Journal of Cancer Therapy
Vol.1 No.1(2010), Article ID:1429,11 pages DOI:10.4236/jct.2010.11002
Adjuvant Treatment for High-Risk Operable Prostate Cancer
![]()
1AP-HP, Groupe Henri Mondor-Albert Chenevier, Service Oncologie, Créteil, France; 2Centre Hospitalier de Versailles, Hôpital André Mignot, unité Thérapeutique, Le Chesnay, France; 3UFR de Médecine Paris Ile-de-France Ouest, Université Versailles Saint Quentin en Yvelines, Guyancourt, France.
Email: bepaule@wanadoo.fr
Received December 14th, 2009; revised December 25th, 2009; accepted December 28th, 2009.
Keywords: Adjuvant Treatment, Radical Prostatectomy, High-Risk Patients
ABSTRACT
Patients who have undergone a radical prostatectomy may have to face high risks of recurrence. The risk of recurrence is elevated due to probable occult metastatic disease at the time of diagnosis. A rationale for using multimodal approach in order to minimize the chance of disease recurrence and to improve the survival of high risk patients is emerging from preclinical and clinical studies. New molecular and genetics assays, may help to select patients most likely to benefit from these approaches. In this review, we will especially discuss the potential benefits of adjuvant therapy after radical prostatectomy. This paper presents the identification of these high-risk patients; the explanation of an adjuvant treatment of residual disease after a radical prostatectomy; the clinical studies with adjuvant androgen deprivation, radiotherapy and/or chemotherapy and the microarrays analysis. This review highlights the importance of these new adjuvant treatments that aims at targeting the factor which triggers metastatic disease following a radical prostatectomy.
1. Introduction
According to the pre-operative d’Amico criteria, patients with localized prostate cancer (Pca) (PSA>20 ng/mL, Gleason 8-10, T2c to T4 disease) are considered to be at high risk, with recurrence rates ranged from 50 to 100 percent after a local therapy alone, especially if they are young, healthy and with a long life expectancy. For these patients, prostate cancer specific survival is significantly compromised [1] and surgery alone won’t be able to control the disease. Instead, these patients can show signs of residual disease at the primary site with likely persistent androgen-dependent and independent subpopulation of malignant cells. They also have high risk to develop asymptomatic or symptomatic metastases. In this case, adjuvant approach may be especially important. It is well known that, in breast or colon cancers, the use of adjuvant treatment after surgery has shown a beneficial improvement in survival [2–6]. In Pca, randomized studies are needed to evaluate the potential effect of adjuvant therapy in these high-risk patients. The optimum adjuvant management for high-risk patients after radical prostate ctomy (RP) may consist in androgen deprivation therapy (ADT), chemotherapy, prostate bed radiotherapy (RT) or some combination of these modalities.
2. Identifying High-Risk Patients
According to CaPSURE study and using D’Amico’s criteria, around 20 to 30 percent of localized prostate cancers would be at high-risk of progression [1,7] and, as well, about 30% to 35% of non metastatic prostate cancers will eventually relapse with distant disease [8]. High-risk Pca has higher biochemical relapse or disease recurrence rate after RP. Prior surgery, the identification of such aggressive cancers can be based on, at least, three well-defined predictors of the disease extent and outcome after treatment: patients with clinical stage T3 or T4 disease, a serum PSA level of above 20 ng/ml, and those with Gleason scores of 8-10 plus some 4+3 Gleason score but with negative bone scan and negative computed tomography (CT) scan of abdomen and pelvis. In addition, a number of additional clinical parameters could potentially be used to identify patients with high risk of recurrence. Those includes PSA velocity of >2.0ng/mL/ year, at least 50% positive biopsies cores or either tissue cores invaded by tumor above 20% [9,10].
D’Amico reported in 2004 a study including 1.095 patients who underwent RP and who did not receive adjuvant therapy [11]. At a median follow-up of 5.1 years, 27 of 84 deaths were attributable to prostate cancer. On multivariable analysis, preoperative PSA velocity>2ng/ ml/year was associated with an increased risk of cancer specific mortality (RR: 0.8, p<0.01). This was also an increased risk of overall mortality (RR: 0.9, p=0.01). Pretreatment Gleason score 8 to 10 correlated with an increase in cancer-specific mortality (RR: 32, p=0.02). Patients with higher clinical stage were at greater risk for death from Pca (RR: 7.4, p=<0.01) and 2.0 (p=0.004) for death from any cause.
Today, clinicians may be able to better characterize high risk patients and predict the probability of Pca recurrence for each patient through the use of several recently developed statistical models called nomograms [12, 13]. The presence of micrometastases remains a major issue since it is likely that many high-risk Pca have micrometastasized at the time of diagnosis [14–16]. Though the literature in this regard is poorly documented in prostate cancer, upcoming methods to detect those types of microscopic diseases would help to decide appropriate therapeutic strategies [17,18]. Finally, gene expression profiling of prostate carcinoma could be an alternative means to distinguish aggressive tumor. Biology and integration of gene expression signature together with clinical variables may improve the outcome prediction for patients treated with RP [19].
3. Adjuvant Treatment
Adjuvant treatment is defined as an additional therapy given in association with primary management. RP alone cannot be considered as an efficient curative treatment for locally advanced Pca, due to the high risk of regional or distant metastases and local failure [20,21]. In these conditions adjuvant treatment may be important so as to control the local and/or distant disease. Importantly systemic adjuvant therapy will not compensate for insufficient local therapy.
3.1 Rationale for Adjuvant Treatment
Clinical data and preclinical models provide a rationale for adjuvant therapy and notably for the concomitant administration of hormonal treatment and chemotherapy in prostate cancer.
1) In human prostate cancer xenografts, Craft et al. [22] have shown that prostate cancers contain heterogeneous mixture of cells that vary in their dependence on androgens for growth and survival, and that treatment with anti-androgen therapy provides a selective pressure. The latter stage of androgen independence could result from the clonal expansion of androgen-independent cells that are present at a frequency of about 1 per 105-106 androgen-dependent cells.
2) Among patients treated by RP with occult distant diseases including metastases and micrometastases, an early adjuvant hormone therapy may destroy the androgen-dependent residual tumour cells. By contrast, if the number of residual tumour cells is too important, the presence of many androgen-independent clones could make the hormone therapy ineffective and chemotherapy necessary. Pound et al. [8] observed that patients relapsing less than two years after RP had particular clinical and pathological characteristics: preoperative PSA>10 ng/ml, Gleason>7 or pT3. Survival without progression was decreased and could justify an adjuvant treatment.
3) Using Dunning R3327 rat prostatic adenocarcinoma model that creates lung metastasis on untreated recipient hosts, studies demonstrated that there was a direct relationship between primary tumor size at the time of surgical removal and the number of lung metastases [23] This concept is in favor of early treatment after local therapy such as RP. Theoretically, when the tumor burden of androgen independent cells is low, chemotherapy could be more effective. In other words, if treatment is delayed, the ability of adjuvant chemotherapy to cure the disease may be lost. These results emphasize the critical requirement of combining surgery and adjuvant chemotherapy as early as possible in the treatment of occult metastases, in order to minimize the total metastatic tumor burden and maximize the possibility of cure. In human, in recent decades, several cytotoxic agents have been tested as monotherapy in metastatic hormone refractory Pca with a certain success, at least in terms of PSA response and quality of life [24–33]. Even if these drugs are still deficient as to cure hormone refractory disease, the observed effects strongly support their significant activity on distant disease.
4) A study using the serially transplantable Dunning R-3327H rat prostatic adenocarcinoma has shown how changing the timing of androgen ablation alone and of hormone-chemotherapy can affect the tumor growth rate and host survival [34]. This study demonstrated three basic points: a) when either androgen ablation or cytoxan chemotherapy were given as a single agent treatment, they were both more effective when given as early as possible; b) when androgen ablation was combined with cytoxan chemotherapy, it was more effective when both therapies were begun simultaneously and as early as possible; and c) when androgen ablation and cytoxan treatments were initiated simultaneously and early, it was possible to increase survival as compared with the groups who were given one of the two therapies alone (i.e., such simultaneous early treatment enhanced the individual therapeutic effectiveness of both treatments).
5) Preclinical data evaluating the optimal timing and combination of androgen deprivating therapy (ADT) in LNCaP and Shionogi prostate cancer xenografts reported that the mice that received simultaneous hormone-chemotherapy had a significant improvement in time to treatment failure compared to sequential therapy. A marked lack of response to castration was observed after initial paclitaxel therapy. Moreover, transcriptional profiling identified, after paclitaxel exposure, an increased expression of several survival genes known to play a role in androgen independence [35]. These findings supported simultaneous chemohormonal therapy-adjuvant trials.
3.2 Adjuvant Post RP Treatment
3.2.1 Adjuvant Hormone Therapy (HT)
Messing et al. have [36] demonstrated that adjuvant HT significantly improves survival in patients with positive lymph nodes. Data were updated [37] regarding the use of immediate versus deferred ADT in patients found to have node-positive disease at the time of PR. At a median follow-up of 11.9 years, the survival results remain unchanged. The median survival for the immediate and differed ADT arms, was 13.9 years (2.1-14.5) and 11.3 years (1.3-14.2) respectively. The median disease specific survival has not been reached in the immediate arm yet (2.1-14.5 years) and in the differed arm, it was 12.3 years (1.3-14.2; p: 0.0004). The data continue to support the use of ADT in node-positive disease but it is unknown whether ADT improves overall survival in high-risk patients with negative lymph node. Mc Leod et al. [38] have recently published the preliminary results of a large trial evaluating the efficacy and the tolerability of bicatulamide (150 mg daily) as adjuvant therapy after PR or RT in patients with locally advanced prostate cancer. A total of 8.133 patients were recruited for this placebo-controlled double-blinded randomized study. With a median follow-up of 7.4 years, bicalutamide significantly reduced the risk of objective progression compared to placebo (HR: 0.75; IC 95%: 0.61-0.91; p: 0.004). There was no statistically significant difference between the two groups in terms of overall survival after RP (HR: 1.09; IC 95%: 0.85-1.39; p: 0.51). Again, in men with locally advanced prostate cancer, by 5 years follow-up, the Study by the Scandinavian Prostate Group suggests benefits in terms of progression free survival (PFS) of adding bicalutamide to RP, RT or watchfull waiting [39]. In contrast, bicalutamide provides no benefit in patients with localized prostate cancer but rather may decrease PFS.
In addition, the optimal duration of the hormonal therapy remains to be established. Adjuvant therapy has been used for duration of 2 years or 3 years in phase III trials in men with high-risk disease and the question of adverse effects should be considered in this setting.
3.2.2 Adjuvant Radiotherapy (RT)
Two large randomized trials, European Organization for research and Treatment of cancer (EORTC) trial 22911 and Southwest Oncology Group (SWOG) trial 8794, have reported on the beneficial outcome of adjuvant RT in patients with pathological risk factors after RP. The EORTC 22911 trial showed that adjuvant RT (60 Gy) was associated with improvement in biochemical progression free survival (74% versus 52.6% ; p<0.0001) [40] but the impact on overall survival awaits maturation on the data. In the South Western Oncology Group (SWOG) trial 8794 [41] which randomized 425 high-risk patients to adjuvant RT after RP versus RP alone, no benefit in terms of overall survival was observed in patients assigned to the adjuvant group. It was shown that adjuvant radiation reduced the risk of biochemical treatment failure by 50% over RP alone. High-risk was defined as extracapsular tumor extension, positive surgical margins, or seminal vesicle involvement. To be eligible, patients had to have histologically negative lymph nodes and a negative bone scan. Adjuvant radiation to the prostate bed (60 to 64 Gy) also seemed to reduce the risk of metastatic disease and biochemical failure at all postsurgical PSA levels [42]. Of note, in this study, the pattern of treatment failure in high-risk patients was predominantly local with a surprisingly low incidence of metastatic failure. SWOG 8794 and EORTC 22911 convincingly showed that the primary risk of treatment failure was local, suggesting that the adjuvant studies treating these patients solely with systemic therapy might have limited benefits. Additionally, offering adjuvant irradiation to all patients with pT3 disease could result in overtreatment for a number of patients, as it was exemplified by the fact that in the observation arm of the EORTC and SWOG studies, respectively 52.6% and 38% of patients did not show any biochemical relapse. Therefore a better definition of highrisk groups is necessary to reduce the overtreatment rate of RT, side effects and care costs.
Wiegel et al. [43], in their preliminary evaluation of a Phase III study comparing RP followed by RT (60 Gy) with RP alone in patients with pT3 disease, have reported a significant improvement of relapse-free survival among patients receiving adjuvant therapy compared to the control arm; particularly in patients with a preoperative PSA> 10 ng/ml, pT3b and Gleason 8 as well as positive margins.
3.2.3 Adjuvant RT and Andogen Deprivation Therapy after RP
RADICALS is a large international Phase III randomized controlled trial addressing the RT to the tumoral bed (66 Gy) and ADT after RP [44]. The first randomization, performed within the 3 months after RP (the RT timing), consists in randomizing patients to immediate RT versus salvage RT. The second randomization is performed before giving RT (RT duration hormonal therapy) between no HT, short-term HT (6 months duration) and long-term HT (24 months). The primary end point will be the cancer-specific survival (CCS), the secondary end point will be overall survival. Especially, The RADICALS trial is designed to identify treatment options that could achieve an absolute increase in 10-years CCS of =>5%. 2600 patients in the RT timing randomization could detect an increase in 10-years CCS from 70 to 75% with 80% power, or from 80 to 85% with 90% power and 5% significant level. 3500 patients would be required for the HT duration randomization. The patients who have a PSA level after RP<0.2 ng/ml with some risk factors for disease recurrence that is pT3, positive margins, Gleason score>6, pre-operative PSA level of >10 ng/ml or a combination of these criteria will be included.
3.2.4 Adjuvant Chemotherapy:
In men with metastatic hormone-refractory Pca, two Phase III trials have demonstrated that chemotherapy based on taxotere is superior to that based on mitoxantrone plus prednisone [45,46]. Patients receiving docetaxel-based chemotherapy had a longer progression-free survival and a better quality of life. They improved overall survival (2 months benefit) as compared with mitoxantrone groups. Overall, the use of Docetaxel is now a standard for men with hormone refractory disease. Conversely, adjuvant chemotherapy is not standard for high risk patients after RP. Should adjuvant chemotherapy be administered? Is chemotherapy the next step? [47] These questions need to be addressed in specific trials.
Schmidt et al. [48,49] from the National Prostate Cancer Group randomly assigned 184 patients with localized advanced prostate cancer to one of the three following arms: 2 years of oral cyclophosphamide, estramustinephosphate for 2 years versus observation. After 10 years of follow-up, the estramustine-phosphate group had an improvement in relapse-free survival but there was no difference in overall survival.
In patients at high-risk for occult distant disease following RP, a phase II trial of adjuvant docetaxel was performed [50]. Treatment consisted in 6 cycles of 35 mg/m2 docetaxel weekly given from 4 to 12 weeks following RP. At a median follow-up of 29.2 months (range 1.6 to 39.2), 26 of 46 evaluable patients (60.5%) relapsed. The observed median PFS was 15.7 months (95% CI: 12.8-25.1). This PFS is longer than the normal predicted 10 months for these patients, and adjuvant docetaxel had significant but acceptable toxicity in high-risk patients. Grade III toxicity occurred in 20 patients (26%) including dyspnea in 4, fatigue and cardiac arrhythmia in 3 and diarrhea, dizziness, nausea, acute vascular leak syndrome and hyperglycemia in 2. The incidence of Grade IV toxicity was relatively low and appeared in 3 patients. Seven patients died including 4 of prostate cancer, 1 with intra-abdominal bleeding during treatment and 2 of pneumonia and sudden cardiac deaths following treatment.
The Veterans Affairs Cooperative Study 553 [51] has been designed to prospectively evaluate the efficacy of early adjuvant chemotherapy, using docetaxel and prednisone added to the standard of care (i.e., surveillance with the addition of androgen deprivation at the time of biochemical relapse) for patients, who are at high risk for relapse after RP. Patients “veterans”, are stratified for PSA, Gleason score, tumor stage, and the presence of positive margins. A planned 636 patients will be accrued and randomized to one of two treatment arms: docetaxel plus prednisone administred every 3 weeks for 18 weeks or surveillance alone. Patients will then be followed for a minimum of 1 yr and a maximum of 5 yr. The study is designed with 90% power to detect a reduction in the 5-year progression rate from 60% to 45% (15% absolute difference, 25% relative difference). The estimated study Completion Date is June 2011.
3.2.5 Ajuvant Chemohormonal Therapy:
Recent neoadjuvant studies have indicated that combining hormonal and chemotherapy is feasible and safe (for review see ref [52]). Although the impact of chemotherapy on survival need to be proved in randomized trials, it is interesting to note that neoadjuvant studies, wherein hormonal therapy was not included, consistently showed declines in preoperative PSA level, ranging from 20% to 60% after chemotherapy. This indicated the likelihood of an antitumoral effect of these drugs in high risk patients irrespective of hormonal treatment [53–56].
In their study, Pummer et al. [57] evaluated whether patients with previously untreated advanced Pca benefit from combining total ADT with weekly epirubicin chemotherapy Patients with either metastatic (n=117) or locally advanced (n=28) were randomly allocated to treatment with ADT by bilateral orchiectomy and flutamide 250 mg or ADT plus weekly epirubicin 25 mg/m2 i.v. for 18 weeks. At a median follow-up of 81 months, progression-free survival and overall survival in the ADT and E-ADT groups were 12 and 18 months (p<0.02) and 22 and 30 months respectively (p=0.12). Subjective quality of life assessment showed no impairment of quality of life by epirubicin treatment. Objective toxicities were generally mild with either treatment. The authors concluded that the combination of ADT and epirubicin was well tolerated by patients with advanced Pca and resulted in a significant extension of progression-free survival.
Wang et al. [58] have randomly assigned 96 patients with clinical T3 or T4 disease or metastatic disease to mitoxantrone plus combined anfrogen bockade versus combined androgen blockade alone. In the 38 patients without metastatic disease, a higher initial objective response (95% versus 53%; p=0.0008) and median survival (80 versus 36 months; p=0.04) were observed in patients healed with mitoxantrone plus combined androgen ablation.
In a prospective randomized Phase II study (trial PR005) [59] of adjuvant paclitaxel and ADT versus ADT alone, 47 patients with high-risk Pca were randomized after PR between paclitaxel 100 mg/m2 once a week for 8 weeks and ADT for 3 years versus ADT for 3 years. The mean age was 58 year-old [51–67], the mean PSA concentration and Gleason score were respectively 18ng/ ml and 7.4 [7–9]. Toxicity, quality of life and functional results were compared between the two arms. With a mean follow up of 36 months, 23 patients receiving paclitaxel and ADT were evaluated. Toxicity and side effets were assessed using the National Cancer Institute’s Common Toxicity Criteria (version.2). Alopecia was observed in 100% of the cases. No hematologic toxicity was noted. 4 patients had neurological disorders in fingers (86% of grade I), 2 patients had nausea and/or vomiting disorders (grade I and II), 2 had asthenia (grade I) and one patient developed cardiac insufficiency not due to chemotherapy. One grade III febrile neutropenia was reported. These preliminary results indicated that adjuvant paclitaxel-based chemotherapy associated with ADT was a safe and well tolerated approach.
4. Local Control and Systemic Therapy
The effect of RT on survival should be considered in the context of systemic therapy, which is thought to be effective against distant disease. In breast cancer, adjuvant systemic therapy reduces the likelihood of both local and distant recurrence. A subgroup analysis in the EBCTCG metanalysis of local therapy showed that the use of RT after mastectomy in node-positive patients improved 15-year survival only in patients who also received adjuvant systemic therapy and not in patients who were treated with mastectomy alone [3]. In high-risk patients for distant metastases, such as women with positive lymph nodes, RT in the absence of systemic therapy can improve survival only in the rare patients with residual local disease who have no distant dissemination. In contrast, in node-positive patients treated with mastectomy and adjuvant systemic therapy, RT will potentially contribute to survival in patients in whom systemic therapy eradicates microscopic metastases but not residual local disease.
What is also pertinent for this review is recent evidence that, in men experiencing an increasing PSA after their primary local treatments (RP or RT), docetaxel -based systemic therapy administred every three weeks was able to reduce PSA level [60]. Authors reported a decreased > or =50% in 17 of 35 patients (48.5%) and > or =75% in seven of 35 patients (20%) with docetaxel. Again, this demonstrated the activity of chemotherapy against prostate cancer cells. In this study, chemotherapy (for up to 6 cycles) was followed by hormone therapy. In five of 33 men, the PSA remains at 0.1 ng/mL at a median of 18.9 months. Herein, it is further interesting to note that three of these five men had soft tissue metastasis at entry but remain in complete remission.
The influence of RT on local control emphazise the need in high-risk patients with Pca:
1) To select patients with high-risk of residual disease after PR (pathological margin).
In stage I or II breast cancer treated with breast-conserving surgery and RT, the patients with close margins and those with negative margins both have a rate of local recurrence (LR) of 7%. It is interesting to note that women with extensively positive margins have an LR of 27%, whereas patients with focally positive margins had an intermediate rate of LR of 14%. The use of systemic therapy adds significant effect on the risk-ratio of LR [61]. In Pca, data from the Mayo clinic indicate that 76% of patients with no positive surgical margin and 65% of patients with a single-positive margin after RP remain biochemically and clinically free from disease by 5 years; 62% with two or more positive margins had no evidence of disease by 5 years [62]. SWOG 8794 and EORTC 22911 showed evidence that the primary risk of treatment failure was local. In recent reports of EORTC 22911 using the grading, staging and surgical margins status determined by a central pathology review, it was shown that the margin status was a stronger predictor for the magnitude of the treatment benefit [63,64]. Thus, while the 5-year biochemical PFS rates were 67.4% (95% CI: 56.1% to 76.3%) and 76.2% (95% CI: 66.1% to 83.6%) for patients with negative margins in the control versus irradiation arm, they were 77.6% (95% CI: 68.8% to 84.2%) and 48.5% (95% CI: 39.6% to 58.9 %) for the patients with positive surgical margin in the control versus irradiation arm [61]. Treatment failure is now documented to be primarily in the area of the prostate fossa, and adjuvant radiation reduces both biochemical and clinical local recurrence (22% versus 8%). In the observation arm of the EORTC study, the rate of clinical local treatment failure was four times the rate of systemic failure. In view of these data, patients with high-risk prostate cancer after PR should be given adjuvant RT as standard treatment [42] however immediate postoperative RT might not be recommended for Pca patients with negative surgical margins.
2) To identify the patients in whom the influence of local RT on mortality will be reduced and possibly eliminated with systemic therapy.
In breast cancer, Marks et al. [65] suggest that benefits of local therapy on survival has an inverted-U-shaped or parabolic relationship with increasing effective systemic therapy, so that the survival benefit derived from better local therapy increases with increasing effective therapy but only to a certain threshold of effectiveness and then declines. In the SWOG study, in the observation group, the rate of clinical local failure was 24% versus 16% distant metastases. By improving the local control, adjuvant RT was associated with a reduction in the proportion of patients with metastases (16 to 7%). Based on the above mentioned observations, these data also suggest that offering these patients systemic therapy could lead to limited improvement in survival [42]. The fact remains that reduction in local recurrence with systemic therapy with and without RT must be reported in further studies. The new EORTC trial 22043-30041 aims at recruiting 600 patients with a PSA level of =<0.2 ng/ml after RP and testing the value of adding 6 months of ADT to RT. The RTOG 0534 trial in patients with PSA level of 0.2-2 ng/ml after RP will compare treatment with adjuvant RT alone or with additional 5 months of ADT or similar ADT with pelvic RT.
5. Clinical Studies with Adjuvant ADT and/or Chemotherapy
The role of adjuvant therapy in the high-risk population following RP needs systematic study. From experience in colon and breast cancer, active agents in the metastatic setting seem to be more beneficial when used in patients with earlier stage disease. Prospects for long-term survival following surgical treatment of localized breast cancer have significantly improved with the widespread use of adjuvant systemic chemotherapy. According to the uptaded 2005, Early Breast cancer Trialist collaborative meta-analysis, combination chemotherapy was associated with an approximate 23% reduction in the risk of breast cancer recurrence. The greatest absolute benefit was noticed in younger women with lymph node–positive disease. In absolute terms, a 5-year course’ of a selective oestrogen receptor modulator tamoxifen for patients with oestrogen receptor-positive tumors reduces the annual breast cancer death rate by 31% largely irrespective of chemotherapy [3].
As with the use of adjuvant treatment in breast cancer, three randomized studies were planned to investigate chemohormonal adjuvant setting in patients considered to be at high-risk Pca after RP.
SWOG 9921 was intiated but it closed prematurely in January 2007 (Table 1). The primary endpoint of this trial was initially overall survival. All patients should have received 2 years of combined ADT using goserelin and bicalutamide, and half should have been treated with 6 cycles of 12 mg/m2 mitoxantrone plus 5 mg prednisone twice daily. RT to the prostate was allowed. Study accrual was held when three cases of leukemia (AML) were reported in the mitoxantrone-containing arm. Therefore, all patients in the chemotherapy-containing arm stopped taking mitoxantrone. Although this phase III has stopped, 2 years of ADT will be continued in active patients in both arms and it may be possible to obtain precious information given the high number of patients included.
A second Phase III TAX 3501 aimed at using docetaxel as an adjuvant treatment for high-risk disease with an accrual goal of 2172 patients (Table 2). The primary endpoint was PFS. Unfortunately, this study failed to meet its accrual target and was closed very recently. The design was a 4 arms randomization to immediate versus delayed therapy with ADT plus or without chemotherapy. This trial was designed to provide valuable information on both hormonal therapy and chemotherapy in the adjuvant setting. In particular, it would have helped to define the optimal timing in adjuvant therapy among immediate postoperative period and delayed when increasing PSA was detected [66]. Of interest is that an important number of men were accrued prior to closure, and useful data on their outcomes might be possible.
A Phase II randomized trial PR005 aims at using paclitaxel as an adjuvant for high-risk disease (Table 3) [59]. All patients will receive 4 years of ADT using LH-RH agonist for three years and bicalutamide 50 mg for 1 month, and half will be treated with 8 weekly cycles of 100 mg/m2 paclitaxel. An approach to maximizing tumor-cell death with adjuvant chemotherapy is to use optimal doses of active chemotherapy drugs administered sequentially with a shortened scheduling interval. This approach, called “dose-dense,” increases dose intensity (drug delivery over time) by reducing the intertreatment interval for chemotherapy delivery. A number of preclinical studies suggest that continuous dosing of chemotherapy with a very short interdose interval, so

Table 1. SWOG 9921 adjuvant trial in patients with Pca at high risk after RP
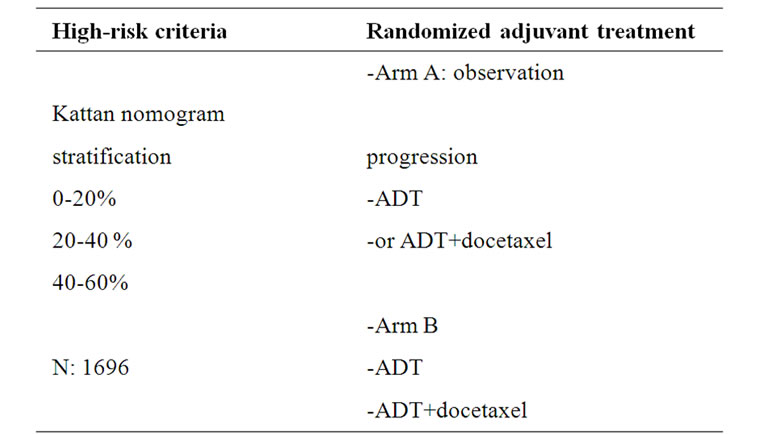
Table 2. TAX3501 adjuvant trial in patients with Pca at high risk after RP

Table 3. PROO5 adjuvant trial in patients with Pca at high risk after RP
called “metronomic” scheduling, may enhance the antiangiogenic effects of chemotherapy in breast cancer [66–71]. This current study tests a sequential dose-dense of weekly administration of paclitaxel (cumulative dose: 800 mg/m2) for which the feasibility and efficacy has been assessed previously [26,27]. In this study, frozen prostate tissue will be obtained from men undergoing RP who are enrolled in either the treatment or the control arms of the trial. These samples will be analyzed for their mRNA level expression patterns in an attempt to draw up outcome prediction models. Likewise, from these array-based methods of expression analysis, it would be ideal if we could predict the sensitivity to chemotherapeutic agents and the response to chemotherapy.
6. Microarrays Analysis
Microarrays analysis has been used to characterize the molecular profiles of breast cancer [72–74]. Important advances are being made in the use of genetic analysis to determine the risk of recurrence and to predict a tumor’s responsiveness to adjuvant chemotherapy or tamoxifen in breast cancer [75–79]. These approaches may be informative to determine high-risk Pca for local recurrence or distant recurrence. Given that initiation and progression of Pca involve multiple changes in gene expression, cDNA microarray technology has been recently used to identify disease-related gene expression patterns in prostate samples [80–82] This approach has successfully detected alterations in several candidate genes associated with Pca progression [83,84]. However, there is not any definitive molecular classification that can consistently and reliably predict the clinical behaviour of Pca yet. Nevertheless, gene expression profiling offers an alternative means to distinguish aggressive tumor biology and may improve the accuracy of outcome prediction for patients with Pca treated by RP [14,85,86]. Interestingly, Lapointe et al. [87], so as to further characterize the clinical relevance of tumor subtypes identified from a gene expression profiling, used immunohistochemistry on tissue microarrays in an independent set of 225 prostate tumors in order to assess two genes as surrogate markers, namely MUC1 and AZGP1, differentially expressed among indentified tumor subgroups. MUC1 and AZGP1were found to be strong predictor of tumor recurrence. In Kaplan-Meier survival analysis, positive MUC1 staining was associated with significantly shorter time to recurrence, while strong immunostaining of AZGP1 was associated with significantly prolonged time to recurrence. Importantly, these genes were found, in multivariate analysis, as additional prognostic information over and above the known risk factors of tumor grade, stage, and preoperative PSA. These genes also provided independent prognostic value, suggesting that using two genes improves the accuracy of tumor subtyping and prognostication.
Glinsky et al. [88] reported a Pca recurrence predictor algorithm that appeared suitable for stratification of patients at the time of diagnosis into poor-and good-prognosis subsets, this, with a statistically significant difference in the disease-free survival after RP. It could provide additional predictive value over conventional prognostic factors such as PSA level and Gleason sum. Tomkins et al. [89] reported that a majority of Pca exhibit fusions between the control region of an androgen regulated gene TMPRSS2 and the coding region of the ETS family of transcription factors, most frequently ERG and much less frequently ETV1 and ETV4. The fusions are associated with an increased risk of cancer progression in patients treated surgically [90,91]. Recently, a fourvariable model predictive of cancer-specific outcome incorporate gene expression of topoisomerase-2a, cadherine-10, the fusion status based ERG, ETV1 and ETVA expression and the aneuploïd status in men with highgrade Pca treated with RP was established [92]. The trial POO5 has used gene expression profiling to define subgroups of high-risk Pca associated with good or poor outcome. The refined gene-expression signature associated with metastases contained three upregulated and thirty down regulated gene (Personal communication).
Collectively, these data illustrate the potential helpfulness of expression profiling in the identification of high-risk patients as well as in the development of new biological markers and prognostic markers.
7. Conclusions
Patients with high-risk Pca after RP should be offered adjuvant RT as standard treatment. However, a policy of adjuvant RT would result in significant overtreatment: in the observation arm of the EORTC and SWOG studies, 52.6% and 38% of patients did not show any biochemical relapse. Thus, a better definition of high-risk groups is warranted to reduce the overtreatment rate of RT and to reduce the side effects and cost of this adjuvant treatment. An alternative approach to reduce the number of treated patients might be to identify subsets of patients who may significantly benefit from immediate post operative RT. Further improved in local control with adjuvant systemic treatment would also enable better results. Accumulating clinical and preclinical data suggests that the use of early HT will improve the outcome in patients with high-risk localized Pca. Yet, two randomized Phase III trials evaluating the effect of adjuvant hormonal therapy with or without chemotherapy in high-risk patients after RP have been prematurely closed. Another problem is to better distinguish diseases that will be cured with local treatement only, from those that will require an adjuvant approach. Gene expression profiling of Pca offers an alternative means to distinguish aggressive tumor biology and may improve the accuracy of outcome prediction for patients with Pca treated by RP.
REFERENCES
- A. V. D'Amico, R. Whittington, S. B. Malkowicz, et al., “Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer,” Journal of the American Medical Association, Vol. 280, pp. 969–974, 1998.
- “Adjuvant systemic therapy for women with node-positive breast cancer,” The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer, Canadian Medical Association Journal, Vol. 158, Supplement 3, pp. S52–64, 1998.
- “Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials,” Early Breast Cancer Trialists’ Collaborative Group, Lancet, Vol. 365, pp. 1687–1717, 2005.
- C. G. Moertel, T. R. Fleming, J. S. Macdonald, et al., “Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma,” New England Journal of Medicine, Vol. 322, pp. 352–358, 1990.
- T. Andre, C. Boni, L. Mounedji-Boudiaf, et al., “Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer,” New England Journal of Medicine, Vol. 350, pp. 2343–2351, 2004.
- C. Twelves, A. Wong, M. P. Nowacki, et al., “Capecitabine as adjuvant treatment for stage III colon cancer,” New England Journal of Medicine, Vol. 352, pp. 2696– 2704, 2005.
- D. P. Lubeck, M. S. Litwin, J. M. Henning, et al., “The CaPSURE database: A methodology for clinical practice and research in prostate cancer,” CaPSURE Research Panel, Cancer of the Prostate Strategic Urologic Research Endeavor, Urology, Vol. 48, pp. 773–777, 1996.
- C. R. Pound, A. W. Partin, M. A. Eisenberger, D. W. Chan, J. D. Pearson, and P. C. Walsh, “Natural history of progression after PSA elevation following radical prostatectomy,” Journal of the American Medical Association, Vol. 281, pp. 1591–1597, 1999.
- S. J. Freedland, E. B. Humphreys, L. A. Mangold, et al., “Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy,” Journal of the American Medical Association, Vol. 294, pp. 433– 439, 2005.
- V. Ravery, C. Chastang, M. Toublanc, L. Boccon-Gibod, V. Delmas, and L. Boccon-Gibod, “Percentage of cancer on biopsy cores accurately predicts extracapsular extension and biochemical relapse after radical prostatectomy for T1-T2 prostate cancer,” European Urology, Vol. 37, pp. 449–455, 2000.
- A. V. D'Amico, M. H. Chen, K. A. Roehl, and W. J. Catalona, “Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy,” New England Journal of Medicine, Vol. 351, pp. 125–135, 2004.
- M. W. Kattan, J. A. Eastham, A. M. Stapleton, T. M. Wheeler, and P. T. Scardino, “A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer,” Journal of the National Cancer Institute, Vol. 90, pp. 766–771, 1998.
- M. W. Kattan, T. M. Wheeler, and P. T. Scardino, “Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer,” Journal of Clinical Oncology, Vol. 17, pp. 1499–1507, 1999.
- D. Weckermann, P. Muller, F. Wawroschek, G. Krawczak, G. Riethmuller, and G. Schlimok, “Micrometastases of bone marrow in localized prostate cancer: Correlation with established risk factors,” Journal of Clinical Oncology, Vol. 17, pp. 3438–3443, 1999.
- P. B. Morgan, A. L. Hanlon, E. M. Horwitz, M. K. Buyyounouski, R. G. Uzzo, and A. Pollack, “Timing of biochemical failure and distant metastatic disease for low-, intermediate, and high-risk prostate cancer after radiotherapy,” Cancer, Vol. 110, pp. 68–80, 2007.
- H. Miyake, T. Kurahashi, I. Hara, A. Takenaka, and M. Fujisawa, “Significance of micrometastases in pelvic lymph nodes detected by real-time reverse transcriptase polymerase chain reaction in patients with clinically localized prostate cancer undergoing radical prostatectomy after neoadjuvant hormonal therapy,” British Journal of Urology International, Vol. 99, pp. 315–320, 2007.
- M. Fukuda, M. Egawa, T. Imao, H. Takashima, K. Yokoyama, and M. Namiki, “Detection of sentinel node micrometastasis by step section and immunohistochemistry in patients with prostate cancer,” Journal of Urology, Vol. 177, pp. 1313–1317, discussion 1317, 2007.
- S. F. Shariat, M. P. Roudier, G. E. Wilcox, et al., “Comparison of immunohistochemistry with reverse transcription-PCR for the detection of micrometastatic prostate cancer in lymph nodes,” Cancer Research, Vol. 63, pp. 4662–4670, 2003.
- A. J. Stephenson, A. Smith, M. W. Kattan, et al., “Integration of gene expression profiling and clinical variables to predict prostate carcinoma recurrence after radical prostatectomy,” Cancer, Vol. 104, pp. 290–298, 2005.
- M. Ohori, J. R. Goad, T. M. Wheeler, J. A. Eastham, T. C. Thompson, and P. T. Scardino, “Can radical prostatectomy alter the progression of poorly differentiated prostate cancer?” Journal of Urology, Vol. 152, pp. 1843– 1849, 1994.
- H. Van Poppel, H. Goethuys, P. Callewaert, L. Vanuytsel, W. Van de Voorde, and L. Baert, “Radical prostatectomy can provide a cure for well-selected clinical stage T3 prostate cancer,” European Urology, Vol. 38, pp. 372– 379, 2000.
- N. Craft, C. Chhor, C. Tran, et al., “Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process,” Cancer Research, Vol. 59, pp. 5030–5036, 1999.
- J. T. Isaacs, “Relationship between tumor size and curability of prostatic cancer by combined chemo-hormonal therapy in rats,” Cancer Research, Vol. 49, pp. 6290– 6294, 1989.
- D. Friedland, J. Cohen, and R. Miller, Jr., et al., “A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: Correlation of antitumor effect to phosphorylation of Bcl-2,” Seminars in Oncology, Vol. 26, pp. 19–23, 1999.
- W. Berry, S. Dakhil, M. A. Gregurich, and L. Asmar, “Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate,” Seminars in Oncology, Vol. 28, pp. 8–15, 2001.
- B. J. Roth, B. Y. Yeap, G. Wilding, B. Kasimis, D. McLeod, and P. J. Loehrer, “Taxol in advanced, hormone-refractory carcinoma of the prostate. A phase II trial of the Eastern Cooperative Oncology Group,” Cancer, Vol. 72, pp. 2457–2460, 1993.
- C. Trivedi, B. Redman, L. E. Flaherty, et al., “Weekly 1-hour infusion of paclitaxel. Clinical feasibility and efficacy in patients with hormone-refractory prostate carcinoma,” Cancer, Vol. 89, pp. 431–436, 2000.
- I. F. Tannock, D. Osoba, M. R. Stockler, et al., “Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points,” Journal of Clinical Oncology, Vol. 14, pp. 1756–1764, 1996.
- P. W. Kantoff, S. Halabi, M. Conaway, et al., “Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: Results of the cancer and leukemia group B 9182 study,” Journal of Clinical Oncology, Vol. 17, pp. 2506–2513, 1999.
- R. Morant, S. F. Hsu Schmitz, J. Bernhard, et al., “Vinorelbine in androgen-independent metastatic prostatic carcinoma–a phase II study,” European Journal of Cancer, Vol. 38, pp. 1626–1632, 2002.
- S. Oudard, A. Caty, Y. Humblet, et al., “Phase II study of vinorelbine in patients with androgen-independent prostate cancer,” Annals of Oncology, Vol. 12, pp. 847–852, 2001.
- K. P. Delaere, H. Leliefeld, F. Peulen, E. W. Stapper, J. Smeets, and J. Wils, “Phase II study of epirubicin in advanced hormone-resistant prostatic carcinoma,” British Journal of Urology, Vol. 70, pp. 641–642, 1992.
- R. Petrioli, A. I. Fiaschi, D. Pozzessere, et al., “Weekly epirubicin in patients with hormone-resistant prostate cancer,” British Journal of Cancer, Vol. 87, pp. 720–725, 2002.
- J. T. Isaacs, “The timing of androgen ablation therapy and/or chemotherapy in the treatment of prostatic cancer,” Prostate, Vol. 5, pp. 1–17, 1984.
- B. J. Eigl, S. E. Eggener, J. Baybik, et al., “Timing is everything: Preclinical evidence supporting simultaneous rather than sequential chemohormonal therapy for prostate cancer,” Clinical Cancer Research, Vol. 11, pp. 4905– 4911, 2005.
- E. M. Messing, J. Manola, M. Sarosdy, G. Wilding, E. D. Crawford, and D. Trump, “Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer,” New England Journal of Medicine, Vol. 341, pp. 1781–1788, 1999.
- E. M. Messing, J. Manola, J. Yao, et al., “Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy,” Lancet Oncology, Vol. 7, pp. 472–479, 2006.
- D. G. McLeod, P. Iversen, W. A. See, T. Morris, J. Armstrong, and M. P. Wirth, “Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer,” British Journal of Urology International, Vol. 97, pp. 247–254, 2006.
- P. Iversen, J. E. Johansson, P. Lodding, et al., “Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6,” Journal of Urology, Vol. 172, pp. 1871–1876, 2004.
- M. Bolla, H. van Poppel, L. Collette, et al., “Postoperative radiotherapy after radical prostatectomy: A randomised controlled trial (EORTC trial 22911),” Lancet, Vol. 366, pp. 572–578, 2005.
- I. M. Thompson, Jr., C. M. Tangen, J. Paradelo, et al., “Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial,” Journal of the American Medical Association, Vol. 296, pp. 2329–2335, 2006.
- G. P. Swanson, M. A. Hussey, C. M. Tangen, et al., “Predominant treatment failure in postprostatectomy patients is local: Analysis of patterns of treatment failure in SWOG 8794,” Journal of Clinic Oncology, Vol. 25, pp. 2225–2229, 2007.
- T. Wiegel, O. Bottke, N. Willich, et al., “Phase III results of adjuvant radiotherapy versus wait-and-see in patients with pT3 prostate cancer following radical prostatectomy (RO 96-02/AUO AP 09/05),” Journal of Clinic Oncology, Annual Meeting Proceedings Part 1, Vol. 25, 18S (June 20, Supplement), pp. 5060, 2007.
- C. Parker, M. R. Sydes, C. Catton, et al., “Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): A new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy,” British Journal of Urology International, Vol. 99, pp. 1376–1379, 2007.
- D. P. Petrylak, C. M. Tangen, M. H. Hussain, et al., “Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer,” New England Journal of Medicine, Vol. 351, pp. 1513–1520, 2004.
- I. F. Tannock, R. de Wit, W. R. Berry, et al., “Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer,” New England Journal of Medicine, Vol. 351, pp. 1502–1512, 2004.
- W. K. Oh and P. W. Kantoff, “Treatment of locally advanced prostate cancer: Is chemotherapy the next step?” Journal of Clinic Oncology, Vol. 17, pp. 3664–3675, 1999.
- J. D. Schmidt, R. P. Gibbons, G. P. Murphy, and A. Bartolucci, “Adjuvant therapy for clinical localized prostate cancer treated with surgery or irradiation,” European Urology, Vol. 29, pp. 425–433, 1996.
- J. D. Schmidt, R. P. Gibbons, G. P. Murphy, and A. Bartolucci, “Evaluation of adjuvant estramustine phosphate, cyclophosphamide, and observation only for node-positive patients following radical prostatectomy and definitive irradiation,” Investigators of the National Prostate Cancer Project, Prostate, Vol. 28, pp. 51–57, 1996.
- A. S. Kibel, E. Rosenbaum, M. W. Kattan, et al., “Adjuvant weekly docetaxel for patients with high risk prostate cancer after radical prostatectomy: A multiinstitutional pilot study,” Journal of Urology, Vol. 177, pp. 1777–1781, 2007.
- U. S. National Insititutes of Health Reference CSP #553 chemotherapy after prostatectomy for high risk prostate carcinoma. Available from URL: http://www. clinicaltrials. gov.
- M. Gleave and W. K. Kelly, “High-risk localized prostate cancer: A case for early chemotherapy,” Journal of Clinic Oncology, Vol. 23, pp. 8186–8191, 2005.
- R. Dreicer, C. Magi-Galluzzi, M. Zhou, et al., “Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer,” Urology, Vol. 63, pp. 1138–1142, 2004.
- T. M. Beer, M. Garzotto, B. A. Lowe, et al., “Phase I study of weekly mitoxantrone and docetaxel before prostatectomy in patients with high-risk localized prostate cancer,” Clinic Cancer Research, Vol. 10, pp. 1306–1311, 2004.
- W. K. Oh, D. J. George, D. S. Kaufman, et al., “Neoadjuvant docetaxel followed by radical prostatectomy in patients with high-risk localized prostate cancer: A preliminary report,” Seminars Oncology, Vol. 28, pp. 40–44, 2001.
- P. G. Febbo, J. P. Richie, D. J. George, et al., “Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer,” Clinic Cancer Research, Vol. 11, pp. 5233–5240, 2005.
- K. Pummer, M. Lehnert, H. Stettner, and G. Hubmer, “Randomized comparison of total androgen blockade alone versus combined with weekly epirubicin in advanced prostate cancer,” European Urology, Vol. 32, Supplement 3, pp. 81–85, 1997.
- J. Wang, S. Halford, A. Rigg, R. Roylance, M. Lynch, and J. Waxman, “Adjuvant mitozantrone chemotherapy in advanced prostate cancer,” British Journal of Urology International, Vol. 86, pp. 675–680, 2000.
- G. Ploussard, B. Paule, L. Salomon, Y. Allory, S. Terry, D. Vordos, A. Hoznek, F. Vacherot, C. C. Abbou, S. Culine, and A. de la Taille, “Pilot trial of adjuvant paclitaxel plus androgen deprivation for patients with high-risk prostate cancer after radical prostatectomy: Results on toxicity, side effects and quality-of-life,” Prostate Cancer and Prostatic Diseases, pp. 1–5, 2009.
- A. Hussain, N. Dawson, P. Amin, et al., “Docetaxel followed by hormone therapy in men experiencing increasing prostate-specific antigen after primary local treatments for prostate cancer,” Journal of Clinical Orthodontics, Vol. 23, pp. 2789–2796, 2005.
- C. C. Park, M. Mitsumori, A. Nixon, et al., “Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: Influence of margin status and systemic therapy on local recurrence,” Journal of Clinical Orthodontics, Vol. 18, pp. 1668–1675, 2000.
- S. J. Kausik, M. L. Blute, T. J. Sebo, et al., “Prognostic significance of positive surgical margins in patients with extraprostatic carcinoma after radical prostatectomy,” Cancer, Vol. 95, pp. 1215–1219, 2002.
- T. H. Van der Kwast, L. Collette, and M. Bolla, “Adjuvant radiotherapy after surgery for pathologically advanced prostate cancer,” Journal of Clinical Orthodontics, Vol. 25, pp. 5671–5672, 2007.
- T. H. Van der Kwast, M. Bolla, H. Van Poppel, et al., “Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911,” Journal of Clinical Orthodontics, Vol. 25, pp. 4178–4186, 2007.
- L. B. Marks and L. R. Prosnitz, “Postoperative radiotherapy for lung cancer: The breast cancer story all over again?” International Journal of Radiation Oncology Biology Physics, Vol. 48, pp. 625–627, 2000.
- I. C. Henderson, D. A. Berry, G. D. Demetri, et al., “Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer,” Journal of Clinical Orthodontics, Vol. 21, pp. 976–983, 2003.
- M. L. Citron, D. A. Berry, C. Cirrincione, et al., “Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of nodepositive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741,” Journal of Clinical Orthodontics, Vol. 21, pp. 1431–1439, 2003.
- Y. Shaked, U. Emmenegger, G. Francia, et al., “Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy,” Cancer Research, Vol. 65, pp. 7045–7051, 2005.
- J. A. Sparano, M. Wang, S. Martino, et al., “Phase III study of AC followed by paclitaxelor docetaxel given every 3 weeks or weekly in patients with axillary nodepositive or high-risk node-negative breast cancer: Results of intergroup trial E1199,” Presented at the 28th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, 8-11 December 2005 (abstract #48).
- G. Bocci, K. C. Nicolaou, and R. S. Kerbel, “Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs,” Cancer Research, Vol. 62, pp. 6938–6943, 2002.
- H. Kaur and G. T. Budd, “Metronomic therapy for breast cancer,” Current Oncology Reports, Vol. 6, pp. 49–52, 2004.
- T. Sorlie, C. M. Perou, R. Tibshirani, et al., “Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications,” Proceedings of the National Academy of Sciences, USA, Vol. 98, pp. 10869– 10874, 2001.
- L. J. van't Veer, H. Dai, M. J. van de Vijver, et al., “Gene expression profiling predicts clinical outcome of breast cancer,” Nature, Vol. 415, pp. 530–536, 2002.
- M. J. van de Vijver, Y. D. He, L. J. van't Veer, et al., “A gene-expression signature as a predictor of survival in breast cancer,” New England Journal of Medicine, Vol. 347, pp. 1999–2009, 2002.
- J. C. Chang, E. C. Wooten, A. Tsimelzon, et al., “Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer,” Lancet, Vol. 362, pp. 362–369, 2003.
- J. C. Chang, E. C. Wooten, A. Tsimelzon, et al., “Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients,” Journal of Clinical Orthodontics, Vol. 23, pp. 1169–1177, 2005.
- K. Iwao-Koizumi, R. Matoba, N. Ueno, et al., “Prediction of docetaxel response in human breast cancer by gene expression profiling,” Journal of Clinical Orthodontics, Vol. 23, pp. 422–431, 2005.
- M. P. Jansen, J. A. Foekens, I. L. van Staveren, et al., “Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling,” Journal of Clinical Orthodontics, Vol. 23, pp. 732–740, 2005.
- M. A. Rubin and A. M. De Marzo, “Molecular genetics of human prostate cancer,” Modern Pathology, Vol. 17, pp. 380–388, 2004.
- S. M. Dhanasekaran, T. R. Barrette, D. Ghosh, et al., “Delineation of prognostic biomarkers in prostate cancer,” Nature, Vol. 412, pp. 822–826, 2001.
- G. V. Glinsky, A. B. Glinskii, A. J. Stephenson, R. M. Hoffman, and W. L. Gerald, “Gene expression profiling predicts clinical outcome of prostate cancer,” Joint Commission International, Vol. 113, pp. 913–923, 2004.
- D. Singh, P. G. Febbo, K. Ross, et al., “Gene expression correlates of clinical prostate cancer behavior,” Cancer Cell, Vol. 1, pp. 203–209, 2002.
- G. K. Reddy and S. P. Balk, “Clinical utility of microarray-derived genetic signatures in predicting outcomes in prostate cancer,” Clinical Genitourinary Cancer, Vol. 5, pp. 187–189, 2006.
- S. M. Henshall, D. E. Afar, J. Hiller, et al., “Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse,” Cancer Reseach, Vol. 63, pp. 4196–4203, 2003.
- S. Bettuzzi, M. Scaltriti, A. Caporali, et al., “Successful prediction of prostate cancer recurrence by gene profiling in combination with clinical data: A 5-year follow-up study,” Cancer Research, Vol. 63, pp. 3469–3472, 2003.
- A. Latil, I. Bieche, L. Chene, et al., “Gene expression profiling in clinically localized prostate cancer: A four-gene expression model predicts clinical behavior,” Clinical Cancer Research, Vol. 9, pp. 5477–5485, 2003.
- J. Lapointe, C. Li, J. P. Higgins, et al., “Gene expression profiling identifies clinically relevant subtypes of prostate cancer,” Proceedings of the National Academy of Sciences, USA, Vol. 101, pp. 811–816, 2004.
- G. V. Glinsky, A. B. Glinsky, A. J. Stephenson, A. J. Hoffman, R. M. Hoffman, and W. L. Gerald, “Gene expression profiling predicts clinical outcome of prostate cancerf,” Joint Commission International, Vol. 113, pp. 913–923, 2004.
- S. A. Tomlins, D. R. Rhodes, S. Perner, S. M. Dhanasekaran, R. Mehra, X. W. Sun, S. Varambally, X. Cao, J. Tchinda, R. Kuefer, C. Lee, J. E. Montie, R. B. Shah, K. J. Pienta, M. A. Rubin, and A. M. Chinnaiyan, “Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer,” Science, Vol. 310, pp. 644–648, 2005.
- F. Demichelis, K. Fall, S. Perner, O. Andrén, F. Schmidt, S. R. Setlur, Y. Hoshida, J. M. Mosquera, Y. Pawitan, C. Lee, H. O. Adami, L. A. Mucci, P. W. Kantoff, S. O. Andersson, A. M. Chinnaiyan, J. E. Johansson, and M. A. Rubin, “TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort,” Oncogene, Vol. 26, pp. 4596–4599, 2007.
- M. Macaluso and A. Giordano, “TMPRSS2: ERG gene fusion a new genetic marker for prostate cancer progression,” Cancer Biology and Therapy, Vol. 6, pp. 46–47, 2007.
- J. C. Cheville, R. J. Karnes, T. M. Therneau, F. Kosari, J. M. Munz, L. Tillmans, E. Basal, L. J. Rangel, E. Bergstralh, I. V. Kovtun, C. D. Savci-Heijink, E. W. Klee, and G. Vasmatzis, “Gene panel model predictive of outcome in men at high-risk of systemic progression and death from prostate cancer after radical retropubic prostatectomy,” Journal of Clinical Orthodontics, Vol. 20, No. 26, pp. 3930–3936, 2008.

