytosis (Hb > 16.5 g/dl). One hundred and forty-five patients (13.7%) had severe anemia (Hb < 8 g/dl), 425 patients (40.1%) had moderate anemia (Hb 8 - 9.9 g/dl), and the remaining 277 patients (26.1%) had mild anemia (Hb 10 - 11.9 g/dl). Epidemiological data, as well as the precise hematological diagnosis and the etiology of the anemia, of all the subjects entered this study, are demonstrated in Table 1.
All subjects were tested at the same time-point for hemoglobin, serum creatinine, liver function tests and serum Epo. Estimation of the serum Epo levels was part of the routine work up for 326 patients with myelodysplastic syndromes, 115 with hemoglobinopathies, 76 patients with anemia of chronic disease or other causes of unexplained anemia and for 72 patients with polycythemia/erythrocytosis. Serum Epo levels were also measured in the majority of patients with multiple myeloma and lymphoproliferative disorders, before the administration of rh-Epo, as supportive treatment for chemotherapy-induced symptomatic anemia. For 348 patients, measurement of serum Epo was not part of their routine work up. These patients were tested during evaluation for anemia, were not selected by any criterion and have provided informed consent to participate in this combined, prospective and retrospective analysis. The study plan has been approved by the Ethical and Scientific Committee of the University Hospital of Patras. Hemoglobin measurements were performed on a CELL-DYN 3700 (Abbott) automatic hematologic analyzer. In all patients with hematological malignancies of myeloid origin, serum Epo measurement was performed at baseline, before the administration of any cytotoxic treatment. Patients with lymphoproliferative disorders had previously received various types of cytotoxic chemotherapy and were evaluated for post-chemotherapy symptomatic anemia.
Table 1. Epidemiological data of the population tested.
Serum Epo levels were measured by an immunoradiometric assay (IRMA) from blood samples obtained in the morning, during routine work-up and after obtaining written informed consent. Samples were kept at −20˚C until the time of the analysis. Measurements were performed between 2003 and 2009 on a weekly basis, according to the scheduled program of the laboratory of Nuclear Medicine of the University Hospital. The commercial kit EPO-Trac® (DiaSorin, Italy) was used, and the lower and upper detection limits of the assay were 3.4 and 280 - 290 mU/ml, respectively. When a sample was measured above the upper normal limit, one or two additional measurements were performed, with a dilution of the initial sample 1:5 or 1:10 or more, when necessary, using the standard 0 solution of the kit. Two control samples at different EPO levels were available in each RIA kit. The study has been approved by the Ethical and Scientific Committee of the University Hospital of Patras.
We then tried to find the most appropriate mathematical model, which would better express the “ability” of a subject to respond to anemia. Since the relationship between hemoglobin and erythropoietin is logarithmic, we investigated various mathematical models, relating Hb and Epo, which would create a strong linear regression. In particular, we investigated the relationship between log-Hb and log-Epo, Hb and log-Epo, as well as that of the products Hb × Epo, (Hb)2 × Epo, Hb × log-Epo, and log-Hb × log-Epo with Hb. For each regression the correlation coefficient r or the Spearman’s r when appropriate, was calculated and for the strongest regression, the function which linked the two variables was determined.
We found that only one of the applied mathematical models, the Hb × log-Epo component, was not influenced by hemoglobin levels and maintained reliability and reproducibility. We have therefore, named this mathematical model “Response to Anemia Index” (RAI) and we have tested and validated it as a measure of the strength of an individual’s response to anemia. We checked the upper and the lower part of the RAI distribution, representing patient populations, who demonstrated a blunted erythropoietin response or an over-response to anemia. We then analyzed these two populations, in terms of type of underlying disease and etiology of the anemia. Moreover, we re-evaluated this index at later time-points in 127 patients, who maintained normal renal and liver function and did not exhibit findings of an active infection or any other inflammatory condition, to investigate its variability in the same patient.
Statistical analysis was performed by using the SPSS V.19 statistical software package (SPSS Inc. USA). The distribution of the data was tested by one sample Kolmogorov- Smirnov test. Comparisons between groups were performed by using Student’s t-test or Mann-Whitney U test. Spearman’s rank correlation coefficient was estimated to assess the association between different variables. The effect of Hb on log-Epo was tested by employing linear regression analysis. The statistically significant level was set at p = 0.05.
3. Results
3.1. Hemoglobin and Serum Erythropoietin Levels
Hemoglobin values ranged between 3.7 and 23.8 g/dl, with a median of 9.9 g/dl. The distribution of Hb values of the whole patient population was slightly skewed (Kolmogorov-Smirnov z = 3.98, p < 0.05). The corresponding Epo values ranged between 3.4 and 2670 mIU/ml, with a median of 41.4 mIU/ml and again, as expected, the distribution of Epo values was not normal. According to our laboratory, the range of Epo levels, which is found among normal subjects, is 6 - 29 mIU/ml. Data regarding hemoglobin and the corresponding serum Epo levels, in relation to the underlying disease and etiology of anemia are demonstrated in Table 2. Analysis and discussion of the results in the different etiological groups of anemia will not be performed, because this analysis is beyond the aim of the present study.
Table 2. Hemoglobin, serum erythropoietin and RAI levels, according to hematological diagnosis.
#Excluding diabetic patients from normal subjects.
Between Hb and Epo, Spearman’s r was −0.753 (p < 0.001) and the regression was semilogarithmic. As expected, the linear regression between Hb and log-Epo was strong (r = −0.706), and in fact this was the strongest correlation between Hb and all the other mathematical expressions of serum Epo. The regression between Hb and log-Epo is presented in Figure 1. The function which linked Hb (in gr/dl) and log-Epo was determined by the following equation: 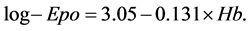
3.2. Determination of the Response to Anemia Index
We then investigated different mathematical formulations, which would parametrically express an individual’s response to anemia. The Hb × Epo component has been used in the literature, as an index of erythropoietin response to hypoxia/anemia. In our population this component ranged between 45.2 and 18156, with a median of 425 and exhibited a skewed distribution of values. Between Hb and the Hb × Epo component there was a significant negative correlation (Spearman’s r = −0.611, p < 0.001), therefore, this index is clearly influenced by the severity of anemia, and cannot be considered an independent variable. Since the regression between Hb and Epo is semilogarithmic we also tested the (Hb)2 × Epo component. This index ranged between 545 and 123461, with a median of 4404 and also showed a skewed distribution of values. Again, there was a significant negative correlation between this component and Hb, (Spearman’s r = −0.434, p < 0.01), suggesting that this index is also influenced by the severity of anemia.
The log-Hb × log-Epo component ranged between 0.384 and 2.853, with a normal distribution of the values, but exhibited a significant negative correlation with Hb (r =
Figure 1. Regression between hemoglobin and log-Epo values. The regression is linear. Response to Anemia Index is the Hb × log-Epo product and represents the area under the regression curve which is formed by the perpendicular lines, connecting the values of the x and y intercept with the regression line. Anemic over-responders were mainly patients with aplastic anemia, myelodysplastic syndromes, and β-thalassemia major with high HbF (area A). Erythremic over- responders had exclusively secondary erythrocytosis (area B). Anemic sub-responders had predominantly anemia of chronic diseases, megaloblastic and β-thalassemia trait (area C).
−0.542, p < 0.001), and therefore, it was influenced by the severity of anemia also, and could not be used as an index of the response to anemia.
The Hb × log-Epo component ranged between 6.63 and 38.74 and exhibited a normal distribution of its values, with a mean of 16.58, a median of 16.45 and a standard deviation (SD) of 3.78 (Figure 2). At a distance of one SD between the mean, i.e. roughly between 12.75 and 19.25, 66.1% of the total number of values (724/1096) were located, a percentage very close to the expected 68%, whereas at a distance of 2 SD between the mean value, (i.e. between 9 and 23) as many as 93.8% of the values (1028/1096) could be identified. The Hb × log-Epo component is equal to the area under the linear segment of the regression curve, which is formed by the perpendicular lines, connecting the values of the x and y intercept with the regression line (Figure 1). This area is the mathematical model of the RAI, and it is obvious that is not affected by the severity of the anemia, since the square area under the curve is roughly equal at any Hb level, when an effective erythropoietin response is achieved. The correlation coefficient r between Hb × log-Epo product and Hb was very low (r = 0.038) and this regression is shown in Figure 3. Among all the other mathematical models tested, only the Hb × log-Epo component was not significantly correlated with Hb, and therefore, appeared not to be influenced by the severity of anemia. This component was therefore nominated Response to Anemia Index (RAI).
3.3. Fluctuation of RAI among the Normal Population
The RAI values of the 54 normal subjects ranged between 10.62 and 19.74 with a normal distribution. As expected [7] , diabetic patients had a significantly lower mean RAI value, compared to non-diabetics (13.66 ± 2.19 versus 16.07 ± 2.06, p < 0.001, Table 2). Considering only the 36 non-diabetic subjects as normal (control) population, we
Figure 2. Histogram of obtained RAI values in the whole population tested. The distribution of the values is normal.
Figure 3. Regression between hemoglobin and the Hb × log-Epo component (RAI). The correlation coefficient r is not significant and tends to 0, suggesting that RAI is a variable independent of the severity of anemia and reflects other individual parameters of response to hypoxic stress.
found that RAI values ranged between 13.04 and 19.74 among them. We therefore, defined arbitrarily, as lower and upper normal limits of RAI, the values of 12 and 21, which roughly stand at a distance of about 1.25 SD from the mean. At this range all normal subjects and 836 out of 1060 subjects with an abnormal Hb value or a known underlying disease or condition (78.9%) were allocated.
Comparing RAI between male and female patients among the non-anemic population of the 54 patients, we did not find any significant difference (15.5 ± 2.4 versus 15.2 ± 2.3, respectively, p = 0.404), and the same was true concerning serum Epo levels (12.3 ± 4.5 versus 15.1 ± 6.4 mIU/ml, p = 0.149), although male patients exhibited significantly higher Hb levels (14.8 ± 1.3 versus 13.3 ± 0.9 g/dl, p = 0.001). The same was true when a comparison between the two genders was performed among the 36 control non-diabetic subjects (16.6 ± 1.7 versus 15.9 ± 2.0, respectively, p = 0.346). However, in the whole patient population tested, male patients had significantly higher RAI, compared to females (17.1 ± 3.7 versus 15.8 ± 3.7 respectively, p < 0.001). Significant difference was also noted in hemoglobin levels (males: 10.7 ± 3.0 versus females: 10.3 ± 2.6, p = 0.018), whereas a borderline significance was obtained in serum Epo levels (median value 44.6 mIU/ml for males, versus 39 mIU/ml for females, Mann-Whitney U test: p = 0.066). This mild discrepancy might be attributed to the imbalance in the etiology of anemia observed among male and female patients.
3.4. Recognition of the Sub-Responders and Over-Responders to Hypoxic/Anemic Stress
We found only 224 subjects (20.4% of the total population) with a RAI outside an arbitrarily defined normal range, between the values 12 and 21. In particular, 106 subjects (9.7%) exhibited a lower than expected erythropoietin response to anemia (RAI ≤ 12) and the remaining 118 subjects (10.8%) demonstrated an over-response (RAI ≥ 21). Analyzing the etiology of the anemia or the underlying diseases and conditions of these two groups, we found that, a sub-response to anemic/hypoxic stress was most frequently encountered among patients with polycythemia vera, anemia of chronic disease, megaloblastic anemia [23] , β-thalassemia trait, as well as in a small proportion of patients with essential thrombocythemia, iron deficiency and hemolytic anemia. Conversely, an over-response was observed in the majority of patients with secondary ery- throcytosis (70.6%), as well as in patients with aplastic [24] and hemolytic anemia, myelodysplastic syndromes and among patients with β-thalassemia intermedia, and sickle-cell/β-thalassemia, exhibiting high proportion of HbF [25] [26] . The whole list of sub-responders and over-responders to anemic/hypoxic stress is shown in Table 3. It is noteworthy that 48 out of 95 sub-responders with known glycemic status were diabetics (50.5%), as were only 6 out of 102 over-responders (5.8%), a statistically very significant difference (p < 0.001). It is also notable that all 5 normal subjects, who exhibited a sub-response to anemia, were diabetics. Moreover, there were subgroups of patients with iron-deficiency anemia, multiple myeloma, other lymphoproliferative disorders, myelodysplastic syndromes, myeloproliferative diseases, who exhibited either an over-response or a normal response or a sub-response to anemia. Such variations may
Table 3. Sub-response and over-response to anemia, according to RAI.
be attributed to other factors and particularly to older age [9] [27] [28] or higher inflammatory status. Conversely, patients with polycythemia vera, anemia of chronic disorders, megaloblastic anemia and β-thalassemia trait, never exhibited an over-re- sponse, whereas patients with secondary erythrocytosis, aplastic anemia, β-thalassemia intermedia and sickle-cell/β-thalassemia, never exhibited a sub-response.
Analyzing patient distribution in the regression between hemoglobin and log of serum Epo (Figure 1), we were capable to distinguish specific patient subgroups, in the different areas of the distribution. In area A, anemic over-responders were mainly patients with aplastic anemia, myelodysplastic syndromes, β-thalassemia major and sickle- cell/β-thalassemia. In area B, erythremic over-responders with secondary erythrocytosis and some normal subjects, mainly heavy smokers were distributed. Finally, in area C anemic sub-responders were mainly patients with anemia of chronic disease, megaloblastic anemia, β-thalassemia trait and some with hemolytic anemia.
3.5. Variation of the RAI in the Same Subject
To evaluate the fluctuations of the RAI in the same subject we performed repeated serum Epo estimations in 142 subjects, 3 months to 2 years following the initial estimation, and compared the results of the second measurements, with those at baseline. Fifteen patients were excluded from the comparative paired analysis, because of deterioration of renal function and increase of creatinine >1.5 mg/dl (7 cases), evolution of the initial hematological disease to a different one (4 cases) and presence of an active inflammatory condition during the second estimation (4 cases). The second Hb estimation varied between −37.9% to +114%, as compared to the initial one. The variation was <10% in 61 cases (48%) and >20% in 31 (24.4%). Serum Epo also exhibited significant variation. It was >25% different in 96 cases (75.6%), >50% different in 68 cases (53.5%) and >100% different in 30 cases (23.6%). The median percentage of serum Epo variation was 23.1% and the range −98% to +1033%. Log-Epo varied a median of 7.3%, with a range of variation from −53.2% to 74.1%. In 51 patients (40.1%) log-Epo varied ≤10%, whereas in 39 patients (30.7%) this index varied >20%.
RAI showed minimal variation, less than any other of the indices tested. Comparing the two consecutive estimations, RAI varied between −17.1% and 37.4%, with a median percentage of variation 3.5%. The variation was ≤10% in 104 cases (81.9%) and >20% only in 5 cases (3.9%). This finding implies that in every individual, there is a relatively uniform responsiveness to anemic/hypoxic stress, which may of course be related to the etiology of anemia, but mainly reflects some individual features of the subject’s and the underlying disease’s background. Figure 4 depicts the variation of log-Epo and that of RAI, in each patient comparatively.
3.6. Usefulness of RAI in Everyday Clinical Practice
3.6.1. Differential Diagnosis of Iron-Deficiency Anemia versus Anemia of Inflammation
Although RAI should not routinely be used for diagnostic purposes, it may be help- ful in the differential diagnosis of some diseases or conditions. In our population,
Figure 4. Variation of the log-Epo and RAI values in 2 sequential estimations in the same individual, in a cohort of 127 subjects, tested at least twice. RAI showed ≤10% variation in 82% of the tested population, implying that in every individual there was a relatively uniform responsiveness to anemic/hypoxic stress, which is not related to the severity of anemia.
excluding diabetic patients, only 3 out of 34 patients with iron deficiency anemia had a RAI value < 15 (8.8%) as did 21/34 patients with anemia of chronic diseases (61.8%, p < 0.001). On the other hand, only 2/34 patients with anemia of chronic disease had a RAI value > 17 (5.9%), as did 18/34 non-diabetic patients with iron deficiency anemia (52.9%, p < 0.001). This overlapping is substantially smaller to that observed, when comparing serum Epo levels in these two groups of anemic patients [29] . Therefore, in the absence of renal failure and in otherwise unclear cases of hypochromic and microcytic anemia, the detection of a RAI value < 15 may not support the diagnosis of iron deficiency anemia, whereas a RAI value > 17 may discourage the diagnosis of anemia of chronic disease.
3.6.2. Differentiation of Polycythemia Vera versus Secondary Erythrocytosis
Although the differential diagnosis of the two conditions is not usually difficult, RAI could contribute to the establishment of the correct diagnosis. Comparing RAI values between the 38 patients with polycythemia vera (Group I) with those of the 34 patients with secondary erythrocytosis (Group II), who were included in this study, we did not find any crossing over values (range of RAI: Group I: 6.63 - 16.87, Group II: 18.55 - 38.74) although there was 11.7% overlapping in serum Epo values between the 2 groups (range of serum Epo values: Group I: <3.4 - 13.4 mIU/ml, Group II: 12.2 - 131 mIU/ml, 4/34 values overlapped). Therefore, patients with a RAI value < 18 might probably have polycythemia vera, whereas those with a RAI value > 18 have rather secondary erythrocytosis.
3.6.3. Prediction of Response to Treatment with rhEpo in Patients with MDS
It is well-known that one parameter affecting response to treatment with recombinant human Epo (rhEpo) in patients with MDS is endogenous serum Epo. In almost all the predictive models, serum Epo levels are taken into consideration and have a recognized predictive power. However, since serum Epo levels are mainly determined by the hypoxic stress, i.e. by the corresponding Hb value, the use of crude Epo levels has not major prognostic value. This is understandable, since a value for instance of 250 mIU/ml, might be considered as an over-response for a patient with a corresponding Hb of 10 g/dl, an acceptable response for a patient with a corresponding Hb of 8 g/dl and probably a sub-response for a patient with a corresponding Hb of 6 g/dl. The proposed limit of 500 mIU/ml, as a negative prognostic factor for response to Epo is exceeded by less than 10% of the patients, and therefore, this model cannot practically take into consideration serum Epo levels. The limit of 200 mIU/ml has not obtained strong predictive significance, since a substantial proportion of severely anemic patients may respond to rhEpo, even if they exhibit endogenous serum Epo above this limit. We therefore, have tested the significance of RAI as a predictive tool to treatment with rhEpo. Among 326 patients with various types of MDS included in this study, 268 were treated with rhEpo, but 232 who completed at least 10 weeks of treatment were evaluable for response. According to the revised International Working Group criteria for response, 112 patients (48.3%) exhibited a favorable response, and among them 76 (32.7%) achieved a complete response (CR) and 36 (15.5%) a partial response (PR). Only 15 patients (6.5%) had serum Epo > 500 mIU/ml, two of whom responded to treatment with rhEpo, and they both, had a RAI value lower than 20. One hundred and forty-five patients had serum Epo < 200 mIU/ml and the remaining 87 had a value higher than 200 mIU/ml. Overall response rate was 55.9% for patients with a serum Epo < 200 mIU/ml, and 34.4% for those with serum Epo > 200 mIU/ml. Likewise, 172 patients had a RAI value < 21 and the remaining 60 a value higher than 21. Overall response rate was 62.2% for patients with a RAI value < 21 and only 8.3% (5/60 patients) for those with a RAI less than 21. Thus, by using the serum Epo cut-off value of 200 mIU/ml, we were capable to predict the favorable response in 81 of the 112 responders (72.3%), whereas by using the RAI cut-off value of 21 we were capable to predict the favorable response in 107/112 patients (95.5%, p < 0.001). These data are presented on Table 4. We have validated the predictive value of RAI, over crude serum Epo levels in an independent group of 437 patients with MDS, treated with Epo and evaluable for response. Results have been presented in the 11th International Symposium of MDS, [30] but further analysis of them is beyond the aims of the present study.
4. Discussion
At any time point, serum Epo levels reflect the response to hypoxic/anemic stress. Even in the absence of clinically detectable hypoxia or anemia, there is a baseline erythropoietin response, aiming to maintain the homeostasis of red blood cell production [31] . The most common cause of impaired Epo production is overt or occult renal failure. In our analysis we have only included patients with normal renal function, as defined by normal serum creatinine levels of ≤1.5 mg/dl and/or by a creatinine clearance of ≥60
Table 4. Comparison of RAI and of serum Epo levels to predict response to treatment with rhEpo in patients with MDS.
ml/min. We thus, have excluded all patients with a possible impairment of Epo production. We have also excluded all patients with impaired liver function, defined as those exhibiting any abnormal liver function test, unless attributed to their basic underlying disease, although it is known that the liver contributes minimally to the Epo production.
The term “response to anemia” has been widely used to describe the compensatory mechanisms, mainly in the form of reactive increase of serum erythropoietin levels, aiming to correct anemia and restore normal hemoglobin levels. There is no acceptable measure to estimate response to anemia, although quantifying the strength of this parameter in an anemic patient would be of particular interest, for both, diagnostic and therapeutic purposes. Serum Epo levels per se, could not be considered such a parameter, because they may vary widely in relation to many factors, sometimes not easily defined. A parametric expression of the response to anemia has not been proposed up to now, and should be useful in evaluating a blunted response, or an over-response to anemic/hypoxic stress. In the literature, the definition of a blunted response to anemia is usually given to anemic patients, who exhibit lower than expected serum Epo levels, compared to a model group of patients, usually with iron-deficiency anemia of comparable severity [28] .
A parametric expression of a “Response to Anemia Index” should take into consideration not only serum Epo levels, but also the corresponding hemoglobin level, with which serum Epo concentration is associated. Moreover, since these two variables are inversely and semilogarithmically related, such a parameter should include at least one of these variables in the logarithmic form. Finally, considering that the response to anemia expresses the maximum capability of an individual to correct his/her anemia, this capability should not be dependent on the severity of anemia. Therefore, any parametric expression, describing a Response to Anemia Index should be independent of the hemoglobin level. Evaluating several mathematical models, based on hemoglobin and serum erythropoietin levels, we found that only one parameter was really independent of the severity of anemia, and this was the Hb × log-Epo component. Of interest, Artunc and Rinsler, analyzing a population of 167 patients without renal failure have found an equation, linking Hb and log-Epo, similar to ours [32] . In some publications the slope of the regression curve between Hb and log-Epo has been used as a measure of the response to anemia, but no quantitative expression for this slope has ever been proposed [33] . We actually propose the same principle, as a measure of the response to anemic stress, but we were capable to create a parametric expression of this response, by using the Hb × log-Epo component.
An impressive and unpredictable finding was that, in the absence of renal failure, or of an active inflammatory process, which are well-known factors reducing circulating serum Epo levels, this component is a rather constant parameter for an individual, exhibiting low degree of variation in consecutive estimations. This finding implies that, in a specified disease group or nosologic background, RAI might ideally characterize an individual’s response to hypoxic stress at any time-point, and for any level of hemoglobin, and therefore, the normal range of RAI could be defined, and might be different among the different etiologic groups of anemia or other underlying diseases and conditions.
Applying this model in a substantial patient population, and arbitrarily defining the normal range, we were able to distinguish patients exhibiting a sub-response, and those showing an over-response to anemia. Both groups of patients represent already-known populations, characterized by this kind of response to hypoxic stress. For instance the two thalassemic patients, exhibiting a low erythropoietin response were transfusion- dependent, whereas 9/10 thalassemic over-responders had thalassemia intermedia or they were not regularly transfused [25] (Table 3). Therefore, these groups of patients have confirmed the descriptive accuracy of the RAI.
An inappropriately low erythropoietin response for the degree of anemia has been previously reported by us and by others, among patients with type II diabetes mellitus [6] [7] . In our current study, the exclusion of 16 diabetic patients from the control group resulted in a substantial increase of the mean RAI of the remaining non-diabetic controls, and hence in the disappearance of significant difference of the mean RAI, between the controls and many groups of anemic patients (Table 2). Moreover, comparing the mean RAI among different anemic etiologic groups, we were able to parametrically describe patient subgroups, demonstrating an inappropriately low erythropoietin response or an over-response to anemia. Thus, more than half of the patients with secondary erythrocytosis were over-responders, suggesting that erythropoietin contributed to the pathogenesis of their condition, even though in many of them serum Epo levels were found in the normal range (Table 2 and Table 3). Finally, by using this parameter as a predictive model for the response to treatment with rh-Epo among patients with myelodysplastic syndromes, we found that RAI could better predict the responders than crude serum Epo levels, as this has been proposed by the Scandinavian MDS Group [21] , and therefore, the use of RAI might be more clinically relevant.
5. Conclusion
In conclusion, we were able to define the Hb × log-Epo component as the only reliable and independent of the severity of anemia mathematical model expressing the “Response to Anemia Index” (RAI). This index can be used for the evaluation of the strength of the response to anemia/hypoxia in all etiological groups, also including non-anemic or erythremic patients.
Cite this paper
Symeonidis, A., Kou- raklis-Symeonidis, A., Labropoulou, V., Lazaris, V., Katsaouni, P., Verigou, E., Spiridonidis, T., Tzouvara, E., Tiniakou, M., Apostolopoulos, D. and Leotsinidis, M. (2016) Quan- tification of the Erythropoietic Response to Anemia and Determination of the Hb × log(Epo) Component as an Independent Pa- rameter, Indicating the Response to Hypoxic Stress. J. Biomedical Science and Engineering, 9, 460-477. http://dx.doi.org/10.4236/jbise.2016.910042
References
- 1. de Klerk, G., Rosengarten, P.C., Vet, R.J. and Goudsmit, R. (1981) Serum Erythropoietin (EST) Titers in Anemia. Blood, 58, 1164-1170.
- 2. de Klerk, G., Wilmink, J.M., Rosengarten, P.C., Vet, R.J. and Goudsmit, R. (1982) Serum Erythropoietin (ESF) Titers in Anemia of Chronic Renal Failure. Journal of Laboratory and Clinical Medicine, 100, 720-734.
- 3. Camaschella, C., Gonella, S., Calabrese, R., Vischia, F., Roetto, A., Graziadei, G., et al. (1996) Serum Erythropoietin and Circulating Transferrin Receptor in Thalassemia Intermedia Patients with Heterogeneous Genotypes. Haematologica, 81, 397-403.
- 4. Galanello, R., Barella, S., Turco, P.M., Giagu, N., Cao, A., Dore, F., et al. (1994) Serum Erythropoietin and Erythropoiesis in High- and Low-Fetal Hemoglobin β-Thalassemia Intermedia Patients. Blood, 83, 561-565.
- 5. Kreuzer, K.A., Rockstroh, J.K., Jelkmann, W., Theisen, A., Spengler, U. and Sauerbruch, T. (1997) Inadequate Erythropoietin Response to Anaemia in HIV Patients: Relationship to Serum Levels of Tumour Necrosis Factor-Alpha, Interleu-kin-6 and Their Soluble Receptors. British Journal of Haematology, 96, 235-239.
http://dx.doi.org/10.1046/j.1365-2141.1997.d01-2031.x - 6. Yun, Y.S., Lee, H.C., Yoo, N.C., Song, Y.D., Lim, S.K., Kim, K.R., et al. (1999) Reduced Erythropoietin Responsiveness to Anemia in Diabetic Patients before Advanced Diabetic Nephropathy. Diabetes Research and Clinical Practice, 46, 223-229.
http://dx.doi.org/10.1016/S0168-8227(99)00097-2 - 7. Symeonidis, A., Kouraklis-Symeonidis, A., Psiroyiannis, A., Leotsinindis, M., Kyriazopoulou, V., Vassilakos, P., et al. (2005) Inappropriately Low Erythropoietin Response for the Degree of Anemia in Patients with Non Insulin-Dependent Diabetes Mellitus. Annals of Hematology, 85, 79-85.
http://dx.doi.org/10.1007/s00277-005-1102-9 - 8. Musso, C.G., Musso, C.A., Joseph, H., De Miguel, R., Rendo, P., Gonzalez, E., et al. (2004) Plasma Erythropoietin Levels in the Oldest Old. International Urology and Nephrology, 36, 259-262.
http://dx.doi.org/10.1023/B:UROL.0000034682.61762.ad - 9. Matsuo, T., Kario, K., Kodoma, K. and Asada, R. (1995) An Inappropriate Erythropoietic Response to Iron Deficiency Anaemia in the Elderly. Clinical and Laboratory Haematology, 17, 317-321.
- 10. Baer, A.N., Dessypris, E.N., Goldwasser, E. and Krantz, S.B. (1987) Blunted Erythropoietin Response to Anaemia in Rheumatoid Arthritis. British Journal of Haematology, 66, 559-564.
http://dx.doi.org/10.1111/j.1365-2141.1987.tb01344.x - 11. Yamashita, H., Kukita, J., Ohga, S., Nakayama, H., Akazawa, K. and Ueda, K. (1994) Serum Erythropoietin Levels in Term and Preterm Infants during the First Year of Life. American Journal of Pediatric Hematology/Oncology, 16, 213-218.
http://dx.doi.org/10.1097/00043426-199408000-00005 - 12. Siciliano, M., Tomasello, D., Milani, A., Ricerca, B.M., Storti, S. and Rossi, L. (1995) Reduced Serum Levels of Immunoreactive Erythropoietin in Patients with Cirrhosis and Chronic Anemia. Hepatology, 22, 1132-1135.
http://dx.doi.org/10.1002/hep.1840220418 - 13. Ebrahim, O., Folb, P.I., Robson, S.C. and Jacobs, P. (1995) Blunted Erythropoietin Response to Anaemia in Tuberculosis. European Journal of Haematology, 55, 251-254.
http://dx.doi.org/10.1111/j.1600-0609.1995.tb00267.x - 14. Corazza, F., Beguin, Y., Bergmann, P., Andre, M., Ferster, A., Devalck, C., et al. (1998) Anemia in Children with Cancer Is Associated with Decreased Erythropoietic Activity and Not with Inadequate Erythro-poietin Production. Blood, 92, 1793-1798.
- 15. Chen, J.S., Lin, K.H., Wang, S.T., Tsao, C.J. and Yeh, T.F. (1998) Blunted Serum Eryth-ropoietin Response to Anemia in Patients Polytransfused for Beta-Thalassemia Major. Journal of Pediatric Hematology/Oncology, 20, 140-144.
http://dx.doi.org/10.1097/00043426-199803000-00010 - 16. Lee, S.J., Kwon, J.H. and Jung, C.W. (2001) Erythropoietin Response Is Inadequate in Cancer Patients Receiving Chemotherapy. International Journal of Hematology, 74, 416-420.
http://dx.doi.org/10.1007/BF02982085 - 17. Bergamaschi, G., Markopoulos, K., Albertini, R., Di Sabatino, A., Biagi, F., Ciccocioppo, R., Arbustini, E. and Corazza, G.R. (2008) Anemia of Chronic Disease and Defective Erythropoietin Production in Patients with Celiac Disease. Haematologica, 93, 1785-1791.
http://dx.doi.org/10.3324/haematol.13255 - 18. Gasché, C., Reinisch, W., Lochs, H., Parsaei, B., Bakos, S., Wyatt, J., Fueger, G.F. and Gangl, A. (1994) Anemia in Crohn’s Disease. Importance of Inadequate Erythropoietin Production and Iron Deficiency. Digestive Diseases and Sciences, 39, 1930-1934.
http://dx.doi.org/10.1007/BF02088127 - 19. Van Vlerken, L.G., Van Soest, H., Janssen, M.P., Boland, G.J., Drenth, J.P., Burger, D.M., Siersema, P.D. and Van Erpecum, K.J. (2010) Suboptimal Endogenous Erythropoietin Response in Chronic Hepatitis C Patients during Ribavirin and PEG Interferon Treatment. European Journal of Gastroenterology & Hepatology, 22, 1308-1315.
http://dx.doi.org/10.1097/MEG.0b013e32833e784d - 20. Inomata, S., Itoh, M., Imai, H. and Sato, T. (1997) Serum Levels of Eryth-ropoietin as a Novel Marker Reflecting the Severity of Diabetic Nephropathy. Nephron, 75, 426-430.
http://dx.doi.org/10.1159/000189580 - 21. Hellstrom-Lindberg, E., Gulbrandsen, N., Lindberg, G., Ahlgren, T., Dahl, I.M., Dybedal, I., et al., Scandinavian MDS Group (2003) A Validated Decision Model for Treating the Anaemia of Myelodysplastic Syndromes with Erythropoietin + Granulocyte Colony-Stimulating Factor: Significant Effects on Quality of Life. British Journal of Haematology, 120, 1037-1046.
http://dx.doi.org/10.1046/j.1365-2141.2003.04153.x - 22. Wallvik, J., Stenke, L., Bernell, P., Nordahl, G., Hippe, E. and Hast, R. (2002) Serum Erythropoietin (EPO) Levels Correlate with Survival and Independently Predict Response to EPO Treatment in Patients with Myelodysplastic Syndromes. European Journal of Haematology, 68, 180-185.
http://dx.doi.org/10.1034/j.1600-0609.2002.01530.x - 23. Remacha, A.F., Bellido, M., García-Die, F., Marco, N., Ubeda, J. and Gim-ferrer, E. (1997) Serum Erythropoietin and Erythroid Activity in Vitamin B12 Deficiency. Haematologica, 82, 67-68.
- 24. Schrezenmeier, H., Noé, G., Raghavachar, A., Rich, I.N., Heimpel, H. and Kubanek, B. (1994) Serum Erythropoietin and Serum Trans-Ferrin Receptor Levels in Aplastic Anaemia. British Journal of Haematology, 88, 286-294.
http://dx.doi.org/10.1111/j.1365-2141.1994.tb05020.x - 25. Chaisiripoomkere, W., Jootar, S., Chanjarunee, S. and Ungkanont, A. (1999) Serum Erythropoietin Levels in Thalassemia Major and Intermedia. Southeast Asian Journal of Tropical Medicine and Public Health, 30, 786-788.
- 26. Papassotiriou, I., Voskaridou, E., Stamoulakatou, A. and Loukopoulos, D. (2000) Increased Erythropoietin Level Induced by Hydroxyurea Treatment of Sickle Cell Patients. The Hematology Journal, 1, 295-300.
http://dx.doi.org/10.1038/sj.thj.6200049 - 27. Nafziger, J., Pailla, K., Luciani, L., Andreux, J.P., Saint-Jean, O. and Casadevall, N. (1993) Decreased Erythropoietin Responsiveness to Iron Deficiency Anemia in the Elderly. American Journal of Hematology, 43, 172-176.
http://dx.doi.org/10.1002/ajh.2830430303 - 28. Joosten, E., Van Hove, L., Lesaffre, E., Goossens, W., Dereymaeker, L., Van Goethem, G. and Pelemans, W. (1993) Serum Erythropoietin Levels in Elderly in Patients with Anemia of Chronic Disorders and Iron Deficiency Anemia. Journal of the American Geriatrics Society, 41, 1301-1304.
http://dx.doi.org/10.1111/j.1532-5415.1993.tb06479.x - 29. Kendall, R., Wasti, A., Harvey, A., Hill, J., Chapman, C., Norfolk, D.R. and Pullar, T. (1993) The Relationship of Haemoglobin to Serum Erythropoietin Concentrations in the Anemia of Rheumatoid Arthritis: The Effect of Oral Prednisolone. British Journal of Rheumatology, 32, 204-208.
http://dx.doi.org/10.1093/rheumatology/32.3.204 - 30. Symeonidis, A., Zikos, P., Galanopoulos, A., Kotsianidis, I., Kouraklis, A., Terpos, E., Protopapa, M., Papadaki, H., Lampropoulou, V., Aktypi, A., Bakarakos, P., Michalopoulou, S., Anastasiadis, A., Michalis, E. and Zoumbos, N. (2011) 67 Response to ESA Treatment in Patients with MDS: Determination of a Predictive Score from a Retrospective Analysis of 669 Patients. Leukemia Research, 35, S25.
http://dx.doi.org/10.1016/s0145-2126(11)70069-8 - 31. Rondon, I.J., Mac-Millan, L.A., Beckman, B.S., Goldberg, M.A., Schneider, T., Bunn, H.F., et al. (1991) Hypoxia Up-Regulates the Activity of a Novel Erythropoietin mRNA Binding Protein. The Journal of Biological Chemistry, 266, 16594-16598.
- 32. Artunc, F. and Risler, T. (2007) Serum Erythropoietin Concentrations and Responses to Anaemia in Patients with or without Chronic Kidney Disease. Nephrology Dialysis Transplantation, 22, 2900-2908.
http://dx.doi.org/10.1093/ndt/gfm316 - 33. El Hassan, A.M., Saeed, A.M., Fandrey, J. and Jelkmann, W. (1997) Decreased Erythropoietin Response in Plasmodium Falciparum Malaria-Associated Anaemia. European Journal of Haematology, 59, 299-304.
http://dx.doi.org/10.1111/j.1600-0609.1997.tb01690.x






