Materials Sciences and Applications
Vol.4 No.3(2013), Article ID:29528,7 pages DOI:10.4236/msa.2013.43021
Novel EPS/TiO2 Nanocomposite Prepared from Recycled Polystyrene
![]()
1Department of Chemistry, University of Puerto Rico at Mayaguez, Mayagüez, USA; 2School of Science and Technology, Universidad Metropolitana, San Juan, USA
Email: *samuel.hernandez3@upr.edu
Received October 16th, 2012; revised January 4th, 2013; accepted February 2nd, 2013
Keywords: Nanocomposite; Expanded Polystyrene; TiO2; Photodegradation; Methylene Blue
ABSTRACT
The synthesis and characterization of a new nanocomposite material that was prepared from recycled expanded polystyrene (EPS) and titanium dioxide (TiO2) is reported here. The EPS was obtained from chemical reagent box insulation. To obtain the nanocomposite, these materials were dispersed in a solvent, mixed with TiCl4 and heated. The resulting new material was characterized with SEM, TEM, TGA, BET, Raman and IR techniques. The Raman and IR spectra provided complementary information regarding the structure of the nanocomposite. The Raman spectra were used to identify the crystalline structure of TiO2 in the nanocomposite. In contrast, the IR spectra were used to identify the organic portion of the nanocomposite. The TEM images indicated that the nanocomposites had an average particle size of 6 - 12 nm. In addition, the adsorption and photocatalytic properties of the new material were evaluated. The EPS/TiO2 nanocomposite was efficient at degrading methylene blue (MB) dye solutions under UV irradiation. Furthermore, according to thermal analysis, this material had greater polymer stability due to the incorporation of TiO2.
1. Introduction
Titanium dioxide  is a common material that is used daily and is present in paints (as a pigment), cosmetics and foods. TiO2 is inexpensive, non-toxic, and thermally stable, and it has a high refractive index and does not absorb visible light [1]. In addition, TiO2 has three different crystalline forms including anatase, rutile and brookite [2]. TiO2 is a semi-conductor material with well-established photocatalytic activity, especially when used in its anatase crystalline form [3-7]. Furthermore, TiO2 is a strong oxidant that can be used to mineralize organic contaminants [8,9]. In recent years, TiO2 was extensively used in the development of new water and air treatment technologies because it is easy to implement and operates across a broad temperature range. However, because of its size, TiO2 must be fixed on a suitable substrate to improve the effectiveness of the oxidation process. Several different types of materials have been used as substrates including metals [10], glass, ceramics [11- 13] and polymers [14]. One specific polymer that has been used for this purpose is polystyrene (PS) [15,16]. However, polymers like PS can cause serious environmental and economic problems because they do not biodegrade well and may be pollution sources. Thus, various polymer recycling methods were developed in the last few years [8,13,16-22]. Very small beads of extruded polystyrene (EPS) can be manufactured that contain between 4% and 7% of the blowing agent (usually pentane or butane). Due to its versatility, dimensional stability, cleanliness and low cost, EPS is widely used, especially in insulation and packing materials.
is a common material that is used daily and is present in paints (as a pigment), cosmetics and foods. TiO2 is inexpensive, non-toxic, and thermally stable, and it has a high refractive index and does not absorb visible light [1]. In addition, TiO2 has three different crystalline forms including anatase, rutile and brookite [2]. TiO2 is a semi-conductor material with well-established photocatalytic activity, especially when used in its anatase crystalline form [3-7]. Furthermore, TiO2 is a strong oxidant that can be used to mineralize organic contaminants [8,9]. In recent years, TiO2 was extensively used in the development of new water and air treatment technologies because it is easy to implement and operates across a broad temperature range. However, because of its size, TiO2 must be fixed on a suitable substrate to improve the effectiveness of the oxidation process. Several different types of materials have been used as substrates including metals [10], glass, ceramics [11- 13] and polymers [14]. One specific polymer that has been used for this purpose is polystyrene (PS) [15,16]. However, polymers like PS can cause serious environmental and economic problems because they do not biodegrade well and may be pollution sources. Thus, various polymer recycling methods were developed in the last few years [8,13,16-22]. Very small beads of extruded polystyrene (EPS) can be manufactured that contain between 4% and 7% of the blowing agent (usually pentane or butane). Due to its versatility, dimensional stability, cleanliness and low cost, EPS is widely used, especially in insulation and packing materials.
Several studies have focused on the synthesis of nanocomposites, such as . These studies have captured a great deal of attention in the past few years [23- 27]. The use of reagents and monomers to produce these nanocomposites generates high energy and, in some cases, produces more contaminated waste [28-35]. This study introduces the use of an alternative material for creating new nanocomposites from TiO2 and recycled expanded polystyrene (EPS).
. These studies have captured a great deal of attention in the past few years [23- 27]. The use of reagents and monomers to produce these nanocomposites generates high energy and, in some cases, produces more contaminated waste [28-35]. This study introduces the use of an alternative material for creating new nanocomposites from TiO2 and recycled expanded polystyrene (EPS).
Because EPS usually ends up in landfills or is incinerated, the large physical volume of EPS poses a severe contamination problem. In the past few years, EPS has been manually recycled as a hardwood replacement for garden furniture, as a slate replacement for roofing tiles and as a medium for new plastic items, such as coat hangers, CDs and video cases [36-40].
This study presents a new and simple way to recycle EPS into a  nanocomposite. This new material was characterized by TEM, SEM, TGA, FT-IR and Raman spectroscopy. Photocatalytic experiments were conducted to evaluate the catalytic properties of this new material. In addition, the results were compared with those from commercially available TiO2.
nanocomposite. This new material was characterized by TEM, SEM, TGA, FT-IR and Raman spectroscopy. Photocatalytic experiments were conducted to evaluate the catalytic properties of this new material. In addition, the results were compared with those from commercially available TiO2.
2. Experimental
2.1. Reagents
Reagent grade titanium tetrachloride , benzene and methylene blue (MB) were used in these experiments. The EPS was obtained from the insulation and packaging materials that were used to ship solvent grade acetone bottles. This recycled material was the main reagent in the synthesis of the
, benzene and methylene blue (MB) were used in these experiments. The EPS was obtained from the insulation and packaging materials that were used to ship solvent grade acetone bottles. This recycled material was the main reagent in the synthesis of the  nanocomposite material.
nanocomposite material.
2.2. Preparation of the EPS/TiO2 Nanocomposite
The method for synthesizing nanocomposite  was simple. The first step of dispersing the recycled polymeric material in benzene was conducted at room temperature. A suitable amount of TiCl4 was slowly added to the EPS that was dispersed in benzene. This mixture was heat treated at 300˚C for approximatly 3 h. After the mixture was allowed to return to room temperature, a black powder product
was simple. The first step of dispersing the recycled polymeric material in benzene was conducted at room temperature. A suitable amount of TiCl4 was slowly added to the EPS that was dispersed in benzene. This mixture was heat treated at 300˚C for approximatly 3 h. After the mixture was allowed to return to room temperature, a black powder product was obtained.
was obtained.
2.3. EPS/TiO2 Nanocomposite Characterization
Particle size and shape distribution are two important nanoparticle characteristics. Thus, the size, shape, surface area and spectroscopic properties of the nanoparticles were determined. Each of the obtained nanocomposite materials was characterized by using a combination of the following techniques.
2.3.1. Surface Area
Nitrogen adsorption isotherms and surface area measurements were performed with nitrogen at 77 K. First, the samples were degassed by heating at a rate of 5 K/min and by evacuating at a rate of 50 mm Hg/s for 6 h. The specific surface area was determined by using the Langmuir method with a relative pressure range of 0.01 - 0.20 Torr. A static volumetric adsorption unit (ASAP 2020 Micrometrics, Norcross, GA, USA) was used for these analyses.
2.3.2. FTIR and Raman Spectroscopy
Raman spectra were collected with a Renishaw RM2000 microspectrometer that was equipped with 532 and 785 nm excitation sources. Raman spectra were acquired under the following conditions: a spectral range of 100 - 3200 cm−1; an accumulation of 2; an acquisition time of 20 s; and laser power levels of 10 - 60 mW. A Bruker Optics IFS 66 series FT-IR spectrometer coupled to a Hyperion Infrared Microscope and the OPUS™ 4.0 data acquisition and analysis suite was used to obtain the IR spectra of the starting materials and the prepared nanocomposites.
2.3.3. SEM and TEM Images
Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM) images were used to determine the average particle size and morphology of the prepared nanocomposites. The SEM images were obtained with a JEOL JSM-541 OL SEM microscope. The TEM images were used to observe the morphologies of the nanocomposite particles. First, the nanocomposite samples were dispersed in benzene. Next, a portion of the dispersed sample was placed (using a dropper) on a copper grid that was coated with Formvar and a carbon film. The solvents were allowed to evaporate on the grid. The TEM images were recorded by a model 1011 Transmission Electron Microscope that had a lattice resolution of 0.2 nm and a magnification between 50 and 106 at an accelerating voltage of between 40 and 100 kV (JEOL, Peabody, MA). The samples were prepared by placing 1.0 μL of the Ag-NPs solution on ultrathin carbon film/ holey carbon 400 mesh copper TEM grids (01824, Ted Pella, Inc., Redding, CA). The solvents were allowed to evaporate at room temperature. The grids were stored in a desiccator to provide a dust free environment. White light images were obtained with an Olympus America, Inc. (Center Valley, Pa, USA) model BH2-UMA high resolution optical microscope that was equipped with 10 - 250´ magnification and a 6.0 MB PAX-Cam image capturing CCD camera controlled by PAX-it!™ Software (Midwest Information Systems, Inc., Villa Park, IL, USA).
2.3.4. TGA Analysis
The thermal degradation behavior of the samples was analyzed with a thermal gravimetric analyzer (TGA), model Q-500 (TA Instruments, New Castle, DE, USA). Aluminum pans (5 mm I.D.) were used to hold the standards, and platinum sample holders were used to hold the nanocomposites. Approximately 5 mg of dried material was heated from 20˚C to 600˚C at a scan speed of 10˚C/ min.
2.3.5. Photocatalitic Degradation Capability of EPS/TiO2
The nanocomposite photodegradation capability was evaluated by using a discoloration method with the methylene blue (MB) dye. For these tests, 30 mg of the EPS/TiO2 composite were mixed with 10 mL of a 14 ppm MB solution. A 2.0 mL aliquot of this reaction mixture was removed with a syringe at pre-determined time intervals. The sampled aliquots were filtered through 0.45 nm Millipore membranes before analysis with a UV/Vis spectrophotometer (Agilent 8453 UV-Visible). The MB concentration was monitored by measuring the absorbance of the sample filtrate at 663 nm. All the experiments were performed in batches. The catalytic efficiency of the nanocomposite was evaluated by measuring the decolorizing efficiency of the MB solutions. All the samples were analyzed in triplicate and commercial TiO2 was used as the control or blank.
3. Results and Discussion
The new EPS/TiO2 nanocomposite was characterized with various analytical techniques. The TEM images are shown in Figures 1(a) and (b) and indicate that the particles were nearly spherical in form. The darker areas in the images correspond with TiO2, and lighter gray and white areas correspond with the recycled EPS. These images confirm the presence of these two materials in the final product. The average nanoparticle sizes were between 5 and 15 nm. In addition, the TEM images show the morphology of the formed nanocomposite. The TiO2 occurred in the form of islands within the polymeric matrix and was agglomerated in some regions. The SEM images (Figures 1(c) and (d)) show a granular morphology with a size of less than 100 µm. In Figure 1(d), the SEM image focuses in on one EPS/TiO2 grain. In this case, a smooth particle surface was observed.
The FT-IR spectra of the TiO2, EPS and EPS/TiO2 were compared to observe the vibrational pattern changes of the nanocomposite spectra relative to those of the parent compounds. Figure 2 shows the FT-IR spectra of the rutile-TiO2, untreated EPS films and EPS/TiO2. Nearly all of the vibrational information that was observed in the starting materials was observed in the nanocomposite. Persistent vibrational signatures that corresponded to phenyl ring vibrations were identified at approximately 1630, 1490 and 1450 cm−1.

Figure 1. SEM and TEM images of the EPS/TiO2 nanocomposite. Micrographs of the TEM images (a) and (b) and the SEM images, (c) and (d).
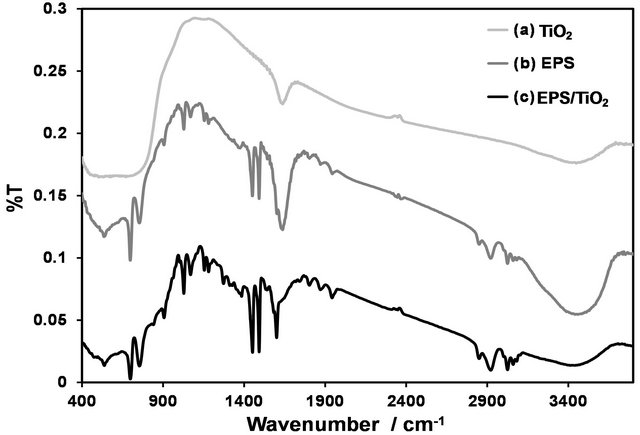
Figure 2. FT-IR spectra of the (a) rutile TiO2; (b) EPS; and (c) EPS/TiO2 nanocomposite.
A broad band at approximately 3400 cm−1 was attributed to surface hydroxyl bending vibrations. Figure 3 shows the Raman spectra of the TiO2 in the anatase and rutile phases and in the nanocomposite. These spectra were obtained to identify which of the bands from these crystalline forms were present in the final product. The main signal corresponded to the anatase phase. The Raman spectra of the new material and TiO2 shows bands that correspond to TiO2, which supports the presence of this material in the final product and indicates that no significant changes in the vibrational profile of the polymer occurred in the low frequency region (Figure 3).
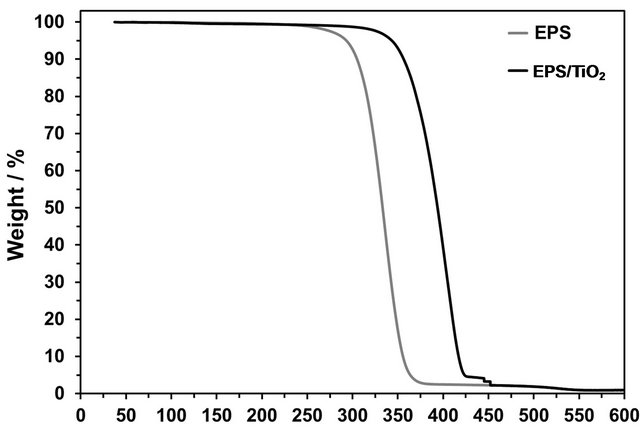
The thermal behavior of the EPS/TiO2 nanocomposite was measured and compared to that of the recycled EPS. TGA analysis of the EPS/TiO2 and EPS resulted in different mass loss profiles at different temperatures. While the thermal degradation of EPS/TiO2 begins at 344˚C with a 5% of weight loss, that of the recycled EPS begins at 293˚C, which is more than 50˚C earlier than that of the EPS/TiO2 nanocomposite. This shift reflects that the incorporation of TiO2 into the polymer improves its thermal stability. The EPS and nanocomposite curves in a nitrogen atmosphere are shown in Figure 4.
These results were similar to those obtained by Dzunuzovic et al. [41]. The final syntheis product that was described in this paper  had similar thermal properties to those of the nanocomposite that was formed by the polymerization of styrene. Thus, this new chemical process provides an important contribution to industrial sustainability and embraces its challenges.
had similar thermal properties to those of the nanocomposite that was formed by the polymerization of styrene. Thus, this new chemical process provides an important contribution to industrial sustainability and embraces its challenges.
In addition, the absorbent and catalytic properties of  were studied and evaluated. The gas adsorption results showed that the new material had a poor adsorption capacity. This result was confirmed by the BET analysis reulsts, in which the suface area was relatively low (BET surface area of
were studied and evaluated. The gas adsorption results showed that the new material had a poor adsorption capacity. This result was confirmed by the BET analysis reulsts, in which the suface area was relatively low (BET surface area of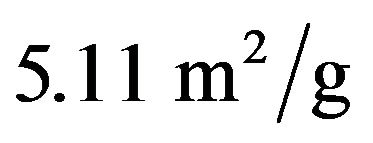 ). Figure 5 contains the
). Figure 5 contains the  composite adsorption results.
composite adsorption results.
The adsorption capacity of the composite was also

Figure 3. Raman spectra of the (a) anatase phase of TiO2; (b) EPS/TiO2 nanocomposite; and (c) rutile TiO2.
Temperature/˚C
Figure 4. Thermogravimetric curves of the EPS (gray trace) and EPS/TiO2 (black trace).
determined to ensure that adsorption was not the only process that occurred. However, this process is important for heterogeneous photocatalysis. The adsorption capacity of  was analyzed by using methylene blue (MB). Approximately 0.01 g of the
was analyzed by using methylene blue (MB). Approximately 0.01 g of the 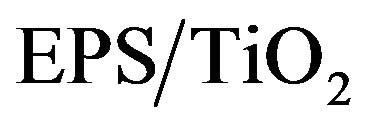 sample were added to aqueous MB solutions and magnetically stirred for 2 h. The samples were placed in the dark to prevent photodecomposition by white light irradiation. After various contact times, the MB concentration was measured to evaluate its dependency on the contact time with
sample were added to aqueous MB solutions and magnetically stirred for 2 h. The samples were placed in the dark to prevent photodecomposition by white light irradiation. After various contact times, the MB concentration was measured to evaluate its dependency on the contact time with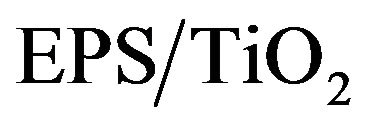 . The MB concentration remained constant during nearly 12 h of contact. The Freundlich Isotherm model best fit the results. The well-known linear form of the Freundlich model is shown below.
. The MB concentration remained constant during nearly 12 h of contact. The Freundlich Isotherm model best fit the results. The well-known linear form of the Freundlich model is shown below.
 (1)
(1)
or
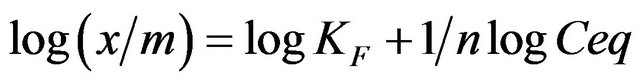 (2)
(2)
where KF is the “Freundlich” equilibrium constant and 1/n is a constant that can be evaluated with the linearized Freundlich equation (Equation (2)). The amount of analyte that is adsorbed at equilibrium (mg, MB) is represented by x/m, where m is the mass of the adsorbent (g) and Ceq is the equilibrium concentration of the adsorbate
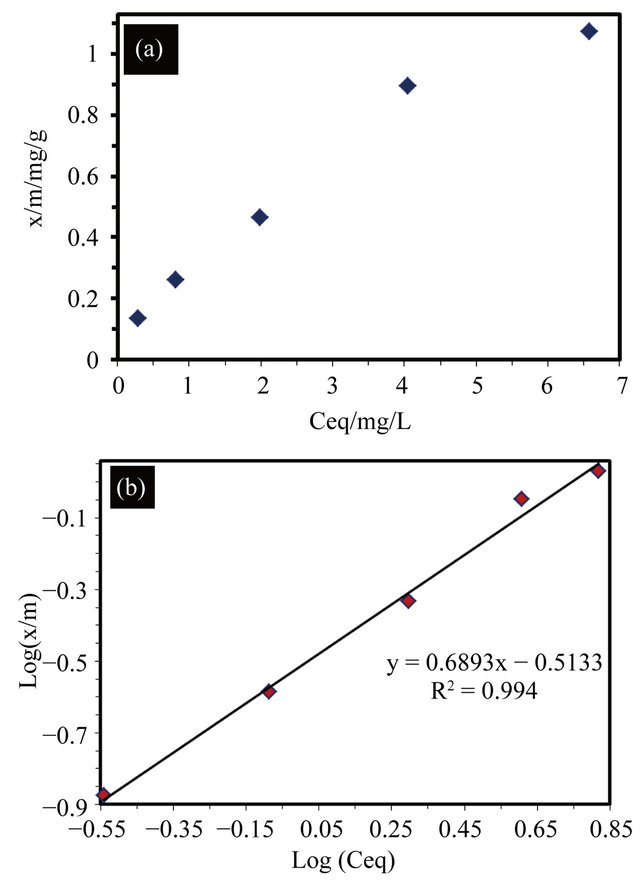
Figure 5. The adsorption capacity of several MB aqueous solution concentrations including (a) normal plot of amount adsorbed vs equilibrium concentration; and (b) the Freundlich plot of the data represented in (a).
(MB).
If (1/n) approaches 1, then the equation is linear. This linear equation occurs for a limited range of the adsorbance values. For the complete range of values, the non-linear isotherm data can be plotted in a linear form by taking the log of both sides of the equation. The linear fit of the adsorption data that is shown in Figure 5(b) is
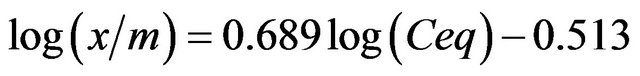 (with a coefficient of determination of
(with a coefficient of determination of 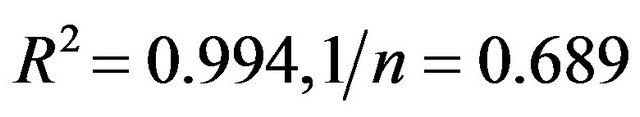 (
(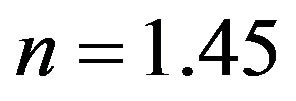 and
and ). The value of
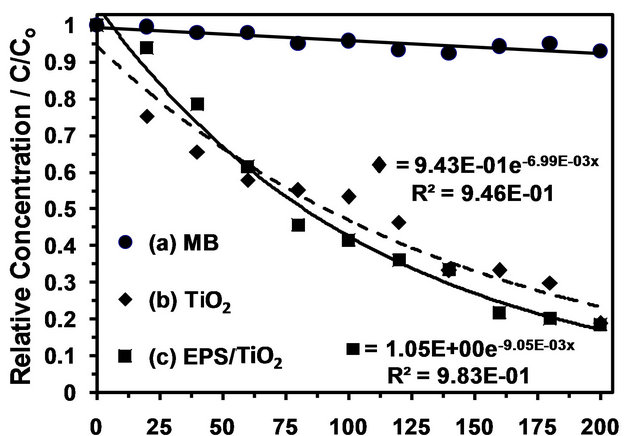
). The value of 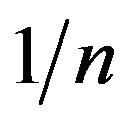 is related to the heterogeneity of the materials surface. Based on these results, the adsorption in this composite is semi-heterogeneous. Another way to evaluate the adsorption efficiency of the material is with a cross comparison experiment that is designed to evaluate the photocatalytic activity of EPS/ TiO2 and TiO2. The results of these experiments are presented in Figure 6. A similar degradation time was observed for
is related to the heterogeneity of the materials surface. Based on these results, the adsorption in this composite is semi-heterogeneous. Another way to evaluate the adsorption efficiency of the material is with a cross comparison experiment that is designed to evaluate the photocatalytic activity of EPS/ TiO2 and TiO2. The results of these experiments are presented in Figure 6. A similar degradation time was observed for 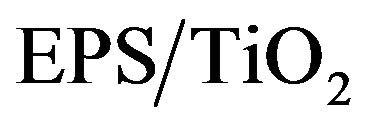 and the comercial TiO2 when 30 mg of material were added to the MB solutions.
and the comercial TiO2 when 30 mg of material were added to the MB solutions.
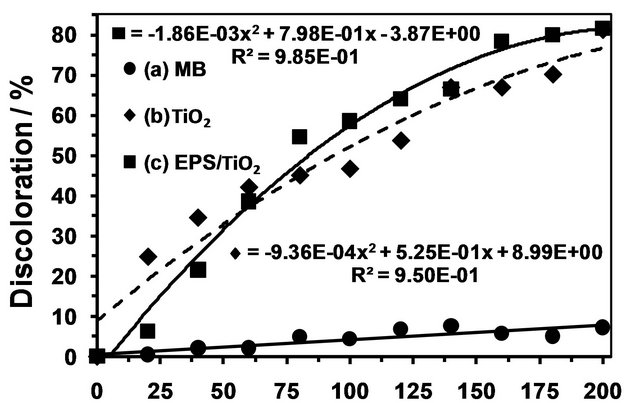
In addition, an alternative method was used to determine the photocatalytic activity of EPS/TiO2. This method was conducted using the discoloration efficiency value (D) of the MB solution as a comparative parameter and was used by Su et al. in 2007 [42]. The catalytic reaction was performed in a flask under UV irradiation at
Time/min
Figure 6. Photocatalytic degradation of MB under UV light irradiation. (a) MB without TiO2; (b) 30 mg of TiO2 in contact with MB; and (c) 30 mg of EPS/TiO2 in contact with MB.
254 nm. Exactly 50 mg of powder were added to 10 mL of a 14 mg/L MB solution. Next, the system was stirred for under UV irradiation at 254 nm before measuring the absorbance as a function of time.
The discoloration efficiency values were calculated by using the MB solution absorbance that was determined after it was in contact with the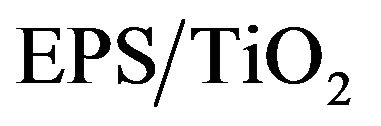 . The discoloration efficiency was evaluated using Equation (3) in terms of percent discoloration (D).
. The discoloration efficiency was evaluated using Equation (3) in terms of percent discoloration (D).
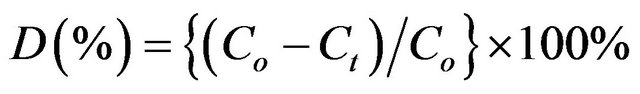 (3)
(3)
where, D represents the discoloration efficiency  of the MB solutions, Co and Ct represent the absorption values of the MB solutions at the initial time t = 0 and at a particular time t, respectively. Commercial TiO2 was used as a reference material. Figure 7 shows the representative discoloration for MB with the
of the MB solutions, Co and Ct represent the absorption values of the MB solutions at the initial time t = 0 and at a particular time t, respectively. Commercial TiO2 was used as a reference material. Figure 7 shows the representative discoloration for MB with the 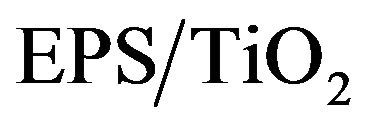 and TiO2 photocatalysts.
and TiO2 photocatalysts.
The best discoloration behavior was observed in the 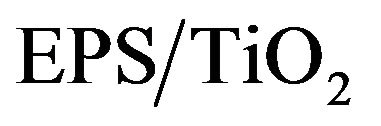 nanocomposite. The percent discoloration of the MB solutions reached 98% (data not shown). Poor discoloration of the MB solutions was observed when the photocatalysts were not added to the solutions (control runs), which confirmed the photocatalytic activity of the nanocomposite.
nanocomposite. The percent discoloration of the MB solutions reached 98% (data not shown). Poor discoloration of the MB solutions was observed when the photocatalysts were not added to the solutions (control runs), which confirmed the photocatalytic activity of the nanocomposite.
4. Conclusion
A new nanocomposite made with extruded polystyrene (EPS/TiO2) was synthesized from recycled polymer sources. The new material was characterized by various techniques, such as SEM, TEM, BET, FT-IR, and Raman spectroscopy. No differences in vibrational spectra were observed between the EPS and EPS/TiO2, which indicated that TiO2 was attached to the polystyrene and produced a black nanocomposite with an average particle size of between 5 and 12 nm. Raman spectroscopy was used as a complementary tool to evaluate the inorganic portion of
Irradiation Time/h
Figure 7. Discoloration of the MB solutions under UV light irradiation. (a) MB with EPS/TiO2; (b) MB with TiO2; and (c) MB without TiO2 or EPS/TiO2 (control).
the new nanocomposite including the anatase phase of the TiO2. The EPS/TiO2 nanocomposite had a high discoloration efficiency for aqueous methylene blue (MB) solutions. The percent of discolorization reached 98% in the MB solution. Better results were obtained for the new nanocomposite than for the commercially available one. Thus,  will be used in future degradation tests of persistent organic pollutants that occur in water.
will be used in future degradation tests of persistent organic pollutants that occur in water.
5. Acknowledgements
Support from the US Department of Homeland Security under Award No. 2008-ST-061-ED0001 is acknowledged. However, the views and conclusions contained within this paper are those of the authors and do not represent the official policies, either expressed or implied, of the US Department of Homeland Security.
Part of the work presented in this study was supported by the U.S. Department of Defense University Research Initiative Multidisciplinary University Research Initiative (URI)-MURI Program under grant No. DAAD19-02-1- 0257.
In addition, Dr. Rong Zhang from Jackson State University is gratefully acknowledged for assisting with the TEM images.
REFERENCES
- G. J. Nohynek, E. Antignac, T. Re and H. Toutain, “Safety Assessment of Personal Care Products/Cosmetics and Their Ingredients,” Toxicology and Applied Pharmacology, Vol. 243, No. 2, 2010, pp. 239-259. doi:10.1016/j.taap.2009.12.001
- A. Fujishima, X. Zhang and D. A. Tryk, “TiO2 Photocatalysis and Related Surface Phenomena,” Surface Science Reports, Vol. 63, No. 12, 2008, pp. 515-582. doi:10.1016/j.surfrep.2008.10.001
- E. Alonso, I. Montequi and M. J. Cocero, “Effect of Synthesis Conditions on Photocatalytic Activity of TiO2 Powders Synthesized in Supercritical CO2,” The Journal of Supercritical Fluids, Vol. 49, No. 2, 2009, pp. 233- 238. doi:10.1016/j.supflu.2009.01.005
- T. Kawahara, T. Ozawa, M. Iwasaki, H. Tada and S. Ito, “Photocatalytic Activity of Rutile-Anatase Coupled TiO2 Particles Prepared by a Dissolution-Reprecipitation Method,” Journal of Colloid and Interface Science, Vol. 267, No. 2, 2003, pp. 377-381. doi:10.1016/S0021-9797(03)00755-0
- T. Ohno, K. Tokieda, S. Higashida and M. Matsumura, “Synergism between Rutile and Anatase TiO2 Particles in Photocatalytic Oxidation of Naphthalene,” Applied Catalysis A: General, Vol. 244, No. 2, 2003, pp. 383-391. doi:10.1016/S0926-860X(02)00610-5
- K. Porkodi and S. D. Arokiamary, “Synthesis and Spectroscopic Characterization of Nanostructured Anatase Titania: A Photocatalyst,” Materials Characterization, Vol. 58, No. 6, 2007, pp. 495-503. doi:10.1016/j.matchar.2006.04.019
- J. Wang, G. Zhao, Z. Zhang, X. Zhang, G. Zhang, T. Ma, Y. Jiang, P. Zhang and Y. Li, “Investigation on Degradation of Azo Fuchsine Using Visible Light in the Presence of Heat-Treated Anatase TiO2 Powder,” Dyes and Pigments, Vol. 75, No. 2, 2007, pp. 335-343. doi:10.1016/j.dyepig.2006.06.007
- L. Zan, L. Tian, Z. Liu and Z. Peng, “A New Polystyrene-TiO2 Nanocomposite Film and Its Photocatalytic Degradation,” Applied Catalysis A: General, Vol. 264, No. 2, 2004, pp. 237-242. doi:10.1016/j.apcata.2003.12.046
- J. I. Lim, B. Yu, K. M. Woo and Y.-K. Lee, “Immobilization of TiO2 Nanofibers on Titanium Plates for Implant Applications,” Applied Surface Science, Vol. 255, No. 5, 2008, pp. 2456-2460.
- V. R. Choudhary, V. P. Patil, P. Jana and B. S. Uphade, “Nano-Gold Supported on Fe2O3: A Highly Active Catalyst for Low Temperature Oxidative Destruction of Methane Green House Gas from Exhaust/Waste Gases,” Applied Catalysis A: General, Vol. 350, No. 2, 2008, pp. 186-190. doi:10.1016/j.apcata.2008.08.008
- S. Cho and W. Choi, “Solid-Phase Photocatalytic Degradation of PVC-TiO2 Polymer Composites,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 143, No. 2-3, 2001, pp. 221-228. doi:10.1016/S1010-6030(01)00499-3
- L.-H. Lin, H.-J. Liu, J.-J. Hwang, K.-M. Chen and J.-C. Chao, “Photocatalytic Effects and Surface Morphologies of Modified Silicone-TiO2 Polymer Composites,” Materials Chemistry and Physics, Vol. 127, No. 1-2, 2011, pp. 248-252. doi:10.1016/j.matchemphys.2011.01.069
- C. Yang, C. Gong, T. Peng, K. Deng and L. Zan, “High Photocatalytic Degradation Activity of the Polyvinyl Chloride (PVC)-Vitamin C (VC)-TiO2 Nano-Composite Film,” Journal of Hazardous Materials, Vol. 178, No. 1-3, 2010, pp. 152-156. doi:10.1016/j.jhazmat.2010.01.056
- B. Lowes, A. Bohrer, T. Tran and D. Shipp, “Grafting of Polystyrene ‘from’ and ‘through’ Surface Modified Titania Nanoparticles,” Polymer Bulletin, Vol. 62, No. 3, 2009, pp. 281-289. doi:10.1007/s00289-008-0016-9
- F. Magalhães and R. M. Lago, “Floating Photocatalysts Based on TiO2 Grafted on Expanded Polystyrene Beads for the Solar Degradation of Dyes,” Solar Energy, Vol. 83, No. 9, 2009, pp. 1521-1526. doi:10.1016/j.solener.2009.04.005
- J. Shang, M. Chai and Y. Zhu, “Solid-Phase Photocatalytic Degradation of Polystyrene Plastic with TiO2 as Photocatalyst,” Journal of Solid State Chemistry, Vol. 174, No. 1, 2003, pp. 104-110. doi:10.1016/S0022-4596(03)00183-X
- W. Fa, L. Zan, C. Gong, J. Zhong and K. Deng, “SolidPhase Photocatalytic Degradation of Polystyrene with TiO2 Modified by Iron (II) Phthalocyanine,” Applied Catalysis B: Environmental, Vol. 79, No. 3, 2008, pp. 216- 223. doi:10.1016/j.apcatb.2007.10.018
- A. P. Kumar, D. Depan, N. Singh Tomer and R. P. Singh, “Nanoscale Particles for Polymer Degradation and Stabilization—Trends and Future Perspectives,” Progress in Polymer Science, Vol. 34, No. 6, 2009, pp. 479-515. doi:10.1016/j.progpolymsci.2009.01.002
- G. Liu, D. Zhu, W. Zhou, S. Liao, J. Cui, K. Wu and D. Hamilton, “Solid-Phase Photocatalytic Degradation of Polystyrene Plastic with Goethite Modified by Boron under UV-Vis Light Irradiation,” Applied Surface Science, Vol. 256, No. 8, 2010, pp. 2546-2551. doi:10.1016/j.apsusc.2009.10.102
- G. L. Liu, D. W. Zhu, S. J. Liao, L. Y. Ren, J. Z. Cui and W. B. Zhou, “Solid-Phase Photocatalytic Degradation of Polyethylene-Goethite Composite Film under UV-Light Irradiation,” Journal of Hazardous Materials, Vol. 172, No. 2-3, 2009, pp. 1424-1429. doi:10.1016/j.jhazmat.2009.08.008
- L. Zan, S. Wang, W. Fa, Y. Hu, L. Tian and K. Deng, “Solid-Phase Photocatalytic Degradation of Polystyrene with Modified Nano-TiO2 Catalyst,” Polymer, Vol. 47, No. 24, 2006, pp. 8155-8162. doi:10.1016/j.polymer.2006.09.023
- X. Zhao, Z. Li, Y. Chen, L. Shi and Y. Zhu, “Enhancement of Photocatalytic Degradation of Polyethylene Plastic with CuPc Modified TiO2 Photocatalyst under Solar Light Irradiation,” Applied Surface Science, Vol. 254, No. 6, 2008, pp. 1825-1829. doi:10.1016/j.apsusc.2007.07.154
- K. Gandhi, B. K. Dixit, and D. K. Dixit, “Effect of Addition of Zirconium Tungstate, Lead Tungstate and Titanium Dioxide on the Proton Conductivity of Polystyrene Porous Membrane,” International Journal of Hydrogen Energy, Vol. 37, No. 4, 2012, pp. 3922-3930. doi:10.1016/j.ijhydene.2011.04.209
- B. Jaleh, M. S. Madad, S. Habibi, P. Wanichapichart and M. F. Tabrizi, “Evaluation of Physico-Chemical Properties of Plasma Treated PS-TiO2 Nanocomposite Film,” Surface and Coatings Technology, Vol. 206, No. 5, 2011, pp. 947-950. doi:10.1016/j.surfcoat.2011.03.136
- H. L. Luo, J. Sheng and Y. Z. Wan, “Preparation and Characterization of TiO2/Polystyrene Core-Shell Nanospheres via Microwave-Assisted Emulsion Polymerization,” Materials Letters, Vol. 62, No. 1, 2008, pp. 37-40. doi:10.1016/j.matlet.2007.04.108
- M. Song, C. Pan, J. Li, R. Zhang, X. Wang and Z. Gu, “Blends of TiO2 Nanoparticles and Poly (N-Isopropylacrylamide)-Co-Polystyrene Nanofibers as a Means to Promote the Biorecognition of an Anticancer Drug,” Talanta, Vol. 75, No. 4, 2008, pp. 1035-1040. doi:10.1016/j.talanta.2008.01.005
- P. J. Wibawa, H. Saim, M. A. Agam and H. Nur, “Design, Preparation and Characterization of Polystyrene Nanospheres Based-Porous Structure towards UV-Vis and Infrared Light Absorption,” Physics Procedia, Vol. 22, 2011, pp. 524-531. doi:10.1016/j.phpro.2011.11.081
- M. Antonietti and K. Landfester, “Polyreactions in Miniemulsions,” Progress in Polymer Science, Vol. 27, No. 4, 2002, pp. 689-757. doi:10.1016/S0079-6700(01)00051-X
- S. Palaniappan and A. John, “Polyaniline Materials by Emulsion Polymerization Pathway,” Progress in Polymer Science, Vol. 33, No. 7, 2008, pp. 732-758. doi:10.1016/j.progpolymsci.2008.02.002
- B. Peng, F. Tang, D. Chen, X. Ren, X. Meng and J. Ren, “Preparation of PS/TiO2/UF Multilayer Core-Shell Hybrid Microspheres with High Stability,” Journal of Colloid and Interface Science, Vol. 329, No. 1, 2009, pp. 62- 66. doi:10.1016/j.jcis.2008.09.069
- B. A. Rozenberg and R. Tenne, “Polymer-Assisted Fabrication of Nanoparticles and Nanocomposites,” Progress in Polymer Science, Vol. 33, No. 1, 2008, pp. 40-112. doi:10.1016/j.progpolymsci.2007.07.004
- E. Tang, H. Liu, L. Sun, E. Zheng and G. Cheng, “Fabrication of Zinc Oxide/Poly(Styrene) Grafted Nanocomposite Latex and Its Dispersion,” European Polymer Journal, Vol. 43, No. 10, 2007, pp. 4210-4218. doi:10.1016/j.eurpolymj.2007.05.015
- Y. Wang, Y. Ke, J. Li, S. Du and Y. Xia, “Dispersion Behavior of Core-Shell Silica-Polymer Nanoparticles,” China Particuology, Vol. 5, No. 4, 2007, pp. 300-304. doi:10.1016/j.cpart.2007.04.004
- Y. Wu, Y. Zhang, J. Xu, M. Chen and L. Wu, “One-Step Preparation of PS/TiO2 Nanocomposite Particles via Miniemulsion Polymerization,” Journal of Colloid and Interface Science, Vol. 343, No. 1, 2010, pp. 18-24. doi:10.1016/j.jcis.2009.11.022
- J. Zhang, G. Gao, M. Zhang, D. Zhang, C. Wang, D. Zhao and F. Liu, “ZnO/PS Core-Shell Hybrid Microspheres Prepared with Miniemulsion Polymerization,” Journal of Colloid and Interface Science, Vol. 301, No. 1, 2006, pp. 78-84. doi:10.1016/j.jcis.2006.05.005
- “05/02386 Production of Biodegradable Plastics from Activated Sludge Generated From a Food Processing Industrial Wastewater Treatment Plant,” Fuel and Energy Abstracts, Vol. 46, No. 5, 2005, pp. 346-346. doi:10.1016/S0140-6701(05)82395-1
- R. Montgomery, “Development of Biobased Products,” Bioresource Technology, Vol. 91, No. 1, 2004, pp. 1-29. doi:10.1016/S0960-8524(03)00154-8
- B. Singh and N. Sharma, “Mechanistic Implications of Plastic Degradation,” Polymer Degradation and Stability, Vol. 93, No. 3, 2008, pp. 561-584. doi:10.1016/j.polymdegradstab.2007.11.008
- M. Suresh Kumar, S. N. Mudliar, K. M. K. Reddy and T. Chakrabarti, “Production of Biodegradable Plastics from Activated Sludge Generated from a Food Processing Industrial Wastewater Treatment Plant,” Bioresource Technology, Vol. 95, No. 3, 2004, pp. 327-330. doi:10.1016/j.biortech.2004.02.019
- S. Yan, S. Bala Subramanian, R. D. Tyagi and R. Y. Surampalli, “Bioplastics from Activated Sludge Treating Pulp and Paper Wastewater,” Journal of Biotechnology, Vol. 136, Supplement 1, 2008, pp. S31-S32.
- E. Dzunuzovic, V. Vodnik, K. Jeremic and J. M. Nedeljkovic, “Thermal Properties of PS/TiO2 Nanocomposites Obtained by in Situ Bulk Radical Polymerization of Styrene,” Materials Letters, Vol. 63, No. 11, 2009, pp. 908-910. doi:10.1016/j.matlet.2009.01.039
- B. Su, Z. Ma, S. Min, S. She and Z. Wang, “Preparation of TiO2/PS Complex Nanoparticles,” Materials Science and Engineering: A, Vol. 458, No. 1-2, 2007, pp. 44-47. doi:10.1016/j.msea.2006.12.111
NOTES
*Corresponding author.

