Journal of Cancer Therapy
Vol.1 No.2(2010), Article ID:2011,7 pages DOI:10.4236/jct.2010.12009
Photothermal Therapy Using TiO2 Nanotubes in Combination with Near-Infrared Laser
![]()
1Department of Materials Science and Engineering, Inha University, Incheon, Korea; 2College of Medicine, Inha University, Incheon, Korea; *Corresponding author.
Email: cmlee@inha.ac.kr
Received April 8th, 2010; revised May 14th, 2010; accepted May 24th, 2010.
Keywords: thermotherapy, TiO2 nanotubes, in vitro cell test, in vivo animal Test, NIR
ABSTRACT
We report the in vitro cell test and in vivo animal test results of titanium oxide nanotubes (TiO2 NTs) as a potential therapeutic agent used for cancer thermotherapy in combination with near-infrared (NIR) laser. The in vitro cell test results show that both the cells exposed to NIR laser without TiO2 NTs treatment and the cells treated with TiO2 NTs but not with NIR irradiation had cell viabilities higher than 96%. Combination of these two techniques, however, shows cell viability less than 1%. The cell death rate strongly depended on the concentration of TiO2 NTs. Also, the cell deaths were mostly due to necrosis but partly due to late apoptosis. The in vivo animal test results show that tumor cells can be completely destroyed without nearly giving damage to surrounding healthy cells by an injection of an adequate amount of TiO2 NTs/NaCl suspension and a subsequent single continuous laser treatment at a moderately low laser illumination intensity for the exposure time optimized for the tumor size. These results suggest that TiO2 NTs can be effectively utilized as a therapeutic agent for cancer thermotherapy due to their excellent photothermal property and high biocompatibility.
1. Introduction
In recent years, cancer thermotherapy techniques based on inorganic nanomaterials and NIR laser have attracted significant interest owing to their advantages over conventional surgical treatments. The inorganic nanomaterials currently demonstrated as thermal coupling agents in thermotherapy are gold nanoparticles (Au NPs) [1], gold nanorods [2], gold nanoshells [3], gold nanocages [4], gold nanocrystals [5], single wall carbon nanotubes (SWCNTs) [6], and porous silicon [7]. The advantages of thermotherapy include the anticipated reduction in morbidity and mortality, low cost, suitability for real-time imaging guidance, and the ability to perform ablative procedures on outpatients because of its non-invasive nature [3].
For irreversible destruction of cancer cells heat treatment at a temperature higher than 46℃ for more than 60 min is required in thermotherapy, but increasing the treatment temperature can shorten the time necessary to induce cytotoxicity [8]. In the conventional thermotherapies based on simple heating [9] most treatment failures result from insufficient temperature rises in the tumor tissues. Therefore, it is very important to develop a thermal coupling agent for thermotherapy with high photothermal effect in order to secure irreversible destruction of tumor cells in a short time without damaging adjacent healthy cells in thermotherapy. In this paper, we report the in vivo animal test results of TiO2 NTs in combination with NIR light. TiO2 is a highly functional material that has numerous interesting properties. As regards biomedical applications of TiO2 NTs, their use in drug delivery applications have been reported recently [10,11]. However, application of TiO2 NTs to cancer photothermotherapy has not been reported before. The thermotherapy based on TiO2 NTs in combination with NIR laser is anticipated to be effectively utilized to cure various cancers such as cancer of the esophagus, gastric cancer, cancer of the colon, cancer of the skin, breast cancer, and liver cancer due to the excellent photothermal property as well as the high biocompatibility of TiO2 NTs [12-16].
2. Methods
TiO2 NTs samples used in the experiments were in a state of fragments. First, TiO2 NTs layers were formed by electrochemical anodization [17] of Ti thin foils in an electrolyte consisting of 0.3 wt.% NH4F and 2 vol.% H2O in ethylene glycol at 60 V for 17 h. The inner diameter of the TiO2 NTs is ~ 100 nm and the thickness of the TiO2 NTs layers is ~ 160 μm (Figure 1). Next, the TiO2 NT layers were fragmented into small pieces with sizes less than 220 nm by using an ultrasonicator (Model: 2510EDTH, BRANSON Ultrasonics Corp., USA) and then filtered through a 220 nm microfilter (Model: SLGV 033 RS, Millipore, USA). TiO2 NTs/NaCl suspensions were prepared by mixing TiO2 NTs with 9% saline solution and then ultrasonicating them for 6 h and finally filtering them with a 220 nm filter (model: MILLEX®–GV, Millipore, USA).
Annexin V-fluorescein isothiocyanate (FITC) apoptosis assays were performed on five different mouse CT-26 cell sample groups to see their modes of cell deaths: the CT-26 cell control group given neither TiO2 NTs nor laser treatment, the CT-26 cell group not treated with TiO2 NTs but with laser, the group not treated with laser but with TiO2 NTs, the group treated with both a low concentration (50 mg/4 ml)-TiO2 NTs/NaCl suspension and laser, and the group treated with both a high concentration (210 mg/4 ml)-TiO2 NTs/NaCl suspension and laser to distinguish between apoptosis and necrosis. For Annexin VFITC Apoptosis assay, the TiO2 NT layer formed by the electrochemical anodization of a Ti thin foil was fragmented into fine particles by ultrasonicated for 24 h in a beaker filled with ethanol and then filtered through a 220 nm filter, first. After evaporation of the ethanol, TiO2/NaCl:PEG suspensions with two different concentrations (50 mg/4 ml and 210 mg/4 ml) were prepared by mixing the TiO2 particles with an 1:1 NaCl:PEG solution. Next, CT-26 cells were treated with one of the two TiO2/NaCl: PEG suspensions and then
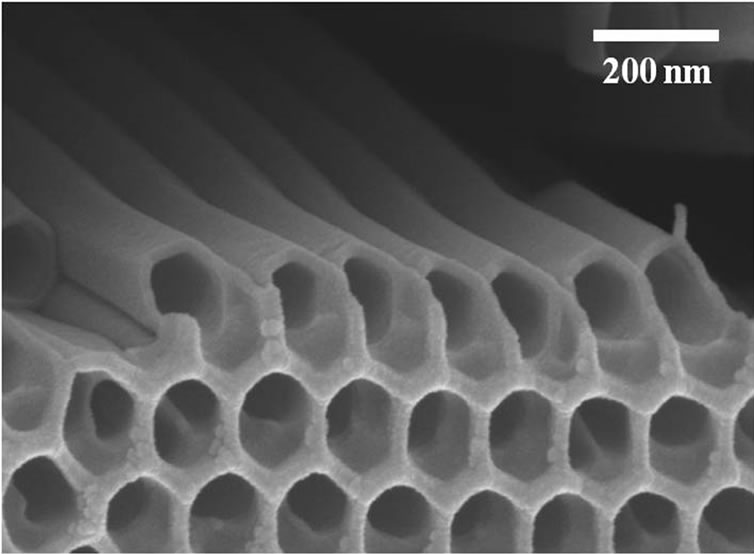
Figure 1. Bird’s eye view SEM images of TiO2 NTs. TiO2 NTs were formed by anodic etching of Ti thin foils in an electrolyte consisting of 0.3 wt.% NH4F and 2 vol.% H2O in ethylene glycol at 60 V for 17 h
NIR laser at 300 mW/cm2 for 20 min, 2 × 106 cells were removed from the culture, washed twice with cold PBS, and double stained with Annexin V-FITC and Propidium iodide (PI) (BD Biosciences, San Jose, CA, USA) in Annexin-binding buffer, followed by analysis on a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with a 488-nm argon laser. To avoid nonspecific fluorescence from dead cells, live cells were gated using forward and side scatter.
Animal care and all experimental procedures were conducted in accordance with “Laws and regulations on animal experiments” [18]. In vivo animal tests were performed on 15 healthy BALB/C male mice, 6-7 weeks old, weighing 28-31 g. CT-26 murine colon carcinoma tumor cells were inoculated into the hollow of the back to transplant them to the mice. After the inoculation the mice were maintained in separate cages and fed with standard laboratory food and water. The tumors grew to ~ 1.0 cm in diameter within a week of the tumor transplant. Details of treatment procedures (TiO2 NTs/NaCl suspension injection and then laser treatment) are as follows: The mice were anesthetized by injecting 40 μl of a 9:1 solution of ketamine (100 mg/ml) and rompun (100 mg/ml), 24 h prior to laser treatment. The overlying skin was shaved. 100 μl of 27.5 or 52.5 mg/ml TiO2 NTs/ NaCl suspension was then directly injected ~ 7 mm into the tumor volume. NIR laser treatment was performed at 300 or 400 mW/cm2 for 20 min. The treatment (the suspension injection plus the subsequent laser treatment) was repeated for 2 or 3 times with a time interval of 48 h between the treatments. The control group received no injection or subsequent laser treatment. Details of the parameters in the suspension injection and the laser treatment are listed in Table 2. The first, second, and fourth groups in Table 1 were given the treatment (the suspension injection and the laser treatment) twice under the condition given to each group and the third given the treatment for three times in a similar manner. On the other hand, the fifth was given a single continuous laser treatment for 60 min.
3. Results and Discussion
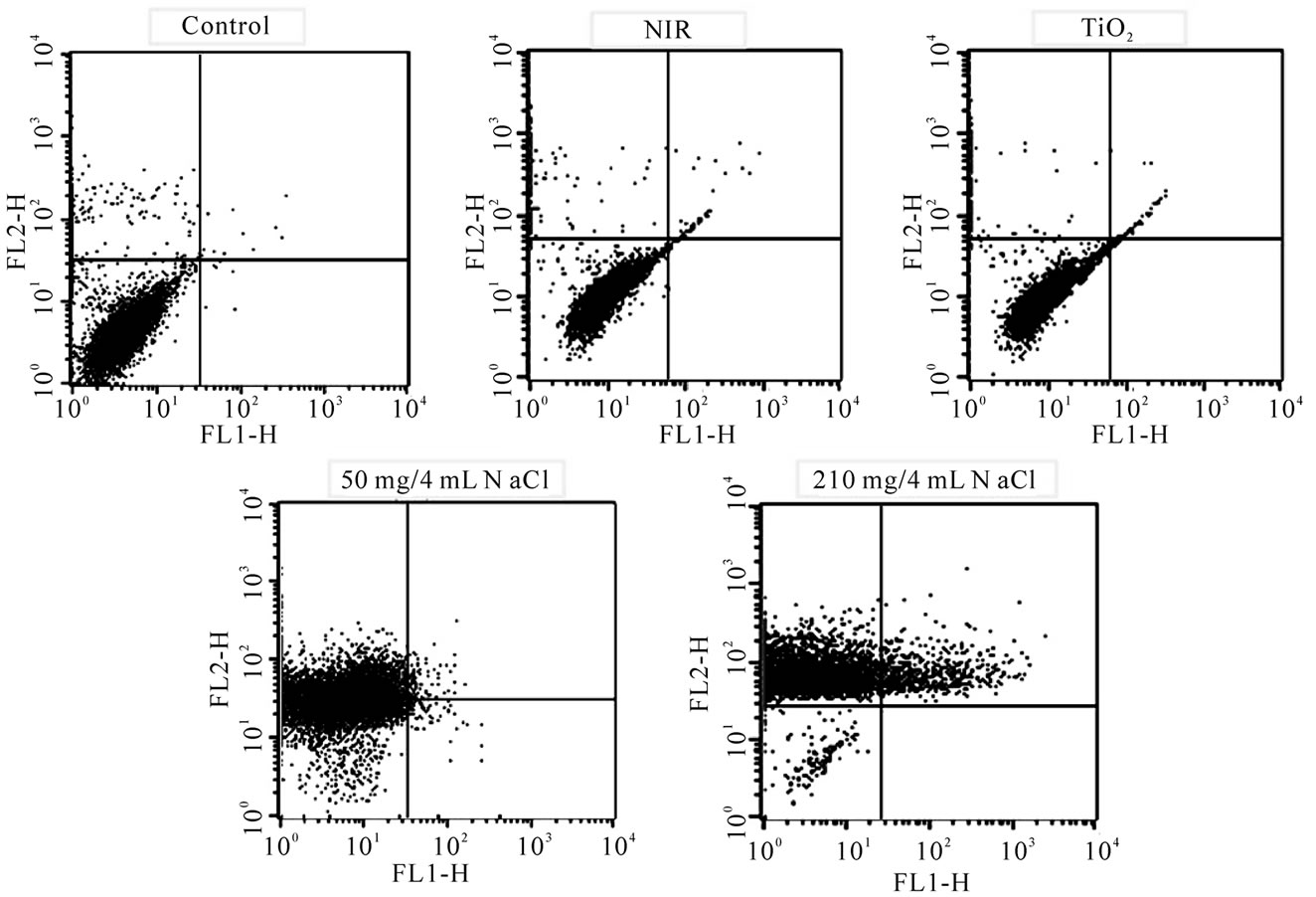
The fluorescent activated cell sorter (FACS) flow cytometry profiles (Figures 2(a) and (b)) obtained as a result of Annexin V-FITC Apoptosis assay represent Annexin V-FITC staining in X-axis and PI in Y-axis. The four sections of the quadrant in each profile from the upper left in a clockwise direction represent necrosis, late apoptosis, early apoptosis, and live cell, respectively. The groups treated with TiO2 NTs/NaCl suspensions show substantially higher cell death (necrosis + late apoptosis) rates than those not given both treatments (Figures 2(a) and (b)). The Annexin V-FITC Apoptosis assay results in (Figures 2(a) and (b)) are summarized in Table 1. The figure show that the cell death rate strongly depends on
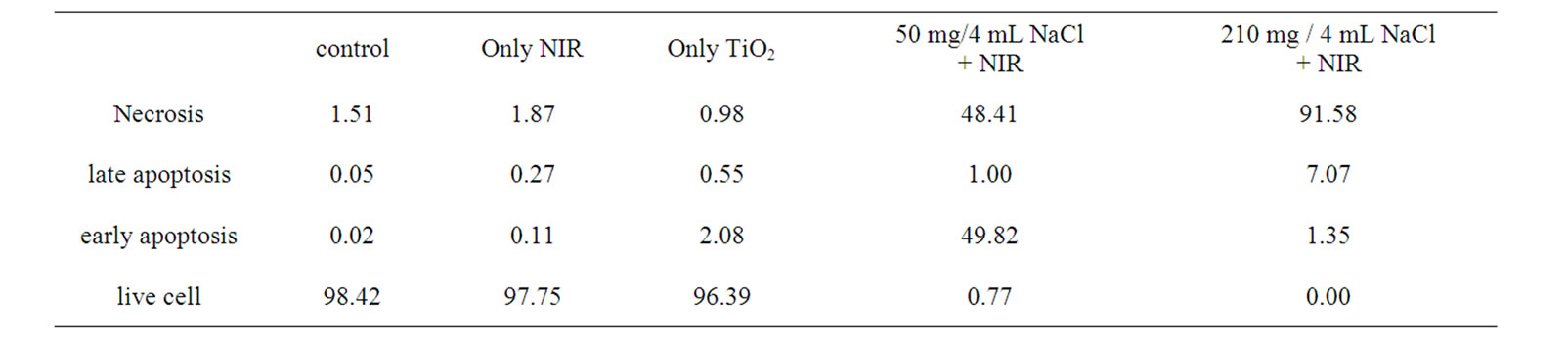
Table 1. A summary of the Annexin V-FITC Apoptosis assay results shown in Figure 2, showing the percentages of cell death modes: necrosis, late apoptosis, early apoptosis, and live cell
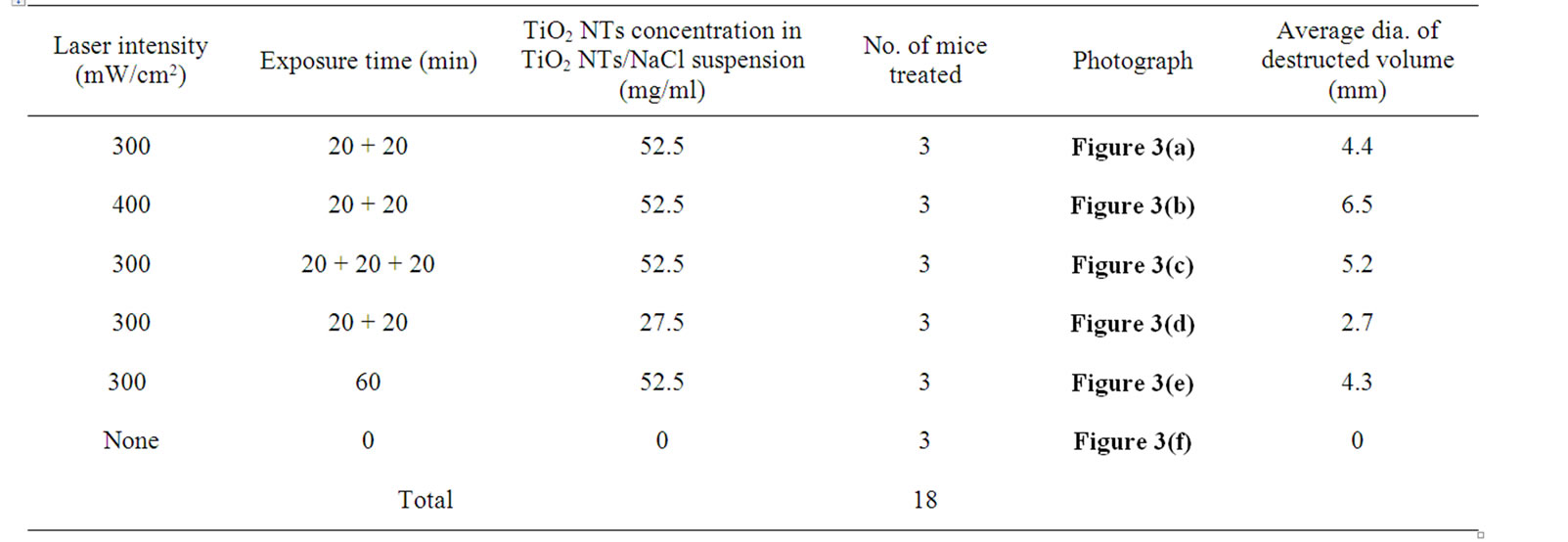
Table 2. Parameters in TiO2 NTs / NaCl suspension injection and NIR laser treatment
the concentration of TiO2 NTs. The group treated with a high concentration (210 mg/4 ml)-TiO2 NT suspension and exposed to NIR laser at 300 mW/cm2 for 20 min without TiO2 NTs treatment shows a cell viability of 97.8%. Likewise, the group treated with a high concentration (210 mg/4 ml)-TiO2 NT suspension but not with NIR irradiation also shows a cell viability of 96.4% (Figure 3). Combination of these two techniques, however, shows cell viabilities of 0.77 and 0.0% for lower (50 mg/4 ml) and higher (210 mg/4 ml) TiO2 NT concentrations, respectively, implying that most cells are killed. The cell deaths are mostly due to necrosis but partly due to late apoptosis. The in vitro cell test results suggest that only combination of both TiO2 NTs and NIR laser treatments can kill cells and that a higher TiO2 NT concentration results in a higher cell death rate, particularly a higher necrosis rate than a lower TiO2 NT concentration.
The in vitro cell test results show the photothermal effect of the thermotherapy based on TiO2 NTs combined with laser on cell death, but it does not guarantee that the thermotherapy can inhibit tumor growth. Therefore, we conducted in vivo animal tests to confirm that the photothermal effect of TiO2 NTs combined with NIR laser could efficiently destroy tumor cells without giving damage to surrounding healthy cells and to investigate the influence of the treatment parameters including the amount and concentration of the suspension, the laser illumination intensity, and the laser exposure time on the destruction efficiency of tumors.
All the mouse CT-26 tumors used in this study were examined and euthanized 48 and 96 h after the last laser treatment, respectively. We chose 48 h after the last treatment as a point of time for examining the photothermal damage since necrosis of the tumor cells by the photothermal effect has usually continued for one or two days after laser treatment. First, we tried a single treatment (for example, an injection of 100 μl of the suspension with a TiO2 NTs concentration of 52.5 mg/ml into tumors and a subsequent laser treatment at 300 mW/cm2 for 20 min), but the photothermal effect by the treatment was lower than we expected. Hence, we decided to repeat treatment twice.
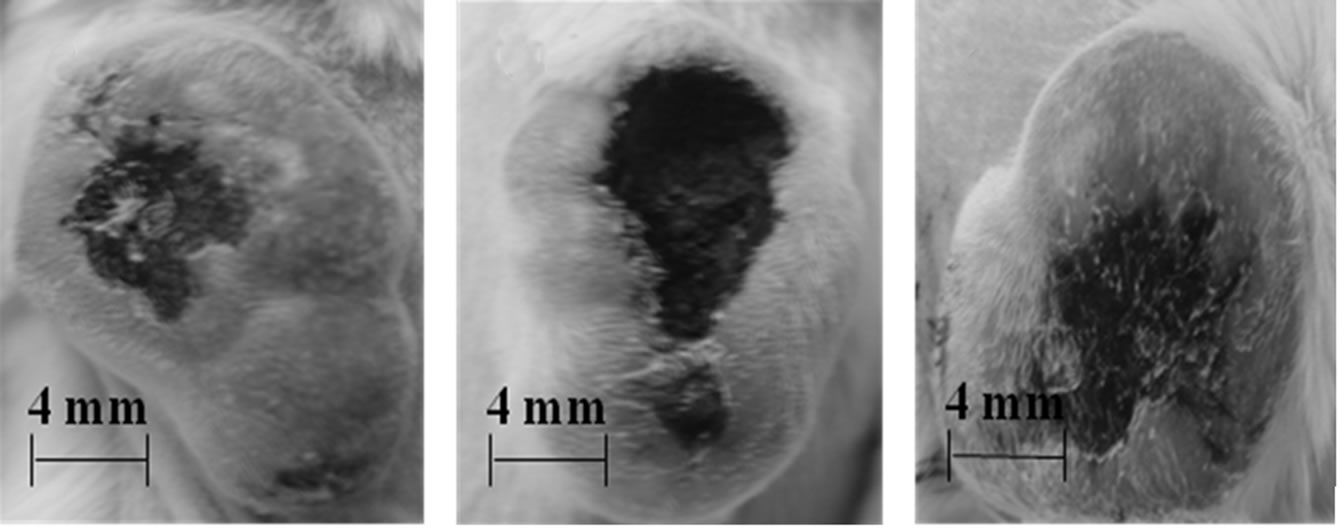
The average diameters and photographs of the tumors 48 h post-treatment of the six mouse tumor groups given different treatments are shown in Table 1 and Figures 3(a)-(f), respectively. The injured areas look black owing to carbonization of the skin tissue as well as the tumor tissue by the photothermal energy generated during laser treatment by the TiO2 NTs/NaCl suspension injected into the tumors. The tumor grew quite a bit after laser treatment (Figure 3(a)) compared with control (Figure 3(f)) due to incomplete destruction of tumor cells and rapid
 (a)
(a)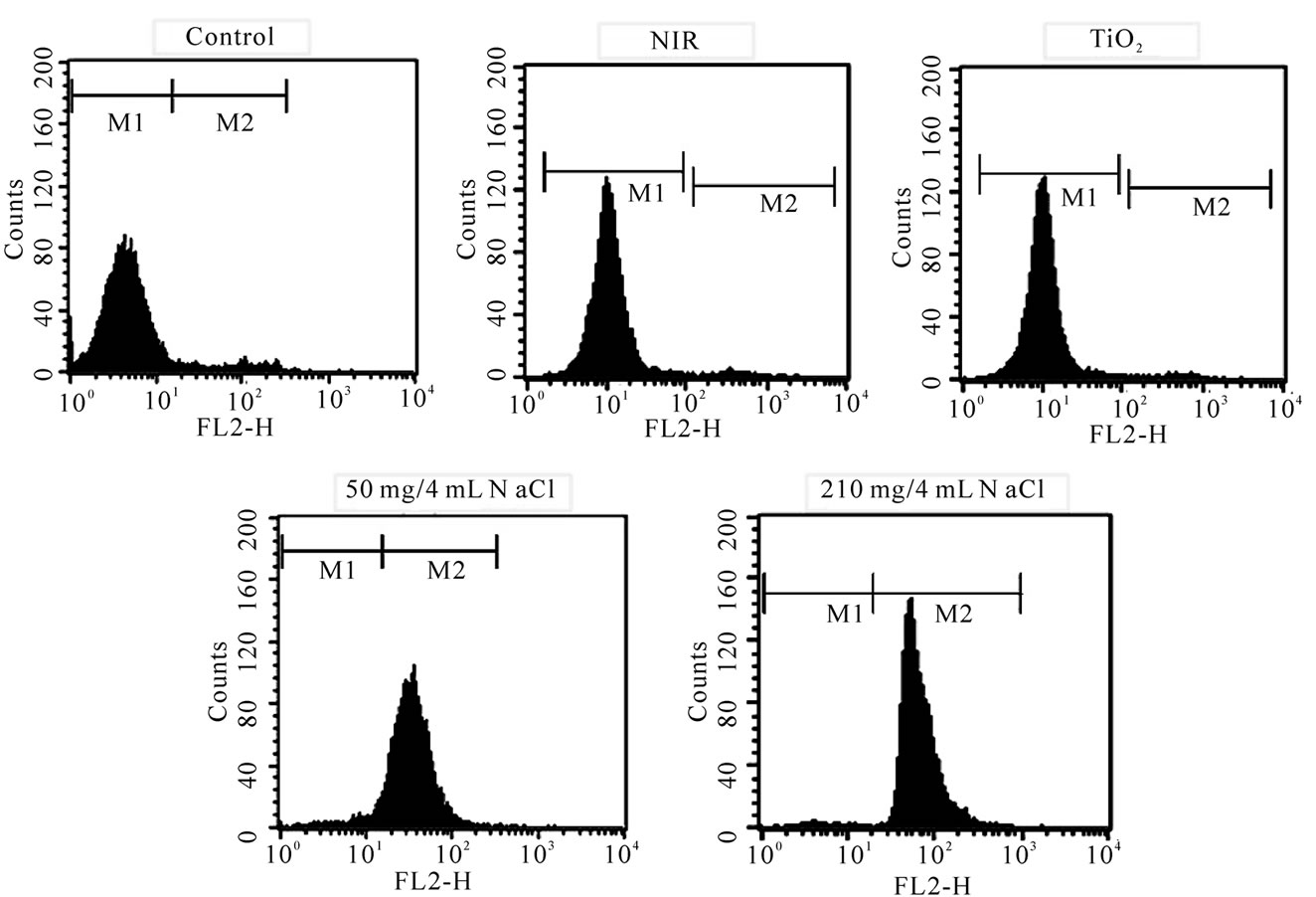 (b)
(b)
Figure 2. FACS flow cytometry profiles ((a) dot plots and (b) histograms) obtained as a result of Annexin V-FITC Apoptosis assay for five different mouse CT-26 cell sample groups to see their modes of cell deaths: the CT-26 cell control group, the CT-26 cell group treated with laser, the group treated with TiO2 NTs, the group treated with both TiO2 NTs (50 mg/4 ml) and laser, and the group treated with both TiO2 NTs (210 mg/4 ml) and laser. The NIR laser irradiation intensity and time in the treatments were 300 mW/cm2 and 20 min, respectively
 (a)
(a)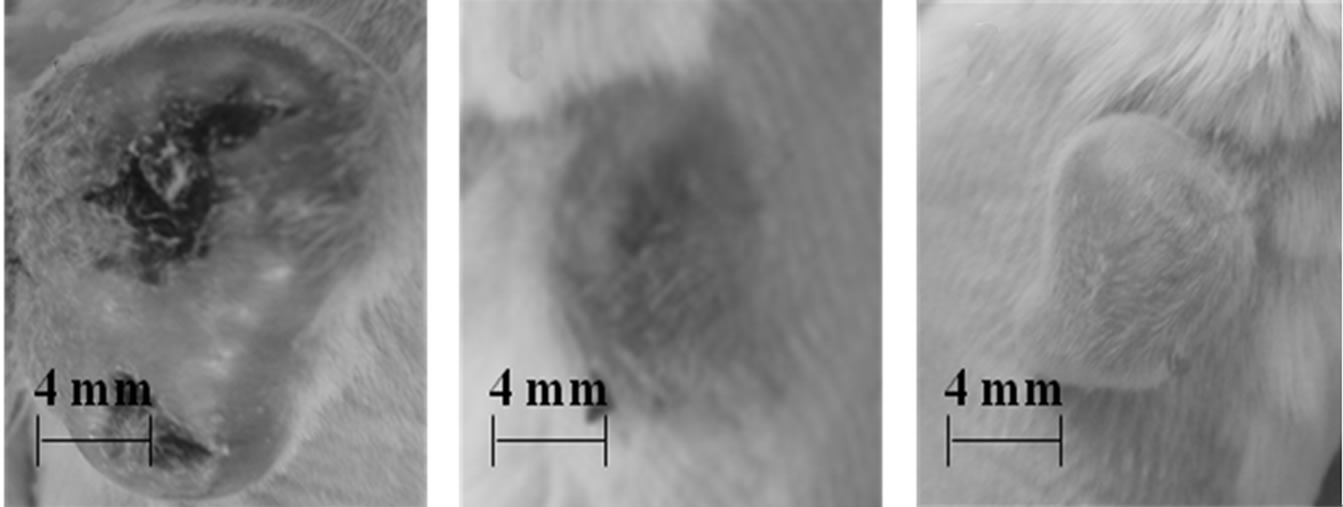 (b)
(b)
Figure 3. Photographs of the tumors of mice 48 h after treatment : (a) double laser treatments ( laser illumination intensity = 300 mW cm-2, exposure time = 20 min, TiO2 NTs concentration = 52.5 mg ml-1); (b) double laser treatments (400 mW cm-2, 20 min, 52.5 mg ml-1); (c) triple laser treatments (300 mW cm-2, 20 min, 52.5 mg ml-1; (d) double laser treatments (300 mW cm-2, 20 min, 27.5 mg ml-1); (e) single laser treatment (300 mW cm-2, 60 min, 52.5 mg ml-1); (f) no laser treatment (control)
growth of survived cancer cells. It appears that only a part of the tumor was destroyed since the laser treatment time of 20 min per treatment was sufficiently long. Comparison of Figure 3(b) with Figure 3(a) reveals that photothermal damage on tumor cells strongly depends on the intensity of the laser delivered. The average diameter (6.5 nm) of the destroyed volume in the tumor samples given a suspension injection and a subsequent laser treatment at a higher illumination intensity is considerably larger than that (4.4 nm) in those given a similar treatment at a lower intensity. Comparing Figure 3(c) with Figure 3(a), we can also see strong dependence of the photothermal destruction efficiency of tumor cells on the number of treatments or the total laser exposure time although the influence of the laser treatment time on the tumor destruction is not as strong as that of the laser illumination intensity. The average diameter of the damaged area (5.2 mm) in the tumor samples given a treatment (a suspension injection plus a subsequent laser treatment at 300 m/cm2) for three times is somewhat larger than that (4.4 mm) in the tumor samples given the same treatment twice. On the other hand, it can be seen by a comparison of Figure 3(d) with Figure 3(a) that the difference in the TiO2 NTs concentration of the TiO2 NTs/NaCl suspension also led to a clear distinction in the photothermal damage on tumor cells. The average size of the tumor samples given a treatment with a higher concentration is about 1.6 times as large as that of the tumor samples given a similar treatment with a lower concentration.
All the tumors given a double treatment in Table 1 were incompletely destroyed (Figures 3(a)-(d)) and have grown much faster than those in the control group (Figure 3(f)) after each treatment. In contrast, two of the three samples in the tumor group given a suspension injection plus a subsequent 60 min laser treatment, i.e. a long single continuous treatment (Figure 3(e)) were so perfectly destroyed that they did not grow at all after the laser treatment and their skin tissue was not nearly damaged in spite of the perfect death of their tumor cells. One of those given the same treatment grew due to incomplete destruction, but its skin tissue was not damaged like those of the other two in the same group. Figure 3(e) shows the intact surface structures and the size of one of the two tumors, whose cells were completely killed, nearly unchanged in comparison with that at the time of laser treatment (Figure 3(f)). By comparing Figure 3(f) with Figures 3(a)-(d), we can clearly see that a long single continuous laser treatment is most effective in destroying tumor cells since it can stop the growth of the tumors without giving damage at all to the skin tissue.
The carbonization of the tissue including the epidermis and subcutaneous tissue overlying the tumors in the tumor samples given a double treatment (Figures 3(a)-(d)) is caused by the reaction of the elements such as carbon, hydrogen, and oxygen comprised in tissue with oxygen molecules in air. It appears that the carbonization of the skin tissue initiated at a localized surface site where TiO2 NT fragments happened to migrate due to the large amount of suspension (100 μl × 2) injected into the tumor and then to expand to neighbors. Once a part of the tumor surface is carbonized, carbonization of the tumor cells is accelerated since the carbonized tissue is an ideal absorber of the NIR light [19]. In contrast to double treatments (Figure 3(a)-(d)), the skin tissue of the tumor given a long single treatment (Figure 3(e)) was not damaged at all since there were less chance of migration of TiO2 NT fragments to the tumor surface due to the smaller amount of suspension (100 μl × 1) in the tumor, but all the tumor cells appears to have been killed since the laser treatment time was sufficiently long. This result suggests that a long single treatment with a smaller total amount of suspension is more efficient in killing tumor cells without injuring adjacent healthy cells than a multiple treatment with a larger total amount of suspension. It is also worthy of noting that all the mice employed in this experiment were alive until the time of euthanasia, suggesting that all those four parameters were properly chosen in safe ranges.
4. Conclusions
The in vitro cell test results show that the cells exposed to NIR laser without TiO2 NTs treatment have a cell viability of 97.8%. Likewise, the cells treated with TiO2 NTs but not with NIR irradiation also have a cell viability of 96.4%. Combination of these two techniques, however, shows a cell viability of 0.0%. The cell death rate strongly depends on the concentration of TiO2 NTs. Also, the cell deaths are mostly due to necrosis but partly due to late apoptosis.
According to the in vivo animal test results the photothermal destruction efficiency of tumor cells can be enhanced by increasing any of the four parameters used in this study, namely, the laser intensity, the laser exposure time, the amount of the TiO2 NTs suspension and the TiO2 NTs concentration, yet excessive increases in these parameters may result in destruction of not only the tumor tissue but also the surrounding tissue including the epidermis and subcutaneous tissue. Therefore, the exposure time should be optimized under the condition of a moderately small amount of suspension, a moderately low concentration of suspension and a moderately low laser intensity in order to destroy all the tumor cells without giving damage to surrounding healthy tissue. It is also worthy of noting that all the mice employed in this experiment were alive until the time of euthanasia, suggesting that all those four parameters were properly selected in safe ranges. These results suggest that TiO2 NTs can be used effectively as therapeutic agents for cancer thermotherapy owing to their excellent photothermal properties and high biocompatibility.
5. Acknowledgements
This work was supported by the National Research Foundation of Korea, through “the 2007 National Research Lab Program”.
REFERENCES
- V. P. Zharov, E. N. Galitovskaya, C. Johnson and T. Kelly, “Synergistic Enhancement of Selective Nanophoto Thermolysis with Gold Nanoclusters: Potential for Cancer Therapy,” Lasers in Surgery and Medicine, Vol. 37, No. 3, 2005, pp. 219-226.
- X. Huang, I. H. El-Sayed and M. A. El-Sayed, “Cancer Cell Imaging and Photothermal Therapy in the NearInfrared Region by Using Gold Nanorods,” Journal of the American Chemical Society, Vol. 128, No. 6, 2006, pp. 2115-2120.
- L. R. Hirsch, R. J. Stafford, J. A. Baukson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas, J. L. West, “Nanoshell-Mediated Near-Infrared Thermal Therapy of Tumors under Magnetic Resonance Guidance,” Proceedings of the National Academy of Sciences USA, Vol. 100, No. 23, 2003, pp. 13549-11554.
- J. Chen, B. Wiley, Z.-Y. Li, D. Campbell, F. Saeki, H. Cang, L. Au, J. Lee, X. Li and Y. Xia, “Gold Nanocages: Engineering their Structure for Biomedical Applications,” Advanced Materials, Vol. 17, No. 18, 2005, pp. 2255- 2261.
- S. Link and M. A. El-Sayed, “Shape and Size Dependence of Radiative, Non-Radiative and Photothermal Properties of Gold Nanocrystals,” International Reviews in Physical Chemistry, Vol. 19, No. 3, 2000, pp. 409-453.
- N. W. S. Kam, M. O. Connell, J. A. Wisdom and H. Dai, “Carbon Nanotubes as Multifunctional Biological Transporters and Near-Infrared Agents for Selective Cancer Cell Destruction,” Proceedings of the National Academy of Sciences USA, Vol. 102, 2005, pp. 11600-11605.
- C. Lee, H. Kim, C. Hong, M. Kim, S. S. Hong, D. H. Lee and W. I. Lee, “Porous Silicon as an Agent for Cancer Thermotherapy Based on Near-Infrared Light Irradiation,” Journal of Materials Chemistry, Vol. 18, No. 40, 2008, pp. 4790-4795.
- S. N. Goldberg, G. S. Gazelle and P. R. Mueller, “Thermal Ablation Therapy for Focal Malignancy,” American Journal of Roentgenology, Vol. 174, No. 2, 2000, pp. 323-331.
- A. Jordan, K. Maier-Hauff, P. Wust and M. Johaunsen, “Nanotechnologies for the Life Sciences” In: C. Kumar, Ed., Nanomaterials for Cancer Therapy, Wiley-VCH, Weinheim, 2006, pp. 242-258.
- C. Yao, G. Balasundaram and T. Webster, “Use of Anodized Titanium in Drug Delivery Applications,” Materials Research Society Symposium Proceedings, Boston, Vol. 951E, 2007, pp. 28-29.
- C. von Wilmowsky, S. Bauer, R. Lutz, M. Meisel, F. W. Neukam, T. Toyoshima, P. Schmuki, E. Nkenke and K. A. Schlegel, “In Vivo Evaluation of Anodic TiO2 Nanotubes: An Experimental Study in the Pig,” Journal of Biomedical Materials Research, Vol. 89B, No.1, 2009, pp. 165- 171.
- Y. T. Sul, C. B. Johansson, Y. Jeong and T. Albrektsson, “The Electrochemical Oxide Growth Behaviour on Titanium in Acid and Alkaline Electrolytes,” Medical Engineering and Physics, Vol. 23, No. 5, 2001, pp. 329-346.
- Y. M. Zhang, P. Bataillon-Linez, P. Huang, Y. M. Zhao, Y. Han, M. Traisnel, K. W. Xu and H. F. Hildebrand, “Surface Analyses of Micro-Arc Oxidized and Hydrothermally Treated Titanium and Effect on Osteoblast Behavior,” Journal of Biomedical Materials Research, Vol. 68A, No. 2, 2004, pp. 383-391.
- K. Sasaki, K. Asanuma, K. Johkura, T. Kasuga, Y. Okouchi, N. Ogiwara, S. Kubota, R. Teng, L. Cui and X. Zhao, “Ultrastructural Analysis of TiO2 Nanotubes with Photodecomposition of Water into O2 and H2 Implanted in the Nude Mouse,” Annals of Anatomy, Vol. 188, No. 2, 2006, pp. 137-142.
- J. M. Jang, S. J. Park, G. S. Choi, T. Y. Kwon and K. H. Kim, “Chemical State and Ultra-Fine Structure Analysis of Biocompatible TiO2 Nanotube-Type Oxide Film Formed on Titanium Substrate,” Metal and Materials International, Vol. 14, No. 4, 2008, pp. 457-464.
- A. Garcia-Ripoll, A. M. Amat, A. Argues, R. Vicente, M. M. Ballesteros Martin, J. A. Sanchez Perez, I. Oller and S. Malato, “Confirming Pseudomonas Putida as a Reliable Bioassay for Demonstrating Biocompatibility Enhancement by Solar Photo-Oxidative Processes of a Biorecalcitrant Effluent,” Journal of Hazadous Materials, Vol. 162, No. 2-3, 2009, pp. 1223-1227.
- V. Zwilling, M. Aucouturier and E. Darque-Ceretti, “Anodic Oxidation of Titanium and TA6V Alloy in Chromic Media. An Electrochemical Approach,” Electrochimica Acta, Vol. 45, No. 6, 1999, pp. 921-929.
- Laws and Regulations on animal Experiments, Ministry of Health & Welfare, Seoul, 19 June 2009.
- W. R. Chen, R. L. Adams, K. E. Bartels and R. E. Nordquist, “Chromophore-Enhanced in Vivo Tumor Cell Destruction Using an 808 Nm Diode Laser,” Cancer Letters, Vol. 94, No. 2, 1995, pp. 125-131.

