Journal of Environmental Protection
Vol. 3 No. 8 (2012) , Article ID: 21756 , 9 pages DOI:10.4236/jep.2012.38083
Environmentally Harmful Low Density Waste Plastic Conversion into Kerosene Grade Fuel
![]()
Department of Research and Development, Natural State Research, Inc., Stamford, USA.
Email: *msarker@naturalstateresearch.com
Received May 12th, 2012; revised June 15th, 2012; accepted July 13th, 2012
Keywords: LDPE; Waste Plastic; Kerosene; Conversion; Fuel; GC/MS; FT-IR
ABSTRACT
Plastics wastes from a municipal solid waste (MSW) plant have a high-energy content and are suitable for fuel generation. Thermal cracking is one of the possible ways to obtain petrochemical feedstock from polymer wastes. Municipal waste plastic of LDPE conversion to kerosene grade fuel experiments were carried out under atmospheric conditions at temperatures between 150˚C and 420˚C. Low density polyethylene (LDPE) plastic waste (Code #2) was thermally depolymerized in batch process into stainless steel reactor without adding catalyst. The maximum kerosene grade fuel yield is 30%, other grade fuel 60%, light gas 6% and left over residue 4%. The composition, sulphur and Btu value of liquid products were determined by ASTM method. Produced fuel was analyzed by gas chromatography and mass spectrometer and FT-IR. Very high conversions from LDPE waste plastic to kerosene grade fuel (up to 35%) were obtained while using this technique. Detailed product analyses and characterization lead to a reasonable explanation of reaction pathways and mechanisms.
1. Introduction
Plastic waste has recently reached a diffusion of ~75 million tonnes per year globally [1]. The European Union (EU) is facing an increasing amount ~18 million tonnes in 2000 [1] of plastic waste which has been increasing by ~4% per year [2]. Municipal waste is by far the largest source of plastic waste with more than 60% of the total; 7% of plastic waste is subjected to material recovery and 15% to energy recovery; and an average of ~80% is disposed of [2]. About 560 thousand tonnes of plastic packaging wastes are annually produced in the Czech Republic: 35% of the sorted plastic waste is subjected to material recovery, 13% to energy recovery and 52% is sent to landfill [3]. A major change has been considered necessary since a European waste directive (1999/31/EC) called for a 30% reduction of the amount of waste sent to landfill by 2010. In Korea, the amount of waste plastics generated in 2002 was about 3.44 million tons, according to data from the National Institute of Environmental Research. Regarding the method of treatment or disposal of waste plastics, incineration and landfill methods were about 50 wt% and 30 wt%, respectively, and the other 20 wt% was through recycling. The majority of recycling methods was material recycling, although the quality of the recovered plastic was very low; only a small percentage was treated by pyrolysis and gasification (chemical recycling). However, material recycling will reach its limits, while chemical recycling will be gradually expanded. Thermal or catalytic cracking of waste plastics is one of the possible methods of their utilization [4-11].
Particularly considering post-consumer plastics, it must be noticed that they pose an attractive opportunity for utilization as a valuable and reusable source of hydrocarbons if broken down into lower molecular weight products. In addition to energy considerations, the development of improved methods for converting these low-cost waste polymers in an environmentally favorable way appears to be the best alternative route to plastics pyrolysis or recycling. With respect to present trends in finding cost-effective energy sources, recent research efforts have focused on the utilization of waste polymeric materials in reacting directly with coal [12,13]. This becomes possible since it is well established that waste plastics undergo depolymerization and degradation above 380˚C by a free radical chain reaction [14]. Although a variety of waste plastics processing methods for obtaining hydrocarbons have been developed in the recent past, there is still a great deal of interest for the mentioned co-liquefaction process due to the fact that coal is an abundant fuel resource [15]. Hence, this case seems to be quite promising since earlier studies indicated that positive synergism was achieved by processing coal and waste plastics targeting oily yields [14]. This process became achievable due to the fact that waste plastics are characterized by high hydrogen content and molecular structures that are suitable for this transformation [16].
Disposal of plastic waste into landfills has become increasingly prohibitive due to high costs, legislative pressures and public opinion. Growing environmental awareness and reductions in available landfill capacity have prompted plastics recycling programs in most developed Countries. Currently, however, only somewhere between 5% and 25% of plastic waste is being recycled. In Europe, recycling rates for post-use plastic waste were as follows: incineration with energy recovery (14%), mechanical recycling (6%) and feedstock or chemical recycling (0.3%). Due to the generation of unacceptable emissions of gases such as nitrous and sulphur oxides, dusts, and dioxins, incineration can no longer be an important mode of waste recycling. Mechanical recycling processes are limited to thermoplastics and technical limitations for the treatment of mixed plastic wastes, together with the limited size of the market for recycled products, difficulties in maintaining product quality and fluctuations in the price. As a consequence the feedstock recycling waste plastics is gaining momentum is an alternative fuel production method. It overcomes many of the limitations (such as contamination and property deterioration) encountered by mechanical recycling. The feedstock recycling of polymers by thermolysis can be applied to transform the waste plastics into high value refinery product. Thermal and catalytic cracking of common plastics such as PE and PP have already been studied extensively [17-21].
2. Materials & Method
Low density waste plastic was collected from local coffee shop and LDPE waste plastic come out from milk container. Milk container cap was LDPE plastic and cap color was red. Collected red color milk container cap stick with some milk and cap was printed with white color. LDPE waste plastic was combination of red and white color. Milk container cap was washed by liquid detergent and make it dried into room temperature. Then cut into small pieces manually with cutting scissor and made size 0.25 - 0.5 inch. Then waste plastic transfer into reactor chamber (showed Figure 1) for set up experiment. After waste plastic transfer experimental accessories was set up properly such as reactor setup, fractional distillation column, collection device according to fuel grading wise, light gas cleaning device, pump and Teflon bag. LDPE waste plastic was used for this experiment only 500 gm and any kind of chemical or catalyst were not use in the experiment. Experiment temperature range was 150˚C - 420˚C because we know LDPE plastic melting point temperature is 120˚C and density is 0.92 - 0.94 gm/ml. LDPE plastic has straight chain hydrocarbon compound and chemical structure is (-CH2-CH2-)n. Starting temperature was 150˚C and temperature was increased gradually up to 420˚C ratio wise and temperature increased rate was 15˚C every 15 minutes interval. This experiment was performed in present of oxygen and system was fully closed. Vacuum was not applied in this experiment because when temperature start to rise up inside moisture come out with vapor and it was not affecting out experiment. In this process none of gas come out outside because it was fully closed system under fume hood. When temperature range was 150˚C to 250˚C cross we noticed that vapor start to come out and fuel start to drop collection device as grading wise. Fractional distillation column was fully insulted by temperature profile range wise low carbon range to high carbon range boiling point. For gasoline grade fuel temperature range was 40˚C - 65˚C, naphtha chemical grade temperature was 110˚C - 135˚C, kerosene grade fuel collection temperature range was 180˚C - 205˚C, diesel fuel collection temperature range was 260˚C - 285˚C and finally fuel oil collection temperature range was 340˚C - 365˚C. In this experiment main goal was kerosene grade fuel collection from LDPE waste plastic. In this experiment light gas also produce which was cleaned by alkali solution light gas cleaning system then light gas transfer into teflon bag via using pump. Produced kerosene grade fuel density is 0.79 g/ml. In this experiment mass balance result showed after finished experiment from 500 gm of LDPE waste plastic to produced gasoline grade fuel 50 gm, naphtha chemical grade 100 gm, kerosene grade 150 gm, diesel grade 100 gm, fuel oil 50 gm, light as sample 30 gm and leftover residue 20 gm. Collected kerosene grade fuel was purified by RCI technology provided RCI purification system and from this fuel was removed water, ash and sediment part. After purification kerosene grade fuel was analysis by ASTM method, GC/MS and FT-IR which is described into result and discussion section.
3. Results and Discussion
From Perkin Elmer GCMS analysis of waste LDPE plastic to kerosene grade fuel (Figure 2 and Table 1) in accordance with retention time and trace mass indicate various types of compound are present. High intensity compounds are preferred in the analysis. An investigated carbon range in the analyzed plastic is C7 to C22 because large carbon chains are breaking down into small chain resulting in lower carbon range. Sometimes noticed that for high peak intensity compound probability factor is low percentage, where as in low peak intensity compound probability factor is high percentage. Most of the
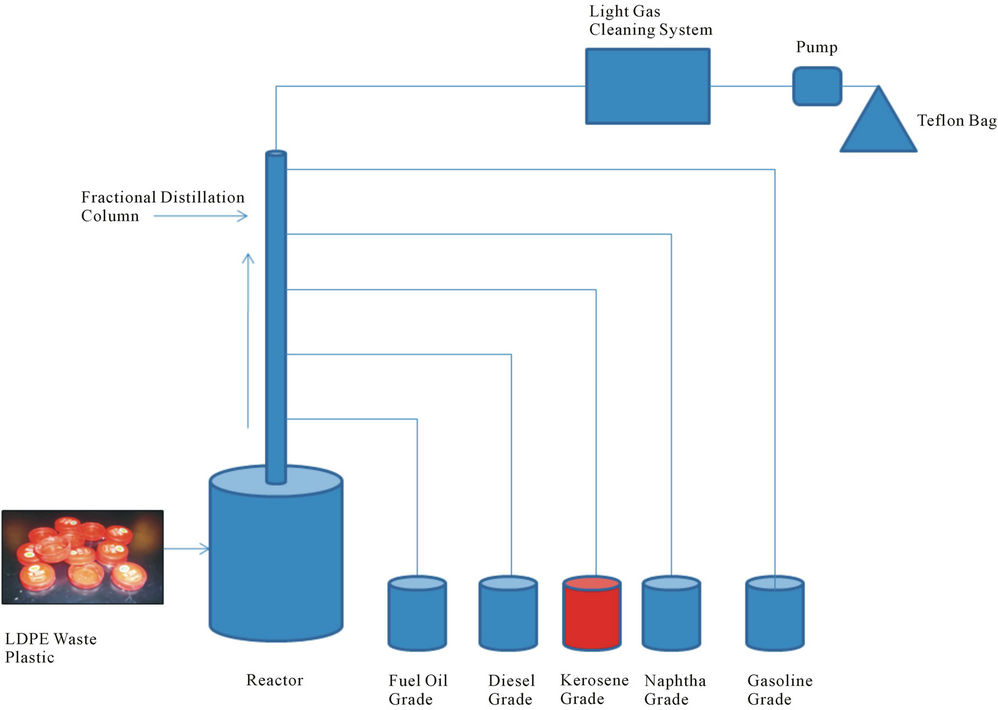
Figure 1. Kerosene grade fuel production diagram.

Figure 2. GC/MS chromatogram of kerosene grade fuel from LDPE waste plastic.
peaks are considered in the analysis and as per their retention time and trace mass maximum peaks are mentioned, in accordance to retention time 4.90 and trace mass 81, derived compound is cyclopentene, 4,4-dimethyl—(C7H12) with probability 7.67%, retention time 5.19 and trace mass 41, compound is 1-octene, (C8H16) with probability 28.6%, retention time 5.43 and trace mass 43, compound is octane (C8H16) with probability 58.7%, retention time 5.96 and trace mass 41, compound is 2-octyn-1-ol (C8H14O) with probability factor 12.2%, retention time 6.29 and trace mass 81, compound is 3-octen-1-ol, (Z)-(C8H14O) with probability 4.95%, retention time 6.92 and trace mass 56, compound is 1-nonene (C9H18) with probability 13.2%, retention time 7.16
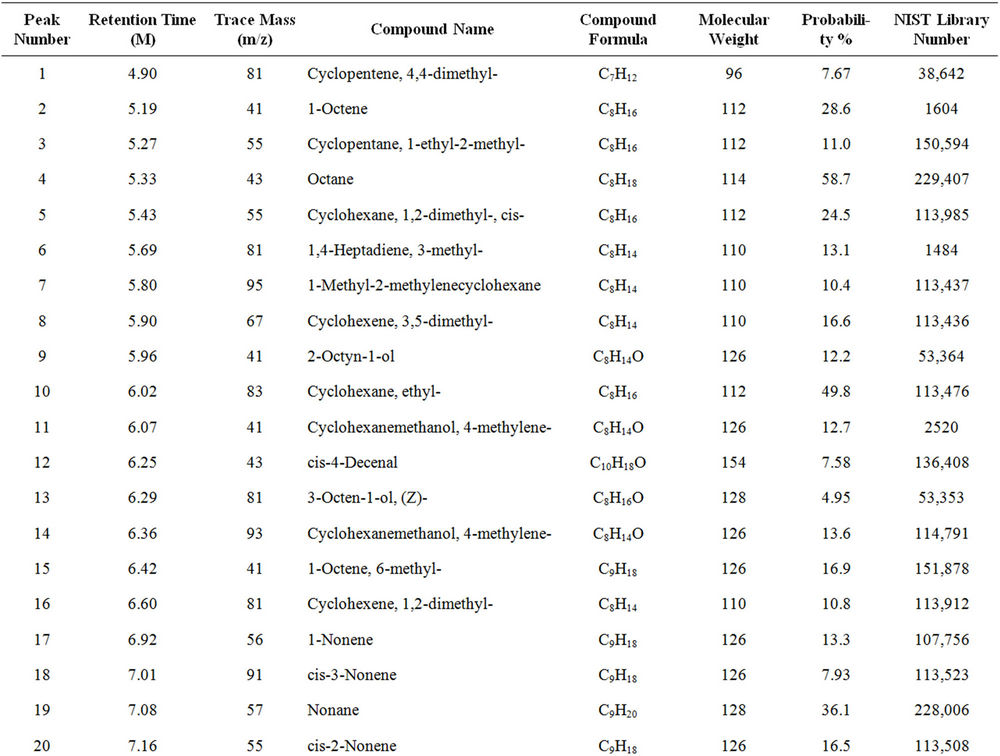
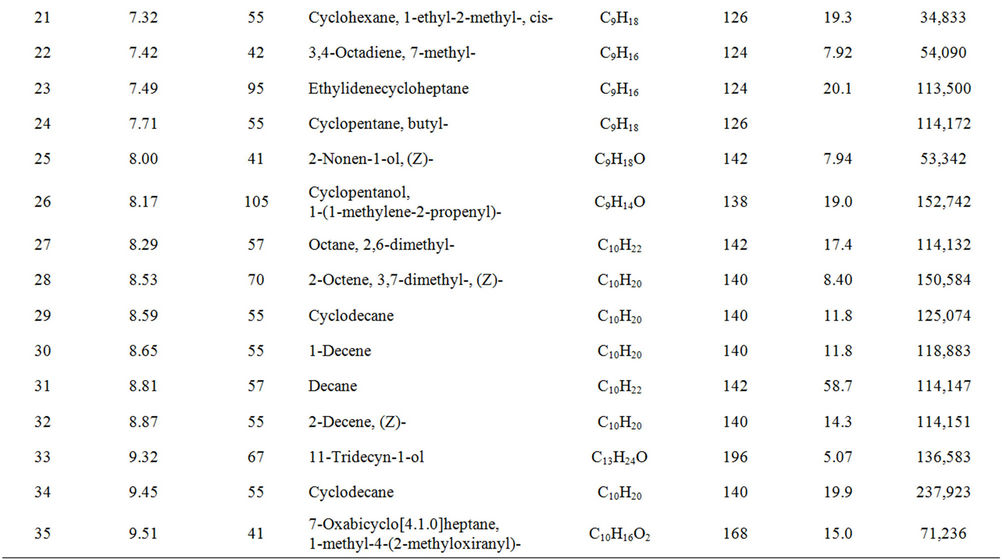
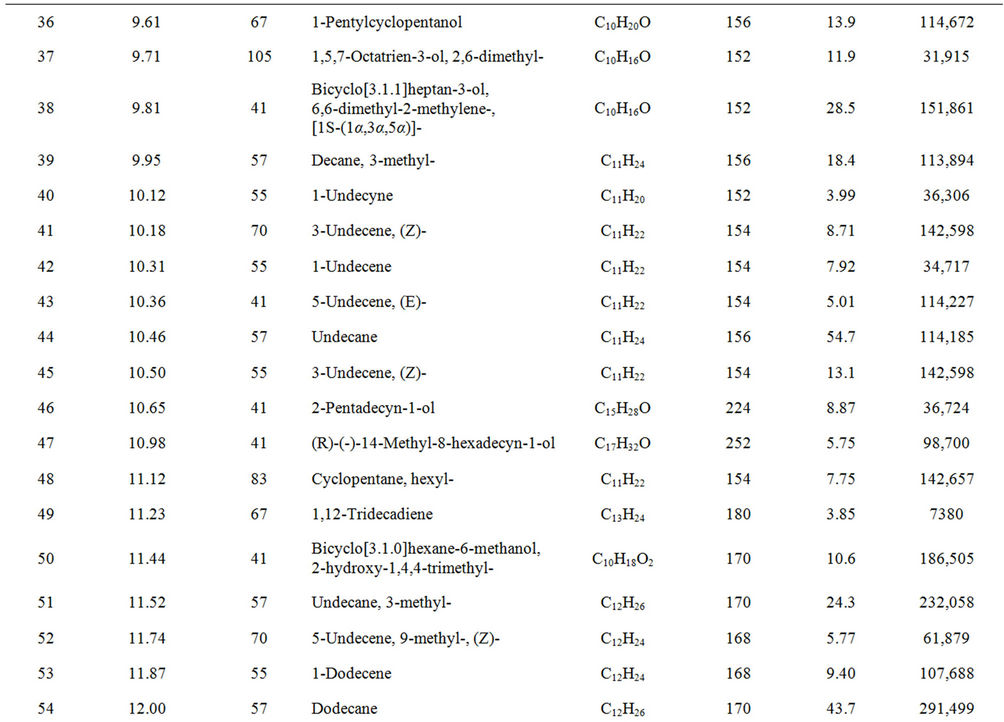
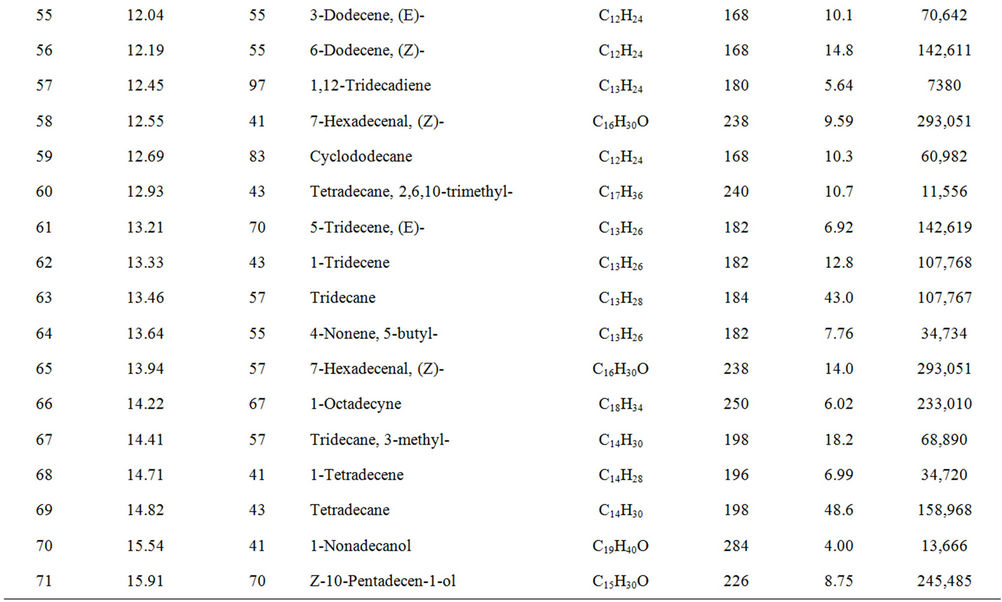
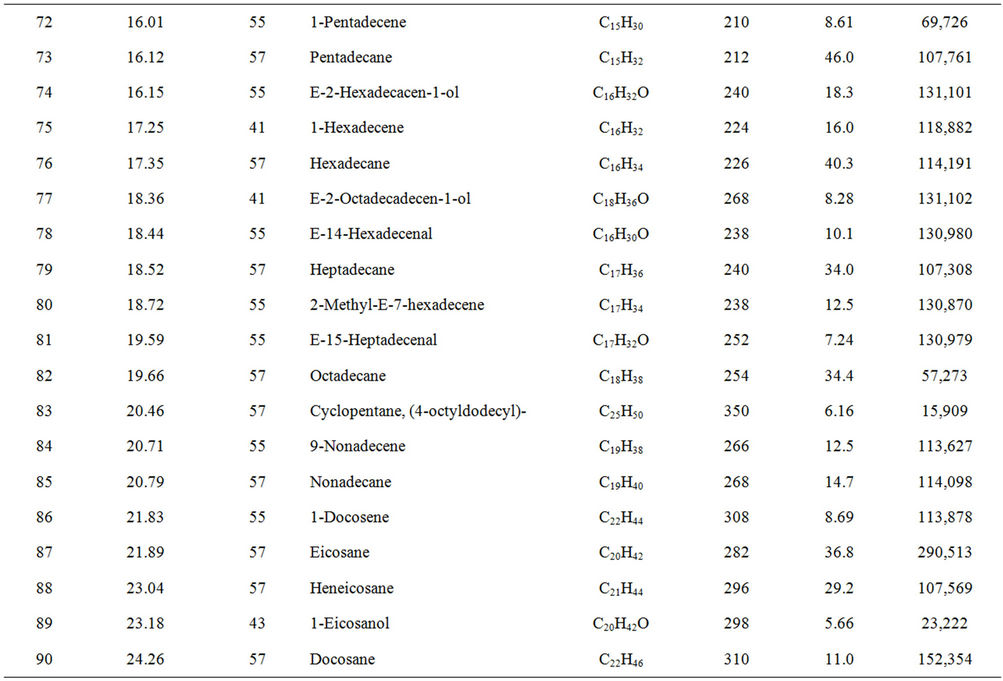
Table 1. GC/MS chromatogram compound list of kerosene grade fuel from LDPE waste plastic.
and trace mass 55, compound is cis-2-nonene, (C9H18) with probability 16.5%, retention time 7.49 and trace mass 95, compound is ethylidenecycloheptane (C9H16) with probability 20.1%, retention time 8.00 and trace mass 41, compound is 2-nonen-1-ol, (Z) (C9H18O) with probability 7.94%, retention time 8.87 and trace mass 55, compound is 2-decene, (Z) (C10H20) with probability 14.3%, retention time 9.32 and trace mass 67, compound is 11-tridecyn-1-ol (C13H24O) with probability 5.07%, retention time 9.51 and trace mass 41, compound is 7-oxabicyclo[4.1.0]heptane, 1-methyl-4-(2-methyloxiranyl) (C10H16O2) with probability 15%, retention time 9.95 and trace mass 57, compound is decane, 3-methyl (C11H24) with probability 18.4%, retention time 10.65 and trace mass 41, compound is 2-pentadecyn-1-ol (C15H28O) with probability 8.87% etc. Also in the middle of the analysis index retention time 11.12 and trace mass 83, compound is cyclopentane, hexyl (C11H22) with probability 7.75%, retention time 11.87 and trace mass 55, compound is 1- dodecene (C12H24) with probability 9.40%, retention time 12.69 and trace mass 83, compound is cyclododecane (C12H24) with probability 10.3%, retention time 12.93 and trace mass 43, compound is tetradecane, 2,6,10- trimethyl (C17H36) with probability 10.7% etc. As well as retention time 13.21 and trace mass 70, compound is 5-tridecene, (E)-, (C13H26) with probability 6.92%, retention time 13.94 and trace mass 57, compound is 7-hexadecenal, (Z) (C16H30O) with probability 14%, retention time 14.82 and trace mass 43, compound is tetradecane (C14H30) with the probability 48.6% etc. Accordingly retention time 15.91 and trace mass 70, compound is Z- 10-pentadecen-1-ol (C15H30O) with probability 8.75%, retention time 16.12 and trace mass 57, compound is pentadecane (C15H32) with probability 46.0%, retention time 17.25 and trace mass 41, compound is 1-hexadecene (E) (C16H32) with probability 16% etc. At the end phase of the analysis index high retention time and trace mass such as retention time 18.72 and trace mass 55, compound is 2-methyl-E-7-hexadecene (C17H34) with probability 12.5%, retention time 19.66 and trace mass 57, compound is octadecane (C18H38) with probability 34.4%, and retention time 20.71 and trace mass 55, compound is 9-nonadecene (C19H38) with probability 12.5% and retention time 21.89 and trace mass 57, compound is eicosane (C20H42) with probability 36.8% and ultimately retention time 24.26 and trace mass 57, compound is docosane (C22H46) as well. In the earlier phase analysis appearing that in several oxygen compounds are produced because in the reactor during reaction oxygen induced from moisture because experiment was not vacuum system. Also noticed in the standard LDPE plastic to kerosene grade fuel including double bond compound as well as benzene and benzene derivatives compounds are available. In the middle of the analysis index also noticed that one or more alcoholic compounds are appeared as well.
From FT-IR (spectrum 100) analysis of LDPE waste plastic to kerosene grade fuel (Figure 3 and Table 2) shows the following types of functional groups appears, at the starting wave number 3619.21 cm−1, derived functional group is free OH group, wave number 3077.53 cm−1, functional group is H bonded NH, wave number 2933.92 cm−1, 2730.89 cm−1 and 2671.02 cm−1 functional group is C-CH3, wave number 1821.63 cm−1 and 1780.15 cm−1 functional group is non-conjugated and 1641.60 cm−1 functional group is non-conjugated, wave number 1606.78 cm−1 functional group is conjugated. As well as wave number 1454.00 cm−1 and 1378.74 cm−1 functional group is CH3, wave number 991.81 cm−1 and 910.93 cm−1, functional group is -CH=CH2 and ultimately wave number 887.81 cm−1 functional group is C=CH2 and wave number 721.84 cm−1 functional group is -CH=CH- (cis). Energy values are calculated for some wave numbers, using formula is E = h·υ, where h = Planks constant, h = 6.626 × 10−34 J, υ = Frequency in Hertz (sec−1), where υ = c/λ, c = Speed of light, where, c = 3 × 1010 m/s, W = 1/λ, where λ is wave length and W is wave number in cm−1. Therefore the equation E = h·υ, can substitute by the following equation, E = h·c·W. According to their wave number such as for 3619.21 (cm−1) calculated energy, E = 7.18 × 10−20 J. Similarly, wave number 3077.53 (cm−1) energy, E = 6.11 × 10−20 J, wave number 2933.92 (cm−1) energy, E = 5.82 × 10−20 J, wave number 2730.89 (cm−1) energy, E = 5.42 × 10−20 J, wave number 2671.02 (cm−1) energy, E = 5.30 × 10−20 J, wave number 1821.63 (cm−1) energy, E = 3.61 × 10−20 J and eventually wave number 1780.15 (cm−1) functional group is 3.53 × 10−20 J respectively.
LDPE waste plastic to kerosene grade fuel (Figure 4) was analysis by DSC and purge Nitrogen gas at 20 ml per minutes. During sample run we observed that the DSC curve started at negative temperature. From negative temperature we can say this fuel has negative boiling
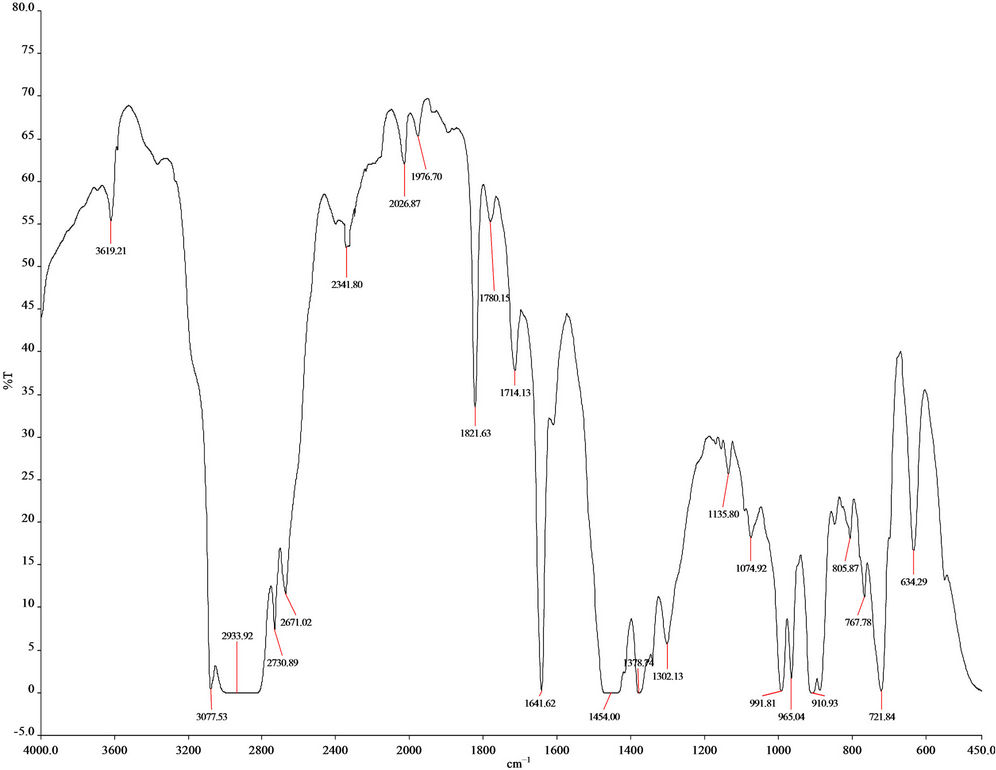
Figure 3. FT-IR Spectrum of LDPE waste plastic to kerosene grade fuel.
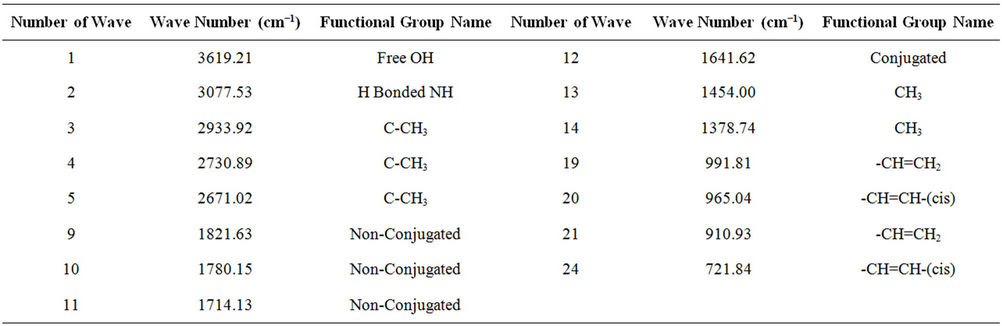
Table 2. LDPE waste plastic to kerosene grade fuel FTIR functional group name.
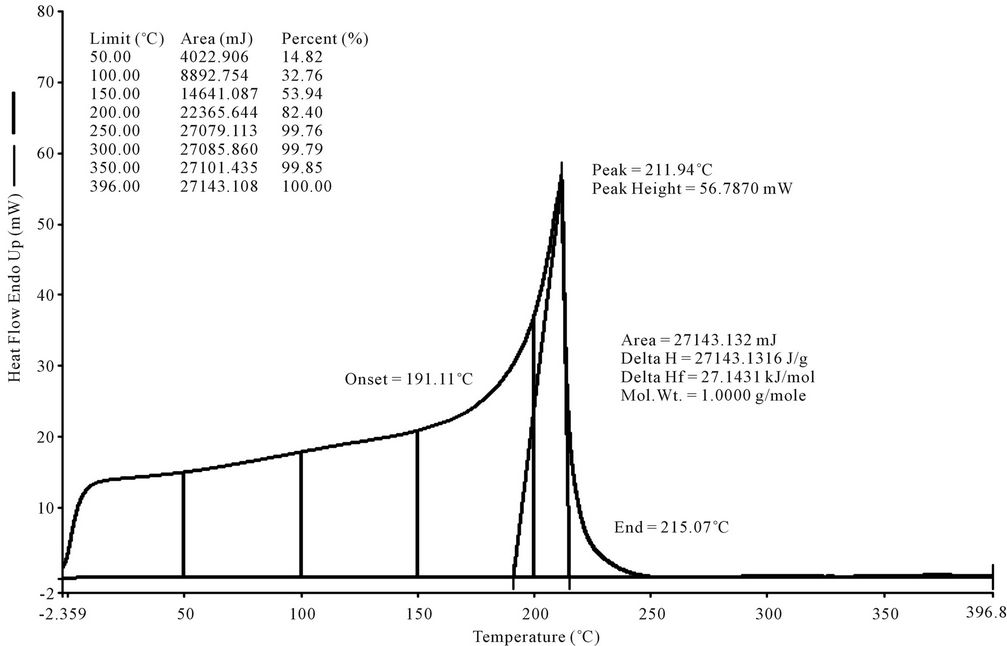
Figure 4. DSC graph of LDPE waste plastic to kerosene grade fuel.
point hydrocarbon compound such as propane and butane. This fuel’s collection fractional temperature program was used 180˚C - 205˚C because we want to collect kerosene group compounds. From DSC analysis we found onset temperature 191.11˚C. DSC analysis is giving us boiling point temperature. Fuel analysis graph is showing peak of 211.94˚C, peak height 56.7870 mW. Heat flow Endo up showing 60% form 100. The area is 27143.132 mJ and enthalpy delta H value is 27143.1316 J/g. From fuel analysis graph was shown at 100˚C fuel was boiled 32.76%, 250˚C temperature fuel was boil almost close to 100%. ASTM Test performed from this fuel API gravity@ 60˚F (ASTM D4052), Corrected flash point (ASTM D56), Freezing Point ˚C/˚F (ASTM D5972), IBP recovery (ASTM D86), Automatic cloud Point ˚C/˚F (ASTM D5773), sulfur (ASTM D2622), Cetane number (ASTM D613), Appearance (ASTM D4176), Smoke point (ASTM D1322), Net heat of combustion/gal (ASTM D3338).
4. Conclusion
LDPE waste plastic such as milk container cap to kerosene grade fuel utilizing thermal degradation was conducted in this research. Approximately 30% of the total obtained product resembled commercial kerosene. The produced fuel’s sulfur content less then what EPA mandates and the fuel has a good Btu value. Fuel was analysis by GC/MS and FT-IR and carbon range showed from GC/MS C7 to C22. Kerosene grade fuel has single bond and double bond compound which is alkane and alkene group compounds and also alcoholic group compound present. The thermal process utilized in this experiment has the capability of converting not only LDPE but all kinds of waste plastics to valuable hydrocarbon fuel like kerosene.
5. Acknowledgements
The author acknowledges the support of Dr. Karin Kaufman, the founder and sole owner of Natural State Research, Inc. (NSR). The authors also acknowledge the valuable contributions NSR laboratory team members during the preparation of this manuscript.
REFERENCES
- P. Picuno and C. Sica, “Mechanical and Spectroradiometrical Characteristics of Agriculture Recycled Plastic Films,” CIGR E-Journal, Vol. 6, 2000. http://ecommons.cornell.edu/handle/1813/10378
- European Environment Agency, “European Topic Center on Waste, Waste Generation and Management—Environment in the EU at the Turn of the Century,” Copenhagen, 1999. http://www.eea.europa.eu/publications/92-9157-202-0/page307.html
- E. Kom, “Annual EKO-KOM Report—2005,” Prague, 2006. http://www.ekokom.cz/uploads/attachments/English/The_Guide.pdf
- N. Shah, J. Rockwell and G. P. Huffman, “Conversion of Waste Plastic to Oil: Direct Liquefaction versus Pyrolysis and Hydroprocessing,” Energy Fuel, Vol. 13, No. 4, 1999, pp. 832-838.
- I. Nakamura and K. Fujimoto, “Development of New Disposable Catalyst for Waste Plastics Treatment for High Quality Transportation Fuel,” Catalysis Today, Vol. 27, No. 1, 1996, pp. 175-179.
- G. de la Puente, J. M. Arandes and U. Sedram, “Recycled Plastics in FCC Feedstocks: Specific Contributions,” Industrial and Engineering Chemistry Research, Vol. 36, No. 11, 1997, pp. 4530-4534.
- A. R. Songip, T. Masuda, H. Kuwahara and K. Hashimoto, “Test to Screen Catalysts for Reforming Heavy Oil from Waste Plastics,” Applied Catalysis B, Vol. 2, No. 2-3, 1993, pp. 153-164.
- W. Ding, J. Laing and L. L. Andersen, “Thermal and Catalytic Degradation of High Density Polyethylene and Commingled Post-Consumer Plastic Waste,” Fuel Processing Technology, Vol. 51, No. 1-2, 1997, pp. 47-62.
- Z. Zhibo, S. Nishio, Y. Moriaka, A. Ueno, H. Ohkita, Y. Tochihara, Y. Mizushima and N. Kakuta, “Thermal and Chemical Recycle of Waste Polymers,” Catalysis Today, Vol. 29, No. 1-4, 1996, pp. 303-308.
- Y. Uemichi, M. Hattori, T. Itoh, J. Nakamura and M. Sugioka, “Deactivation Behaviors of Zeolite and SilicaAlumina Catalysts in the Degradation of Polyethylene,” Industrial and Engineering Chemistry Research, Vol. 37, No. 3, 1998, pp. 867-872.
- H. S. Joo and J. A. Guin, “Continuous Upgrading of a Plastics Pyrolysis Liquid to an Environmentally Favorable Gasoline Range Product,” Fuel Processing Technology, Vol. 57, No. 1, 1998, pp. 25-40.
- L. L. Anderson and W. Tuntawiroon, “Coliquefaction of Coal and Polymers to Liquid Fuels,” Preprints of ACS Meeting, Chicago, 1993, pp. 816-822.
- M. M. Taghici, F. E. Huggins and G. P. Huffman, “Coliquefaction of Waste Plastics with Coal,” Preprints of ACS Meeting, Chicago, Vol. 38, No. 4, 1993, pp. 810- 815.
- L. A. Wall, S. L. Madorsky, D. W. Brown and S. Strauss, “The Depolymerization of Polymethylene and Polyethylene,” Journal of the American Chemical Society, Vol. 76, 1954, pp. 3430-3437.
- R. D. Leaversuch, “Chemical Recycling Brings Real Versatility to Solid-Waste Management,” Modern Plastics, Vol. 68, No. 7, 1991, pp. 40-43.
- X. Xiao, W. Zmierczak and J. Shabtai, “Depolymerization-Liquefaction of Plastics and Rubbers. 1. Polyethylene, Polypropylene and Polybutadiene,” ACS Division of Fuel Chemistry, 1995, Vol. 40, No. 1, pp. 4-8.
- M. A. Uddin, K. Koizumi, K. Murata and Y. Sakata, “Thermal and Catalytic Degradation of Structurally Different Types of Polyethylene into Fuel Oil,” Polymer Degradation and Stability, Vol. 56, No. 1, 1997, pp. 37-44.
- R. C. Mordi, R. Fields and J. Dwver, “Thermolysis of Low Density Polyethylene Catalysed by Zeolites,” Journal of Analytical and Applied Pyrolysis, Vol. 29, No. 1, 1994, pp. 45-55.
- W. Zhao, S. Hasegawa, J. Fujita, F. Yoshii, T. Sasaki, K. Makuuchi, J. Sun and S. Nishimoto, “Effects of Zeolites on the Pyrolysis of Polypropylene,” Polymer Degradation and Stability, Vol. 53, No. 1-2, 1996, pp. 129-135.
- P. T. Williams and E. A. Williams, “Fluidised Bed Pyrolysis of Low Density Polyethylene to Produce Petrochemical Feedstock,” Journal of Analytical and Applied Pyrolysis, Vol. 51, No. 1-2, 1999, pp. 107-126.
- Y. Uemichi, Y. Makino and T. Kanazuka, “Degradation of Polypropylene to Aromatic Hydrocarbons over Ptand Fe-Containing Activated Carbon Catalysts,” Journal of Analytical and Applied Pyrolysis, Vol. 16, No. 3, 1989, pp. 229-238.
NOTES
*Corresponding author.

