Journal of Cancer Therapy
Vol.2 No.4(2011), Article ID:7784,10 pages DOI:10.4236/jct.2011.24060
Quality of Life in Men Treated for Early Prostate Cancer: A Prospective Patient Preference Cohort Study
![]()
1Department of Clinical Oncology and Surgery, The Christie NHS Foundation Trust, Manchester, UK;
2Department of Urology, Salford Royal Hospitals NHS Foundation Trust, Salford, UK;
3Department of Urology, Stockport NHS Foundation Trust, Stockport, UK;
4Department of Medical Statistics, The Christie NHS Foundation Trust, Manchester, UK;
5Department of Urology, East Lancashire Hospitals NHS Trust, Blackburn, UK;
6Department of Urology, Wrightington, Wigan and Leigh NHS Trust, Wigan, UK;
7Department of Urology, Mid Cheshire Hospitals NHS Trust, Cheshire, UK;
8Department of Urology, University Hospital South Manchester NHS Foundation Trust, Manchester, UK.
Email: carmelanandadas@yahoo.co.uk
Received June 30th, 2011; revised July 25th, 2011; accepted August 20th, 2011.
Keywords: Early Prostate Cancer, Quality of Life, Patient Preference, Prostatectomy, Hypo-Fractionated Radiotherapy, Brachytherapy
ABSTRACT
Objectives: In 1997, a study was launched to investigate the treatment of early prostate cancer. Using a patient preference design, health-related quality-of-life (HRQOL) and disease specific HRQOL was assessed prospectively to compare men undergoing radical prostatectomy (RP), hypo-fractionated conformal radiotherapy (CRT) or brachytherapy (BT). Methods: Patients with localised prostate cancer were counselled by a urological surgeon, clinical oncologist and specialist uro-oncology nurse. Patients received treatment according to individual preference. 430 men chose and received RP (n = 217), CRT (n = 161) and BT (n = 52). 354 (82%) completed pre-treatment RAND 36-Item Short-Form Health survey version-2 (SF36v2) and University of California, Los Angeles Prostate cancer index (UCLA-PCI) questionnaires. HRQOL score changes from baseline to 24 months were compared using Kruskall-Wallis test. Results: Pretreatment, the CRT cohort scored lower for physical function (p = 0.0029) and general health perception (p = 0.0021). The BT cohort reported better baseline scores for urinary function (p = 0.0291), urinary bother (p = 0.0030), sexual function (p = 0.0009) and bowel bother (p = 0.0063). At 24 months, bowel function was similar for CRT and BT but both modalities were worse than RP (p = 0.0010). Urinary continence deteriorated most following RP (p < 0.0001) but BT had worse urinary bother (p = 0.0153). Sexual function deteriorated most following RP and BT (p < 0.0005). Percentages of patients achieving erections adequate for sexual activity (from baseline to 24 months) were 66% to 29% for RP, 62% to 49% for CRT and 88% to 65% for BT. Conclusions: This data demonstrates significant differences in disease specific quality-of-life between RP, CRT and BT and should be available for men with early prostate cancer making treatment decisions.
1. Introduction
Men undergoing treatment for early prostate cancer are faced with a decision about which therapeutic option to choose. This is due to the increasing number of available treatments, each with differing side effects. Patients make their decisions following advice from their specialist, which may be biased towards the treatment they offer [1].
PSA testing and screening programmes have changed the profile of prostate cancer, increasing the proportion of patients with early disease at low risk of becoming symptomatic. In this group of patients, with long life expectancy, treatment-related side effects (urinary, bowel and sexual) are of major concern as they can seriously affect quality of life over years. Therefore quality of life after primary definitive treatment is an important outcome as many would contemplate reduction in life expectancy for treatments with fewer side effects [2,3].
The reliability of health-related quality of life (HRQOL) information in localised prostate cancer is compromised because few randomised controlled trials (RCTs) have compared treatments. Many have been attempted but abandoned due to poor accrual [4]. The only RCT comparing radical prostatectomy to watchful waiting reported a modest survival advantage with surgery [5] and HRQOL data showed important differences in urinary and sexual dysfunction between treatment groups. However, treatment choice had little effect on general HRQOL and no pre-treatment data was available [6].
RCTs are currently underway studying screened populations such as the UK based Protect study, which is evaluating survival and HRQOL after radical prostatectomy, external beam radiotherapy and active surveillance. Accrual was improved using qualitative methods [7] and important data will be provided by these studies in the future.
In view of the limited data currently available from RCTs, non-randomised prospective studies can give important information. Several studies have compared HRQOL after different treatments but unfortunately, most do not include pre-treatment data [8-13] and of those that do, pre-treatment data usually does not directly compare all 3 major treatment options [14-16]. A couple of recent non-randomised cohort studies that compared all three treatments with pre-treatment data have not differentiated patients receiving hormone therapy as part of their treatment [17-19]. Patients receiving hormones for their prostate cancer were excluded from our study to remove the confounding variable of hormone treatment on HRQOL. This was done because for the majority of patients in this early stage group, hormone therapy would not be necessary at the time of treatment decision and hormone treatment has its own side effect profile. This study has used a hypofractionated conformal radiotherapy schedule for the CRT option. In recent years hypofractionated schedules for prostate radiotherapy are becoming more popular as increasing evidence from experimental studies show that prostate cancers have a higher sensitivity to fraction size reflected in a low alpha/beta ratio. This centre has used hypofractionated schedule as standard treatment since 1993 and therefore these patients are a unique cohort in this type of study.
After a pilot study where acceptance to randomisation proved problematic, our investigators embarked upon a prospective audit of outcome in men with early prostate cancer. Treatment choice was based on the individual patient’s preference following full counselling by a urologist, clinical oncologist and uro-oncology nurse practitioner. The rationale for this study design was to minimise investigator-related selection bias and produce a more balanced population by comparison with other longitudinal studies. The aims of this report were to assess the impact of treatment side effects for each of the three main treatments for early prostate cancer: radical prostatectomy (RP), conformal hypofractionated radiotherapy (CRT) and brachytherapy (BT), by measurement of generic and disease-specific HRQOL. Importantly, this study evaluated HRQOL in a non-screened population of men typical of uro-oncology centres in the UK and elsewhere.
2. Materials and Methods
2.1. Patients
Patients were recruited prospectively with full ethical committee approval between 1st Dec 1997 and 1st April 2004 from 7 Urological Cancer centres based in the North West of England. Inclusion criteria included:
1) Biopsy confirmed prostate adenocarcinoma
2) Gleason score ≤ 7;
3) Stage T1/T2;
4) PSA ≤ 20;
5) Patient suitable for radical prostatectomy or radiotherapy;
6) No previous malignancy (except non-melanomatous skin cancers);
7) No previous treatments for prostate cancer (except TURP);
8) No previous treatment with hormone manipulation.
All eligible men recruited were counselled by a clinical oncologist and urological surgeon and received information leaflets about treatment options. Participants had a concluding discussion with a trained specialist nurse and were invited to choose their treatment after a period of reflection.
2.2 Treatment Options
2.2.1. Radical Prostatectomy
Surgery consisted of radical retro-pubic prostatectomy in all cases with nerve-sparing where appropriate and/or possible. No adjuvant treatment was given.
2.2.2. Conformal Radiotherapy—Hypo-Fractionated
All patients choosing conformal radiotherapy received photon beams to the prostate with a standard technique in use in this centre since 1993. Patients were treated supine, without formal immobilisation, with an empty bladder. They were treated with a linear accelerator equipped with a multileaf collimator delivered with 8 - 20 MV x-rays with a four-field technique (opposed anterior and posterior and opposed lateral portals). The planning target volume (PTV) was defined as the clinical target volume (CTV) with the addition of a 1 cm margin anteriorly and laterally and 0.7 cm posteriorly. The tumour stage, Gleason score and PSA of each patient determined inclusion of the seminal vesicles with the CTV as per department protocol. A dose of 50 Gy in 16 fractions over 3 weeks was given to the isocentre without neo/adjuvant hormone manipulation.
2.2.3. Brachytherapy
Brachytherapy using transrectal ultrasound guided permanent seed implant became a treatment option in 2000 in our centre. Additional inclusion criteria were
1) prostate volume ≤ 60 ml and
2) International Prostate Symptom Score (IPSS) score < 16 and
3) No previous TURP.
If these criteria were not met, brachytherapy was not offered. After ultrasound assessment of prostate volume, brachytherapy was performed by transrectal ultrasound guided permanent I-125 seed implant. The dose was 145 Gy prescribed to the peripheral margin of the prostate. Patients did not receive supplementary external beam radiotherapy or neo-adjuvant hormone manipulation/downsizing.
2.3. HRQOL Questionnaires
Self-assessment questionnaires were posted to each patient on four occasions. Pre-treatment measurement preceded the patients’ decision on therapy. Post-treatment assessments occurred at 3, 12 and 24 months. Non-respondents were sent a second reminder questionnaire.
Validated questionnaires measured general and disease specific HRQOL. General HRQOL was measured with the RAND 36-Item Short-Form Health survey version 2 (SF36v2) [20]. This contained 36 questions to assess eight aspects of HRQOL. The University of California, Los Angeles Prostate cancer index (UCLA-PCI) measured disease specific HRQOL [21]. The questions assessed bowel, urinary and sexual function and bother. Each question’s score was linearly translated to a score from 0 to 100, and a median score was obtained for each [21].
Stage, PSA and Gleason grade were recorded prospectively. PSA progression was defined by the 1997 American Society for Therapeutic Radiation Oncology criteria for CRT and brachytherapy patients and by the presence of a serum PSA > 0.2 ng/ml for post-radical prostatectomy patients. Data for patients with biochemical or clinical failure were excluded from the point of PSA progression as this was thought to influence HRQOL. In addition, patients receiving hormone manipulation during the follow up period were excluded from the study for the same reason.
2.4. Statistics
Parametric data (e.g. Age and PSA) was analysed using ANOVA followed by Tukey’s multiple comparison test. Non-parametric data (e.g. comparisons between the three treatment groups for HRQOL, baseline stage and Gleason score) were performed using the Kruskal-Wallis test (KW) unless otherwise stated. The Mann-Whitney U-test (MW) was used for inter-group comparison (if the KW test was significant) with a Bonferroni multiple comparison adjustment to preserve the overall significance level. Chi square tests were performed on all proportional data (e.g. response rates and comorbidity). A non-responder analysis was performed to assess baseline differences from those that responded in each treatment group and here T-test were used for age and PSA whereas Mann-Whitney test were used for stage and Gleason grade. Analysis was not adjusted for baseline differences as the aim was to find relationships between treatment choice and baseline differences.
HRQOL scores at 3, 12 and 24 months were compared with pre-treatment quality of life scores to calculate therapy-specific changes in each of the HRQOL domains. Again Kruskall-Wallis test was used to determine treatment effect on the change in HRQOL scores between each time point and pre-treatment assessments.
The 5% significance level was used in all primary tests. Statistical analyses were performed using SPSS version 11.
2.5. Funding
AstraZeneca contributed towards data management for the initial 6 months.
3. Results
3.1. Study Population
Between 1st December 1997 and 1st April 2004, 490 men registered in the quality of life aspect of the study. Patients who received a different treatment to their original choice were excluded from analysis (n = 38) as this may have affected how they perceived the therapy to work and therefore confound the quality of life results related to the treatment. Despite patients being referred to this study if they were seeking active treatment, 22 men chose active monitoring/watchful waiting and therefore these men too were excluded from analysis. The remaining 430 patients chose and received either RP (n = 217), CRT (n = 161) or Brachytherapy (n = 52). Pre-treatment questionnaire responses were obtained from 354 patients (82.3%), 178 (RP), 129 (CRT) and 47 (BT). There was no significant difference in response rates between treatments: RP (82%), CRT (80%) and BT (90%) (p = 0.2381). Patients were counselled by experts in the radical treatment of localised prostate cancer. These comprised of 10 different urological surgeons, 3 clinical oncologists and 14 specialist nurses.
There was no significant difference in baseline characteristics between patients who responded to pre-treatment questionnaires and non-responders. Table 1 summarises the baseline patient characteristics. There was a significant difference in age between the treatment groups (KWp < 0.0005). Inter-group analysis (Bonferroni significance level a = 0.017) showed that compared to RP and BT groups, CRT patients were significantly older. (Between CRT and RP MWp = 0.0002, between CRT and BT MWp < 0.0005). There was no difference in age between the RP and BT patients (MWp = 0.2201).
3.2. Compliance
Table 2 illustrates the compliance to the questionnaires (i.e. the percentage of patients responding to the questionnaire of the patients given follow up questionnaires) and shows the number of eligible questionnaires at each time point, taking into account patients who died and those who had recurrent disease. Brachytherapy patients had better compliance than the other two groups. However, the questionnaire subsection that fell below 85% compliance was the sexual function and bother questions for patients treated with brachytherapy.
3.3. Quality of Life Results
3.3.1. Generic HRQOL at Baseline
There were important baseline differences between treatment groups. Patients undergoing CRT had worse scores than other treatments for physical function (KWp = 0.0029) and general health perception (KWp = 0.0021). They also scored significantly worse than BT patients (but not RP patients) for role-limitation physical (KWp = 0.0352) and bodily pain (KWp = 0.0056). Emotional well-being scores were worse in RP patients compared to the brachytherapy group (KWp = 0.0186) but were the same as those in the CRT group.
3.3.2. Generic HRQOL over Time
For each subscale of SF-36, the median change between the score at each time point and baseline was calculated for the patient groups. No significant changes in SF-36 measures were observed between treatment groups at each time point (data not shown).
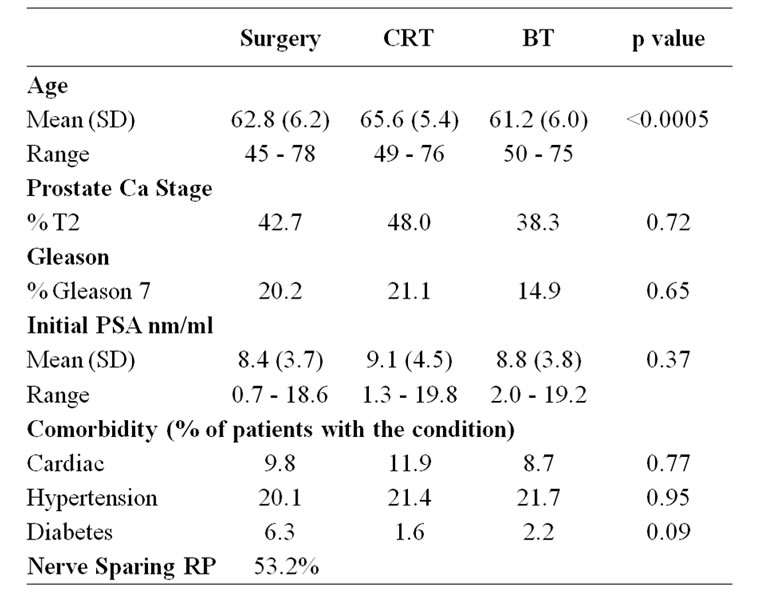
Table 1. Baseline characteristics of study population.
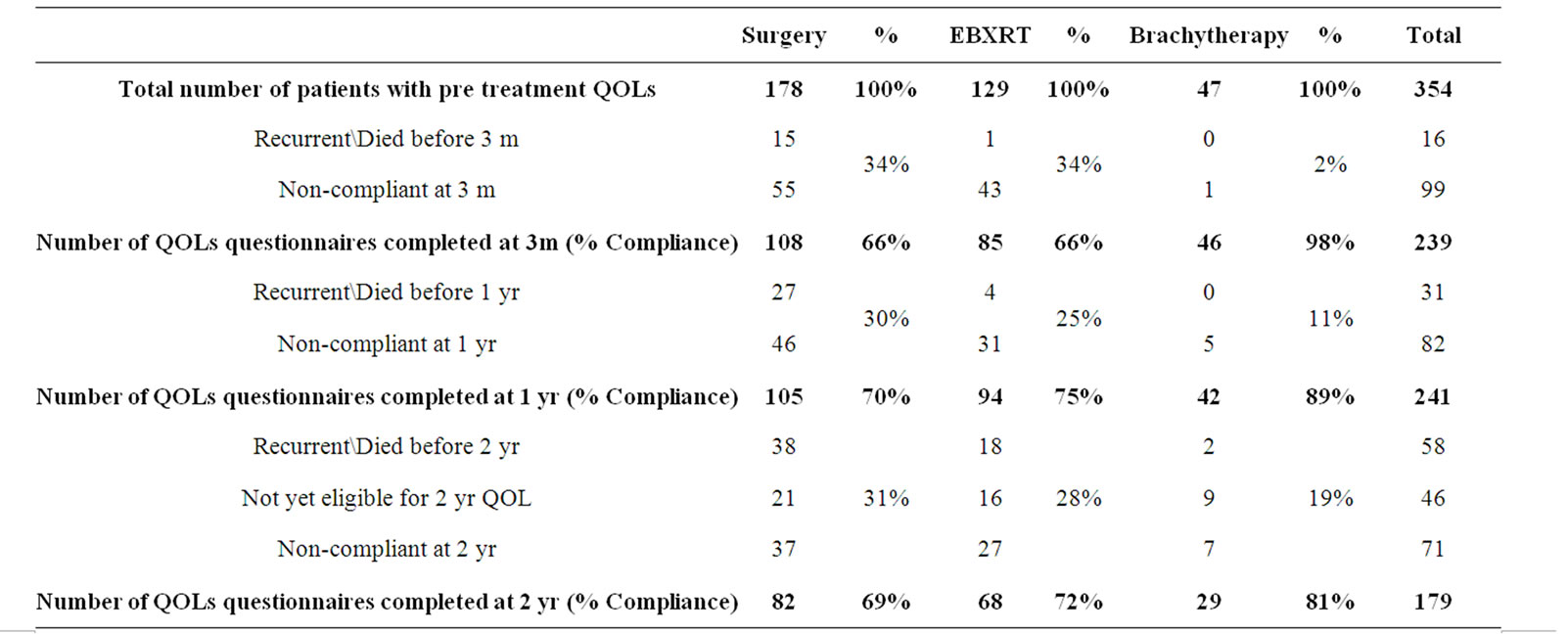
Table 2. Compliance of study population over follow up period.
3.3.3. Disease Specific HRQOL Scores at Baseline
Pre-treatment evaluation revealed that the BT cohort reported significantly better scores than other treatment alternatives for four out of six domains of the UCLA-PCI, including urinary function (KWp = 0.0291), urinary bother (KWp = 0.0030), sexual function (KWp = 0.0009) and bowel bother (KWp = 0.0063). For bowel function and sexual bother there were no differences between treatment options and similarly, there were no differences in any of the subscales between surgical and CRT groups.
3.3.4. Disease Specific HRQOL Scores over Time
Results of repeated measures over time for function items are shown in Figure 1" target="_self"> Figure 1. The decline in urinary function after prostatectomy improved over the follow-up period but did not return to pre-treatment levels (Figure 1(a)). A similar, though less pronounced pattern was seen in bowel function for the CRT group (Figure 1(b)). Sexual function was more complex. All treatments were associated with a decline in function. RP and CRT cohorts had comparable scores at baseline but the impact of RP was greater than CRT (Figure 1(c)). With BT and RP groups there was slight improvement in sexual function over time whereas CRT patients continued to deteriorate over the follow-up period.
3.3.5. Change in HRQOL from Baseline
Figure 2 shows the change in QOL score from baseline at each time point. The data suggests CRT patients had a significantly worse bowel function score at 2 years compared to RP patients, but not compared to BT patients (KWp = 0.0010). The most marked urinary function change from baseline was in the prostatectomy group
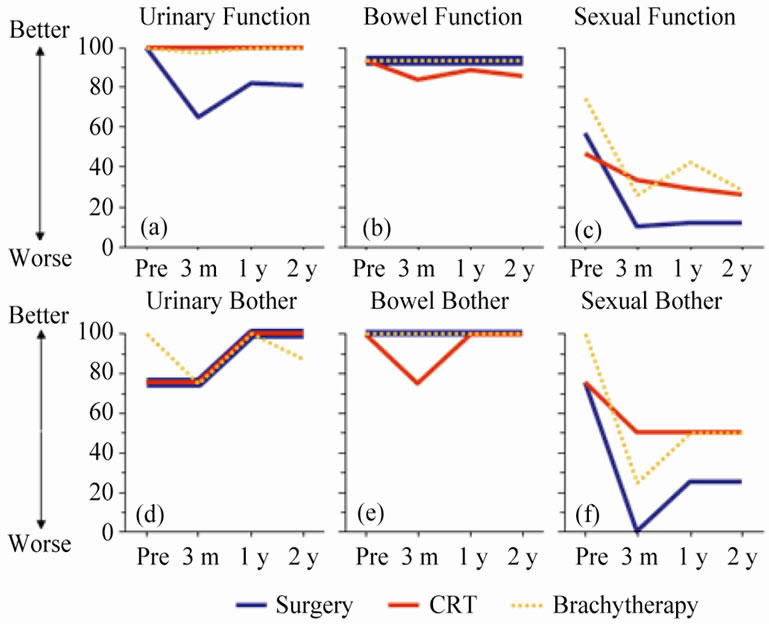
Figure 1. Results showing median scale scores for each treatment group: UCLA-PCI scores (for function and bother), urinary (a and d), bowel (b and e), sexual (c and f).
(KWp < 0.0001). Sexual function deteriorated for all three treatment cohorts. It can be concluded that as baseline functions for surgery and CRT were comparable, the impact of surgery on sexual function was greater than CRT over the two year follow up period (KW and MW p values show 3 month p < 0.0001, 1 year p < 0.0001 and 2 year p < 0.0005). In this study, there was no difference in change in sexual function scores for those undergoing nerve sparing or non-nerve sparing surgery at any time point (p > 0.05) even though their baseline scores were comparable. At all three time points, the deterioration in score from baseline for BT patients was significantly greater than CRT (but not as great as the RP patients).
The proportions of patients able to have erections adequate for sexual activity were 66% to 29% for RP, 62% to 49% for CRT and 88% to 65% for BT (baseline and 24 months respectively). Similarly, assuming patients with poor baseline sexual function remained low post-treatment, we stratified “sexually potent” patients with baseline scores greater than 80 and assessed how each treatment affected sexual function. Figure 3 demonstrates the change in score from pre-treatment to the two-year time point. The numbers were small in each group but brachytherapy patients had better sexual function scores at 2 years.
Bowel bother results showed that both radiotherapy options affected HRQOL significantly compared to RP at three months (KWp = 0.0134) but at 2 years there was no difference between any of the groups correlating with function scores (KWp = 0.0527). Recalling that there was no statistical difference between baseline sexual bother scores for all three treatments, at the three month (KWp = 0.0093) and 1 year (KWp = 0.0071) time points, RP patients fared significantly worse for sexual bother scores. However at 2 years, change in bother scores had deteriorated equally from baseline levels in all three groups (KWp = 0.0982).
4. Discussion
Although the gold standard for comparing treatment approaches is a RCT, it is recognised that in certain circumstances patients are reluctant to be randomised between different treatment options. Many patients choosing not to be randomised are thus excluded from comparison. Our pilot study of randomisation [4] in early prostate cancer eventually led us to the patient preference study where all patients were included. In assessing the impact of treatment on QOL we compared the change in QOL pre and post treatment for each patient. Thus, our aim was to describe the natural history of different groups of patients expressing a preference for their treatment.
Previous cross-sectional studies have demonstrated that sexual, urinary and bowel dysfunction remain preva-
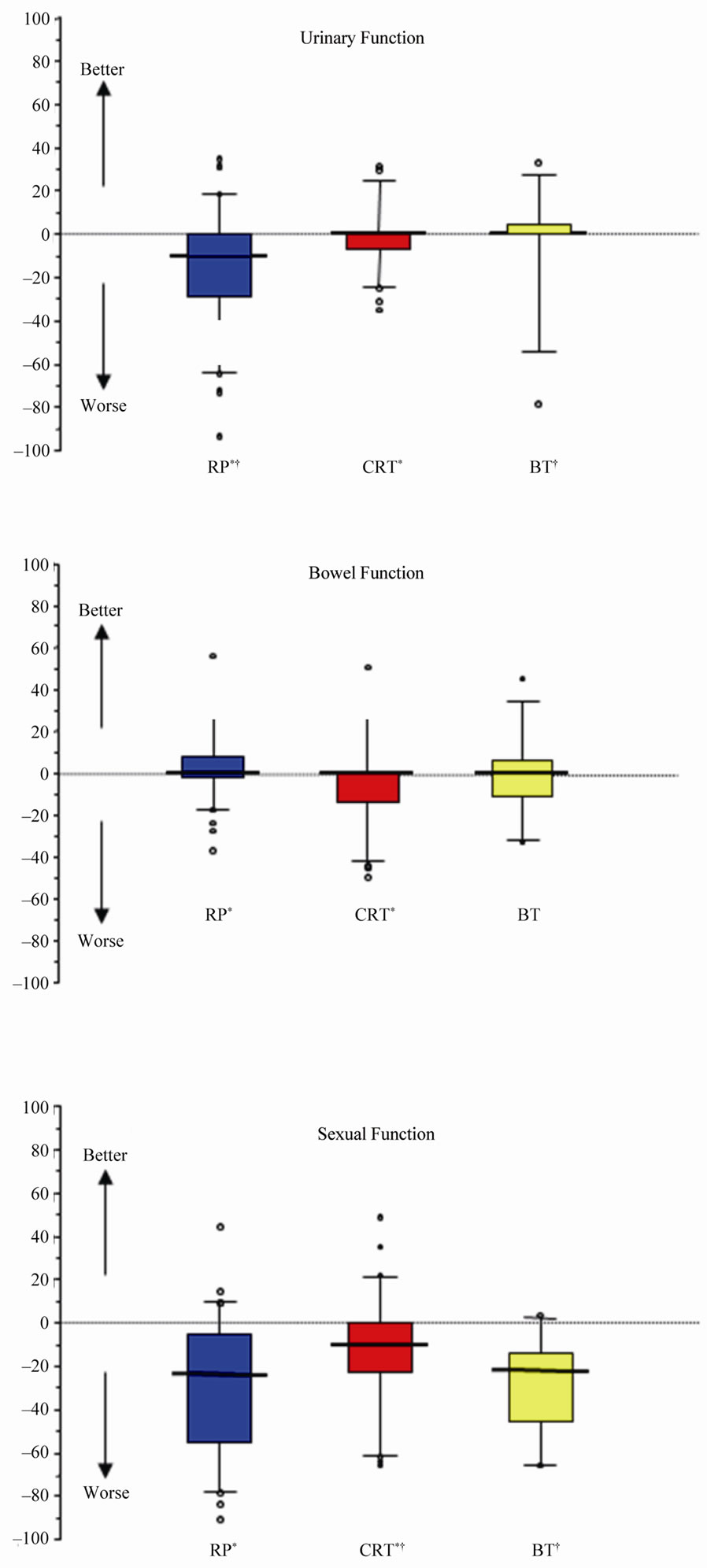
Figure 2. Box and whisker plot showing the change in median score from baseline at 2 year time point. * and † both denote a statistically significant difference between the two treatments indicated, using Mann-Whitney U test with Bonferroni correction (p < 0.001). Circles represent outliers (beyond 95% CI).
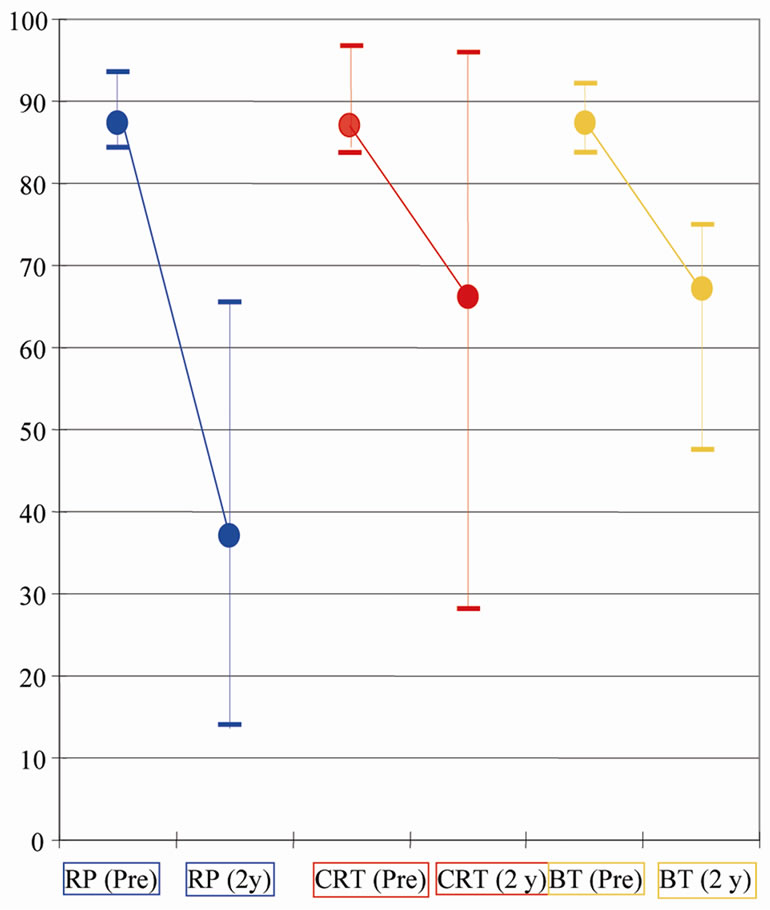
Figure 3. A graph showing the scores for sexual function at baseline (pre) and at the two year (2 y) time points for the cohort of patients who had baseline sexual function scores above 80. Dots represent median scores. Whiskers represent inter-quartile ranges.
lent among men undergoing treatment for localised prostate cancer [8-11,13,22-24]. Longitudinal studies have illustrated treatment effect over time and those with pretreatment data are of greatest value in interpreting the true extent of post treatment change [14,15]. Amongst these studies, those directly comparing all three major treatments are of particular interest [17-19,25,26].
This prospective patient preference study directly compared HRQOL outcomes for the 3 main treatments in a uniform cohort of men without the confounding variable of hormone treatment and with data recorded both pre and post treatment. All men were counselled by both urological surgeon and clinical oncologist (to reduce bias from either specialty) prior to making their treatment choice. This study also incorporates a unique cohort of radiotherapy patients receiving hypofractionated conformal external beam treatment in a European oncology centre.
Previous HRQOL research found that prostatectomy affected predominantly urinary and sexual function, whereas CRT mostly influenced bowel and sexual function. These studies also showed that general HRQOL is relatively unaffected despite changes in disease specific HRQOL [17,27]. This study confirmed these conclusions and also demonstrated brachytherapy and hypo-fractionated CRT were well tolerated when compared to other treatment modalities.
As this study was non-randomised we observed baseline differences between men choosing different treatment options. BT patients had better compliance in replying to questionnaires. Interestingly when brachytherapy was first introduced many men actively sought out this treatment. This degree of motivation may explain the higher compliance rate for completing questionnaires. As expected the median age of the CRT group was significantly older than the other cohorts. Brachytherapy patients had better baseline urinary function, explained by the exclusion criteria of a high IPSS score in this cohort.
This investigation studied the impact of different treatments over time. Interestingly, when evaluating urinary bother, the BT group had a worse score at the two year time point compared to baseline, whereas patients in other cohorts improved despite greater deterioration in urinary function scores. One possible reason is different expectations between patient groups. Patients with almost normal urinary baseline function (e.g. BT cohort) were more likely to be troubled by small declines in function compared with those with poor baseline urinary function that are possibly better adapted to their disability. Treatment itself may improve symptoms for some patients. It should be remembered that UCLA-PCI was designed for patients undergoing surgery. The use of this assessment tool is therefore a major limitation of this study as it has more sensitivity to detect urinary incontinence, rather than irritative/obstructive symptoms and it maybe that BT patients experienced more symptoms than were recorded in function questions. It is worth noting that bother is represented by only one question for each function in the UCLA questionnaire. Detecting irritative voiding symptoms can be achieved using the Expanded Prostate Cancer Index Composite (EPIC) which was modified from the UCLA-PCI and captures irritative voiding complaints more pertinent to BT patients [28].
This research concurred with previous investigators in that bowel-related HRQOL was adversely affected by radiotherapy when compared with surgery. It could be hypothesised that hypofractionated radiotherapy used in this study would increase the chance of late effects to normal tissues such as the rectum and therefore lead to worse bowel function and bother. However, these results show that the bowel HRQOL scores are fairly similar to the other treatments and also comparable to other studies using the same scoring systems for more conventional radiotherapy [29]. In previous studies comparing the observed toxicity of this centre to other published results using more conventional radiotherapy schedules, it has been again demonstrated that treatments are equivalent [30]. To truly assess late effects in this study, longer follow up trends would be needed. Also, further studies evaluating differential bowel symptoms for the available management options should include a tool designed to assess bowel treatment effect, rather than a broadly based QOL tool.
Interpretation of sexual dysfunction is complex because of the multifactorial aspects that make up a person’s sexuality. Previous investigations have established the deleterious effect of surgery and CRT on sexual function. This study additionally confirms that surgery had the greatest initial impact with only slight improvement over time, whereas CRT caused a less marked initial decline followed by a continued slow reduction in function over the follow-up period. A component of this deterioration in CRT men may be attributed to their older age at baseline, though there is undoubtedly a radiation related effect over a 2 year period following treatment. The brachytherapy cohort illustrated a similar pattern to the RP group but with a less marked deleterious effect in the initial phases. It is important to emphasize that the confounding factor of concomitant hormone therapy was not an issue in this study as patients given endocrine therapy were excluded from analysis thereafter. Therefore, comparison of this study to other research should be done with caution as most of these other studies included patients receiving hormone therapy. Previous studies have advocated stratifying patients according to their baseline function (as shown for sexual function in Figure 3) as it has been demonstrated that there is little change to poor baseline sexual function post treatment [18]. Our study confirms the findings from Chen et al. [18] that BT patients with good baseline function preserve function better compared with similar patients receiving the other treatment options. Nerve sparing RP made no difference to sexual function in the patients undergoing surgery even though the surgery was undertaken by experienced urological oncologists, all of whom were trained and had adequate experience of radical retropubic RP with nerve sparing. It may be suggested that the figures are appropriate for the type of population involved (i.e. nonscreened population with a high index of cases from socially deprived areas) where 50% - 60% of patients who present to the hospitals involved in this study have erectile dysfunction prior to treatment.
In this study we have demonstrated a marked variation in the consequences of treatment experienced by our patients. There will be an element of inherent selection bias that is inevitable in non-randomised questionnaire based studies. Evenso it is clear that information from studies like this is highly relevant for men diagnosed with early prostate cancer faced with a difficult choice. To date, there has been a paucity of accurate information on the sequelae of treatment in early prostate cancer. As clinicians it is important we collect good quality data on treatment effects and make this available to our patients to allow them to make an informed choice. These important differences between sexual function should prompt further research taking into account co-morbidities, sexual relationships and aids used to help improve dysfunction in the post treatment period.
5. Conclusions
This novel inclusive study design demonstrated significant differences in treatment sequelae, the most marked of which is sexual function. We plan to include this data in the new information we impart to our patients with the intention of auditing the impact on patient choice.
6. Acknowledgements
We would like to thank the research nurses at the participating centres. We are also grateful for the financial support from AstraZeneca towards the salary of Lynne Gilmore (Data management) in the first 6 months of this study.
REFERENCES
- F. J. Fowler, M. M. Collins, P. C. Albertson, A. Zietman, D. B. Elliott and M. J. Barry, “Comparison of Recommendations by Urologist and Radiation Oncologists for Treatment of Clinically Localized Prostate Cancer,” Journal of the American Medical Association, Vol. 283, No. 24, 2000, pp. 3217-3222. doi:10.1001/jama.283.24.3217
- M. Schulpher, S. Bryan, P. Fry, P. de Winter, H. Payne and M. Emberton, “Patients’ Preferences for the Management of Non-Metastatic Prostate Cancer,” British Medical Journal, Vol. 328, No. 7436, 2004, pp. 382-384. doi:10.1136/bmj.37972.497234.44.
- P. A. Singer, E. S. Tasch, C. Stocking, S. Rubin, M. Siegler and R. Weichselbaum, “Sex or Survival: TradeOffs between Quality and Quantity of Life,” Journal of Clinical Oncology, Vol. 9, No. 2, 1991, pp. 328-334.
- P. O’Reilly, L. Martin and G. Collins, “Few Patients with Prostate Cancer Are Willing to be Randomised to Treatment,” British Medical Journal, Vol. 318, 1999, p. 1556.
- A. Bill-Axelson, L. Holmberg, M. Ruutu, et al., “Radical Prostatectomy versus Watchful Waiting in Early Prostate Cancer,” The New England Journal of Medicine, Vol. 352, No. 19, 2005, pp. 1977-1984. doi:10.1056/NEJMoa043739
- G. Steineck, F. Helgesen, J. Adolfsson, et al., “Quality of Life after Radical Prostatectomy or Watchful Waiting,” The New England Journal of Medicine, Vol. 347, No. 11, 2002, pp. 790-796. doi:10.1056/NEJMoa021483
- J. Donovan, N. Mills, M. Smith, et al., “Improving Design and Conduct of Randomised Trials by Embedding Them in Qualitative Research: Protect (Prostate Testing for Cancer and Treatment) Study,” British Medical Journal, Vol. 325, No. 7367, 2002, pp. 776-780. doi:10.1136/bmj.325.7367.766
- D. C. Miller, M. G. Sanda, R. L. Dunn, et al., “LongTerm Outcomes among Localized Prostate Cancer Survivors: Health-Related Quality-of-Life Changes after Radical Prostatectomy, External Radiation and Brachytherapy,” Journal of Clinical Oncology, Vol. 23, No. 12, 2005, pp. 2772-2780. doi:10.1200/JCO.2005.07.116
- J. W. Davis, D. A. Kuban, D. F. Lynch and P. F. Schellhammer, “Quality of Life after Treatment for Localized Prostate Cancer: Differences Based on Treatment Modality,” Journal of Urology, Vol. 166, 2005, pp. 947-952. doi:10.1016/S0022-5347(05)65870-3
- J. T. Wei, R. L. Dunn, H. M. Sandler, et al., “Comprehensive Comparison of Health-Related Quality of Life after Contemporary Therapies for Localized Prostate Cancer,” Journal of Clinical Oncology, Vol. 20, No. 2, 2002, pp. 557-566. doi:10.1200/JCO.20.2.557
- S. Namiki, T. Tochigi, M. Kuwahara, et al., “Health Related Quality of Life in Japanese Men after Radical Prostatectomy or Radiation Therapy for Localized Prostate Cancer,” International Journal of Urology, Vol. 11, No. 8, 2004, pp. 619-627. doi:10.1111/j.1442-2042.2004.00860.x
- M. S. Litwin, S. C. Flanders, D. J. Pasta, M. L. Stoddard, D. P. Lubeck and J. M. Henning, “Sexual Function and Bother after Radical Prostatectomy or Radiation for Prostate Cancer: Multivariate Quality-of-Life Analysis from CaPSURE. Cancer of the Prostate Strategic Urologic Research Endeavour,” Urology, Vol. 54, No. 3, 1999, pp. 503-508.
- S. J. Frank, L. L. Pisters, J. Davis, A. K. Lee, R. Bassett and D. A. Kuban, “An Assessment of Quality of Life Following Radical Prostatectomy, High Dose External Bean Radiation Therapy and Brachytherapy Iodine Implantation as Monotherapies for Localized Prostate Cancer,” Journal of Urology, Vol. 177, 2007, pp. 2151-2156. doi:10.1016/j.juro.2007.01.134
- I. J. Korfage, M. L. Essink-Bot, G. J. Borsboom, et al., “Five-Year Follow-Up of Health-Related Quality of Life after Primary Treatment of Localized Prostate Cancer,” International Journal of Cancer, Vol. 116, No. 2, 2005, pp. 291-296. doi:10.1002/ijc.21043
- A. L. Potosky, W. W. Davis, R. M. Hoffman, et al., “Five-Year Outcomes after Prostatectomy of Radiotherapy for Prostate Cancer: The Prostate Cancer Outcomes Study,” Journal of the National Cancer Institute, Vol. 96, No. 18, 2004, pp. 1358-1367. doi:10.1093/jnci/djh259
- H. Borchers, R. Kirschner-Hermanns, B. Brehmer, et al., “Permanent 125I-Seed Brachytherapy or Radical Prostatectomy: A Prospective Comparison Considering Oncological and Quality of Life Results,” British Journal of Urology International, Vol. 94. No. 6, 2004, pp. 805-811. doi:10.1111/j.1464-410X.2004.05037.x
- M. Ferrer, J. F. Suarez, F. Guedea, et al., “Health-Related Quality of Life after Treatment with Clinically Localized Prostate Cancer,” International Journal of Radiation Oncology Biology Physics, Vol. 72, No. 2, 2008, pp. 421- 432. doi:10.1016/j.ijrobp.2007.12.024
- R. C. Chen, J. A. Clark and J. A. Talcott, “Individualizing Quality-of-Life Outcomes Reporting: How Localizzed Prostate Cancer Treatments Affect Patients with Different Levels of Baseline Urinary, Bowel, and Sexual Function,” Journal of Clinical Oncology, Vol. 27, No.24, 2009, pp. 3916-3922. doi:10.1200/JCO.2008.18.6486
- G. J. Huang, N. Sadetsky and D. F. Penson, “Health Related Quality of Life for Men Treated for Localized Prostate Cancer with Long-Term Followup,” Journal of Urology, Vol. 183, No. 6, 2010, pp. 2206-2212. doi:10.1016/j.juro.2010.02.013
- J. E. Ware, “How to Score Version 2 of the SF-36® Health Survey,” Quality Metric Incorporated, Lincoln, 2000.
- M. S. Litwin, R. D. Hays, A. Fink, P. A. Ganz, B. Leake and R. H. Brook, “The UCLA Prostate Cancer Index. Development, Reliability and Validity of a Health-Related Quality of Life Measure,” Medical Care, Vol. 36, No. 7, 1998, pp. 1002-1012.
- A. J. Lim, A. H. Brandon, J. Fiedler, et al., “Quality of Life: Radical Prostatectomy versus Radiation Therapy for Prostate Cancer,” Journal of Urology, Vol. 154, No. 4, 1995, pp. 1420-1425. doi:10.1016/S0022-5347(01)66881-2
- Y. Jo, H. Junichi, F. Tomohiro, I. Yoshinari and F. Masato, “Radical Prostatectomy versus High-Dose Brachytherapy for Prostate Cancer: Effects on Health-Related Quality of Life,” British Journal of Urology International, Vol. 96, 2005, pp. 43-47.
- F. Mols, I. J. Korfage, J. J. M. Vingerhoets, et al., “Bowel, Urinary and Sexual Problems among Long-Term Prostate Cancer Survivors. A Population Based Study,” International Journal of Radiation Oncology Biology Physics, Vol. 73, No. 1, 2009, pp. 30-38. doi:10.1016/j.ijrobp.2008.04.004
- J. A. Talcott, J. Manola, J. A. Clark, et al., “Time Course and Predictors of Symptoms after Primary Prostate Cancer Therapy,” Journal of Clinical Oncology, Vol. 21, No. 21, 2003, pp. 3979-3986. doi:10.1200/JCO.2003.01.199
- W. R. Lee, M. C. Hall, R. P. McQuellon, L. D. Case and D. L. McCullough, “A Prospective Quality-of-Life Study in Men with Clinically Localized Prostate Carcinoma Treated with Radical Prostatectomy, External Beam Radiotherapy, or Interstitial Brachytherapy,” International Journal of Radiation Oncology Biology Physics, Vol. 51, No. 3, 2001, pp. 614-623. doi:10.1016/S0360-3016(01)01707-2
- D. F. Penson, M. S. Litwin and N. K. Aaronson, “Health Related Quality of Life in Men with Prostate Cancer,” Journal of Urology, Vol. 169, 2003, pp. 1653-1661.
- J. T. Wei, R. L. Dunn, M. S. Litwin, H. M. Sandler and M. G. Sanda, “Development and Validation of the Expanded Prostate Cancer Index Composite (EPIC) for Comprehensive Assessment of Health-Related Quality of Life in Men with Prostate Cancer,” Urology, Vol. 56, No. 6, 2000, pp. 899-905.
- M. G. Sanda, R. L. Dunn, J. Michalski, et al., “Quality of Life and Satisfaction with Outcome among ProstateCancer Survivors,” The New England Journal of Medicine, Vol. 358, No. 12, 2008, pp. 1250-1261. doi:10.1056/NEJMoa074311
- J. E. Livsey, R. A. Cowan, J. P. Wylie, et al., “Hypofractionated Conformal Radiotherapy in Carcinoma of the Prostate: Five Year Outcome Analysis,” International Journal of Radiation Oncology Biology Physics, Vol. 57, No. 5, 2003, pp. 1254-1259.

