Smart Grid and Renewable Energy
Vol.06 No.01(2015), Article ID:53340,11 pages
10.4236/sgre.2015.61002
Hydrogen Production from an Alkali Electrolyzer Operating with Egypt Natural Resources
Mohamed H. Shedid*, S. Elshokary
Department of Mechanical Engineering, Faculty of Engineering, Mattaria, Helwan University, Cairo, Egypt
Email: *mshedid@hotmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 November 2014; accepted 17 January 2015; published 19 January 2015
ABSTRACT
Egypt is bordered by coastal sea 2450 km , while incident solar radiation is in the order of magnitudes of 1900-2200 W/m2 in that area of the world. The present work is aimed to assess the hydrogen production from the solar-hydrogen system composed of photovoltaic cell motivated by solar energy and supplies electricity to alkali electrolyzer. The electrolyzer uses sea and Nile water as electrolytes. Indoor tests are done to identify the optimum concentration ratio of the sea water to produce hydrogen. Experimental results showed that stand-alone sea water gives a higher production rate. The results of the outdoor tests revealed the need for about seven units of electrolyzer working with the sea water to produce the same amount of hydrogen that KOH solution electrolyte would provide. However, the efficiency of solar-hydrogen units working with the sea water gives a lower constant efficiency of about 0.13%, followed by Photovoltaic/electrolyzer unit using Nile water of approximately 0.005%. The KOH solution electrolyte provides an efficiency of approximately 8% at solar noon. The sea water is recommended to be used instead of KOH solution in all coming electrolyzers.
Keywords:
Alkali Electrolyzer, Hydrogen Production, Sea Water, Photovoltaic, Egypt Climate, Egypt Resources

1. Introduction
Hydrogen reveals distinctive features that in conjunction with electricity allow it to form a one of future energy systems, so-called hydrogen economy [1] . Hydrogen, which has a higher energy density than most current fossil fuels [2] [3] , can be stored for long periods with slight or no energy losses. Hydrogen can be a renewable fuel and has the availability of storage in a variety of forms. It can be easily transported and efficiently produced from and transformed into electricity.
Energy Policy Act of 2005 (EPACT 2005) and the Advanced Energy Initiative of 2006, aimed to develop hydrogen to be an energy carrier of the future. Expected result will contribute in many industries to be readily replacing the traditional energy resources with quietly, efficiently and without pollution. Nonetheless, pure hydrogen is not found naturally in high concentrations on Earth. Instead, hydrogen must be extracted from other compounds such as water, biomass, and fossil fuels.
Egypt is well-qualified to be involved in that trace as it possesses abundant in natural resources such as water, wind and solar energy. Egypt is bordered to the north by the Mediterranean Sea over 995 km, to the east by the Red Sea over 1941 km while it has 1530 km of the Nile river [4] . Incident solar radiation is in the order of magnitudes of 1900-2200 W/m2 in that area of the world.
There are several approaches to produce a renewable hydrogen [5] . Among these methods, water electrolysis is a process in which the electrical energy is converted into chemical energy. When water electrolysis is implemented using renewable energy (e.g. Hydro, Wind, and Solar) the hydrogen can be considered as an entirely renewable and clean energy carrier. Water electrolysis offers several advantages over other production methods such as steam reforming. Water electrolysis gives very pure hydrogen with no carbon emissions as no dependence on hydrocarbon sources. Also, it has the simplicity in a small scale/real time supply, utilization of renewable primary energy sources and pure oxygen as a by-product [6] . Although water electrolysis is a matured technology, the electrolyzers currently available in the market are designed for stationary grid-connected operation, whereas there is a lack of technical data and operational experience regarding the use of these systems for intermittent and long-term operations. Thus, for the efficient performance of such a complex scheme, appropriate components with a well-designed control system are required to achieve autonomous operation and energy management in the system [7] . However, the development of a massive use of hydrogen as an energy carrier is not well-defined and depends on the further development of end-use technologies, significant cost reductions, the build-up of a sufficient hydrogen infrastructure and the competition with alternative technologies [8] [9] . Pregger [9] mentioned that the increasing industrial hydrogen needs in countries with high direct solar radiation will be the most important driver for the development of solar thermal processes and the realization of pilot plants within the next two decades.
Charvin [10] mentioned that the increase of operation hours of a water electrolysis, the hydrogen quantity produced is larger for a given plant size. This leads to a reduced hydrogen production cost.
While the hydrogen production cost from water electrolysis depends strongly on the electricity price, the solar thermochemical cycles is not the case. The energy cost and reactant (water) inputs are negligible, which warrants a stable hydrogen production cost.
Santarelli [11] supplied a simulation program for the complete electric and part of the heat requests of a small residential user in an isolated building in a valley of the Alps in Italy during a whole year of operation without any integration of a traditional energy that depends on the fossil fuels. The hydrogen price is not competitive in the real energy market. However, considering the system is a standalone placed in a remote area and it eliminates of the costs of the distribution voltage lines are benefits that cannot overlook.
Ahmad [12] established a PV/electrolyzer system based on a typical year of meteorological conditions of Cairo, Egypt. It was found that the photovoltaic module current is directly affected by the solar radiation intensity. The hydrogen production flow rate is proportionally directed with the electrolyzer input current.
El Shenawy [13] reported a small PV power system for hydrogen production using the photovoltaic module connected to the hydrogen electrolyzer. It was shown from the experimental results that the hydrogen produced can be more useful by using the photovoltaic energy from the side view of the environmental considerations. Wang [14] examined the solar-to-hydrogen reaction mechanisms, including thermo-chemical cycles that utilize heat to split water molecules, electrolysis that utilizes electric potential to split water molecules, and photochemical processes that utilize photon-activated electrons of auxiliary reagents (sensitizer and catalyst) to activate and split water molecules. He concluded that water electrolysis powered by solar generated electricity is more mature than other technologies. Ramdan [15] optimized a PV-Hydrogen generation system by both PV module and hydrogen cell. He recommended to use Potassium Hydroxide (KOH) over Sodium Chloride (NaCl) as an electrolyte and fixed some distances between electrodes to produce a higher hydrogen flow rate.
Chennouf [16] , in his work for NaOH, noted that increasing temperature and electrolyte concentration led to the net increase of volume flow, current intensity. So, better hydrogen production is reached at high temperature and optimal NaOH concentration. Allebrod [17] remarked the conductivity of the commonly used potassium hydroxide (KOH) is well-described from 0˚C to 100˚C and concentrations up to 45 wt% while optimal conductivity occurred in the range of 20% - 30%. Ramadan [15] extracts the concentration ratio of 30 wt% to give higher hydrogen production rate while Fatouh [18] mentioned that this ratio is 26 wt%.
Cold temperatures produce a more efficient photo conversion for single-crystal solar cells. The efficiency of a single-crystal solar cell decreases as the operating temperature of cells increases. It is reported in the literature that the decrease in the efficiency is approximately 0.06 in absolute value per one ˚C increase [19] .
The literature survey revealed that hydrogen production from an alkali electrolyzer has not been fully explored under different operating environments. This research is a concerned with investigating the hydrogen production extracted from alkali electrolyzer working in Egypt climate (outdoor test). The electrolytes are sea and Nile water, natural resources available in Egypt and compared with KOH solution. Proposed research starts by determining the optimum concentration ratio of pure and sea water to produce a maximum hydrogen rate in the indoor test. This ratio will be investigated in the outdoor test and compared with Nile water and a KOH solu- tion of optimum concentration ratio in alkali electrolyzer.
2. Principle of Water Electrolysis
Water electrolysis is a particular electrochemical process in which water is split into its main components, hydrogen and oxygen through the use of continuous electric current [20] .
The electrolytes, more commonly utilized are liquid solutions that may be acidic or alkaline. Alkaline electrolyzer uses an aqueous solution such as, KOH solution (caustic) as an electrolyte that, usually, circulates through the electrolytic cells as shown in Figure 1. The following reactions take place inside the alkaline electrolysis cell with electrical energy supplied [21] :
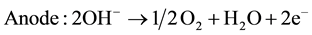 (1)
(1)
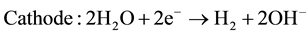 (2)
(2)
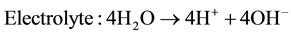 (3)
(3)
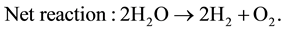 (4)
(4)
3. Experimental Apparatus and Test Procedure
A particular photovoltaic/hydrogen unit is designed and constructed for the hydrogen production from incident solar radiation. The system consists mainly of a photovoltaic module (PV) and an alkali electrolyzer. Figure 2 demonstrates the schematic diagram of the water electrolysis system assisted with photovoltaic cell.
Figure 1. Principle of alkaline water electrolysis.
Figure 2. Alkali electrolyzer assisted with photovoltaic module.
3.1. Photovoltaic Module
This photovoltaic module is a cell of single (mono) crystal module type. It is of commercial use that has a maximum power of 75 Watt. The characteristics of the used module are supplied by the manufacturer and listed in Table 1.
3.2. Alkali Electrolyzer
An experimental alkaline water electrolysis system is designed and constructed in the laboratory. The electrolyzer cell consists of a Perspex cylindrical tank of 20 cm diameter and 20 cm height. A Perspex plate worked as a separator is placed in the middle of the tank at a height of 5 cm from the bottom. In the upper part of the tank, there are two openings of 1 cm diameter for oxygen and hydrogen exit. Oxygen exits to the atmosphere while the hydrogen opening is connected to a digital flow meter. The apparatus showed no leakage after testing seven days.
The two electrodes are stainless steel of 8 mm diameter that placed in both sides of the separator plate. The separator plate splits the tank into two equal halves. The two electrodes are placed at span of 5 cm.
4. Experimental Program
The experimental program includes the influences of Egypt climatic condition on hydrogen produced from alkali electrolyzer working with photo voltaic cell. The program consists of two primary tests; indoor test and outdoor test.
Indoor test investigates the concentration ratio of the sea water that mixed with pure water to identify the concentration ratio that produces the higher hydrogen production flow rate. Also, input voltage to the electrolyte with the different concentrations is examined. Indoor experiment is started when preset input voltage is applied to the electrodes; hydrogen begins to be produced at the cathode then flows to hydrogen opening, next to the flow meter.
The outdoor test explores the productivity and the efficiency of the alkali electrolyzer connected by the photovoltaic cell working in an uncovered area in Cairo, Egypt. Three electrolytes namely; KOH solution, sea water and water of the Nile river are used. KOH solution is a solution of water and KOH with a concentration ratio of 26% that recommended by [15] [18] . Outdoor Experiment is starting at 8 AM and ending at 5 PM approximately. This period covers all the times of sun lights in the winter.
Table 1. The PV characteristics supplied by the manufacturer (at 25˚C ambient temperature and 1000 W/m2 global radiation).
In order to calculate the system efficiency, composed from the electrolyzer unit and photovoltaic cell, the ratio between the energy of hydrogen and incident solar radiation from the sunlight, can be calculated using Equation (5)
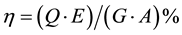 (5)
(5)
η: System efficiency, %
Q: Hydrogen flow rate (ml/min)
E: Calorific value of hydrogen (J/ml)
G: Solar radiation (W/m2)
A: Module area (m2)
Error analysis is performed for the measured quantities and yielded percentage errors listed in Table 2.
5. Results and Discussions
The experimental optimization process was divided into two categories; indoor and outdoor tests. Indoor test investigates the concentration ratio of Egyptian Mediterranean Sea coast with pure water while the outdoor test is concerned with the effect of solar radiation on hydrogen flow rate and the efficiency of the system.
5.1. Effect of Sea Water Concentration
The sea water is a solution of salts that, usually, has a constant composition. It is dissolved in variable amounts of water. The analysis of ions in the Egyptian Mediterranean Sea coast is listed in Table 3 with typical standard sea water analysis according to [22] . All elements are dissolved in sea water and are found as ions-electrically charged atoms or groups of atoms.
Saline water solution (composed from pure water and sea water) is used as electrolyte with concentrations of 25%, 37.5%, 50%, 70%, 85% and 100% of sea water.
Figure 3 shows the hydrogen production rate changes with saline water concentrations at different input voltages. It illustrates that the increase in the concentration of saline water, the higher the hydrogen gas flow rates of hydrogen is obtained. The maximum hydrogen flow rate attained for sea water only. It gives H2 flow rates of 17.4, 26.6 and 43.1 cm3/min for input voltages of 20, 26 and 38 volts, respectively. The sea water has the maximum conductivity without any pure water. The hydrogen flow rates for standalone sea water are presented in Figure 4. The hydrogen flow rate is continuously increasing with input voltage till reach a maximum value of (46 cm3/min) is obtained at the input voltage of 45 Volt, after which the flow rate is decreasing with input voltage increase. This due to that Sulfate and Magnesium salts precipitate on electrode surface due to high voltage forming an insulation layer on the electrode causing decrease in the electrode contact surface area and an extra resistance to ions flow from/to electrodes. Hence, the H2 flow rate tends to decrease.
The maximum hydrogen flow rate achieved from standalone sea water therefore; standalone seawater will be used in the outdoor test for investigation and comparison with KOH and river Nile electrolytes.
5.2. Outdoor Test
The PV module is used to supply power to one alkali electrolyzer. The measured parameters for the module are
Figure 3. The change of hydrogen flow rate with KOH concentration ratio for dif- ferent input voltages.
Figure 4. The hydrogen flow rate as a function of input voltage.
Table 2. Error analysis for measured quantities.
Table 3. Values of the dissolved elements in the Mediterranean Sea coast.
the total solar radiation (G), output current and voltage, module surface temperature and the flow rate of hydrogen at Egypt local civil times. The previous parameters are measured under the climatic conditions of Cairo city, Egypt using the different available electrolytes in Egypt. These electrolytes are the sea water and Nile river water and will be compared with 26% KOH electrolyte as recommended by [15] [18] .
The experiments were taken place in three similar winter days with nearly the same distribution of the incident solar radiation with local time as shown in Figure 5. The distribution in the Figure 5 represents the intensity on the day with a single electrolyte under test. The maximum solar radiation is about 650 W/m2 at solar noon.
The resulting hydrogen flow rate during the operation of the system is demonstrated in Figure 6. The hydrogen flow rate for KOH solution gives the higher production rate (7 - 14.6 ml/min) followed by sea water (0.6 - 1.8 ml/min) and the minimum production rate was for Nile water (0.01 - 0.07 ml/min). Higher flow rate of hydrogen is due to higher conductivity of the elements that is founded with high concentration in the sea water rather than in Nile water like Potassium and Sodium (Table 3). It can be noticed that the maximum production rate is in the solar noon for all used electrolytes. This is due to that the maximum solar radiation was in the solar noon as previously depicted in Figure 5. It is worthwhile that output electric power from a photovoltaic cell highly depends on the solar intensity as shown in Figure 7 which represents the relation between the hydrogen production rate and the incident solar radiation.
Accumulated hydrogen quantities produced during the experiment period (around 9 hours), hence, per day are 4762, 665 and 31.4 ml for KOH solution, sea water and Nile water, respectively, as seen in Figure 8. In other words, it needs about 7 and 153 units of sea water and Nile water, respectively, as an electrolyte to produce the same amount of hydrogen that KOH solution produces daily.
Figure 5. Incident solar radiation with time.
Figure 6. Hydrogen flow rate with time.
Figure 7. Hydrogen production rate as a function of incident solar radiation.
Figure 8. Accumulated hydrogen for each used electrolyte.
In order to assess the competence of the solar-hydrogen unit, the system efficiency is calculated according to Equation (5) for each used electrolyte. The variation in the efficiency of the system with electrolyte replace is plotted in Figure 9 as a function of local time. The higher efficiency is for the system uses a KOH solution followed by sea water followed by Nile water. In a time domain, the efficiency is starting high in the morning, and decreasing to a minimum value at the solar noon and then come back to increase. The higher solar intensity accompanied with higher surface temperatures at the noon are the main factors that contribute in lowering system efficiency to the minimum [15] [19] . The variation of module surface temperature with local time is plotted in Figure 10. The solar-hydrogen system with KOH solution reaches a minimum efficiency of 1% at solar noon
Figure 9. Hydrogen production rate with time.
Figure 10. The change of surface temperature of the PV module with time.
while it attains about 8% at both extremes. Excluding the relatively small periods of beginning and the end of the experiment, the efficiency of the system uses both sea and Nile water gives a nearly constants efficiencies of 0.13% and 0.005% along the experimentation time.
These results are more pronounced in Figure 11 which is the investigation of the efficiency of the solar-hydro- gen unit with incident radiation from the sun according the electrolyte used. The KOH solution has the highest efficiency that decreases from 8% to 1% with increasing solar radiation while it is slightly decreasing and can be assumed constant when sea water and Nile water are used.
6. Conclusion and Recommendations
Egypt has 2940 km of coastal boundaries and the Nile River with the length of 1536 km with abundant solar radiation in this area of the world. The hydrogen producing systems from renewable energy sources are a good alternative to the fuel that can replace conventional fossil fuels in almost every application. Based on the experimental results of according to Egypt climate, the following conclusions can be drawn for maximum hydrogen production rate:
Standalone sea water produces the maximum flow rate of 47 ml/min pure hydrogen when supply voltage is 45 Volt.
It needs about seven units of solar-hydrogen system uses sea water as an electrolyte to produce the same amount of pure hydrogen from a single unit uses KOH solution as an electrolyte. While it requires about 153 units when Nile River is the electrolyte.
Sea water is recommended over the water of Nile River as a good replacement for KOH/water solution electrolyte. Taking into consideration that sea water is abundant and free of charge and, KOH solution is factory made with a specified cost, the hydrogen production using solar-hydrogen unit with sea water as an electrolyte will be effective operation.
Sea water and Nile water give a relatively constant efficiency over the wide range of light time, incident solar radiation and hydrogen production rate.
KOH solution gives a higher efficiency rate than sea water followed by Nile water.
It is recommended for the future work the following.
The research is to be done in different days around the year;
Figure 11. System efficiency change with incident solar radiation.
Investigate the water of the Red sea as an electrolyte;
The future study must focus on the large scale of the unit; and
An economic study has to be applied to the operation of the unit operating with the different electrolytes.
References
- McDonall, W. and Eames, M. (2006) Forecast, Scenarios, Visions, Backcasts and Roadmaps to the Hydrogen Economy: A Review of the Hydrogen Futures Literature. Energy Policy, 34, 1236-1250. http://dx.doi.org/10.1016/j.enpol.2005.12.006
- Envestra Limited (2010) About Natural Gas. http://www.natural-gas.com.au/about/references.html
- ForestBioEnergy (2010) Sustainable Forestry for Bioenergy and Biobased Products. Energy Basics. Fact Sheet 5.8.
- Information and Decision Support Center (IDSC) (2014) Geographical Location. http://www.eip.gov.eg/AboutEgypt/GeoInfo.aspx
- Turner, J.A. (2004) Sustainable Hydrogen Production. Science, 305, 972-974.
- Zavieh, A.H. (2011) Optimization and Modeling of Electrode Structure and Composition for Novel PEM Water Electrolyser MEAs. Norwegian University of Science and Technology, Norway.
- Agbossou, K., Kolhe, M., Hamelin, J. and Bose, T.K. (2004) Performance of a Stand-Alone Renewable Energy System Based on Energy Storage as Hydrogen. IEEE Transactions on Energy Conversion, 19, 633-640.
- Guo, W.L., Li, L., Li, L.L., Tian, S., Liu, S.L. and Wu, Y.P. (2011) Hydrogen Production via Electrolysis of Aqueous For- mic Acid Solutions. International Journal of Hydrogen Energy, 38, 9415-9419. http://dx.doi.org/10.1016/j.ijhydene.2011.04.127
- Pregger, T., Graf, D., Krewitt, W., Sattler, C., Roeb, M. and Möller, S. (2009) Prospects of Solar Thermal Hydrogen Pro- duction Processes. International Journal of Hydrogen Energy, 34, 4256-4267. http://dx.doi.org/10.1016/j.ijhydene.2009.03.025
- Charvin, P., Stéphane, A., Florent, L. and Gilles, F. (2008) Analysis of Solar Chemical Processes for Hydrogen Production from Water Splitting Thermochemical Cycles. Energy Conversion and Management, 49, 1547-1556. http://dx.doi.org/10.1016/j.enconman.2007.12.011
- Santarelli, M. and Macagno, S. (2004) A Thermoeconomic Analysis of a PV-Hydrogen System Feeding the Energy Requests of a Residential Building in an Isolated Valley of the Alps. Energy Conversion and Management, 45, 427- 451. http://dx.doi.org/10.1016/S0196-8904(03)00156-0
- Ahmad, G.E. and Shenawy, E.T.E. (2006) Optimized Photovoltaic System for Hydrogen Production. Renewable En- ergy, 31, 1043-1054. http://dx.doi.org/10.1016/j.renene.2005.05.018
- El Shenawy, E.T. and Hamdy, H. (2012) Annual Performance of a Photovoltaic Hydrogen Electrolyzer System in Egypt. The Pacific Rim Energy and Sustainability Congress, Hiroshima, 6-9 August 2012.
- Wang, Z., Roberts, R.R., Naterer, G.F. and Gabriel, K.S. (2012) Comparison of Thermochemical, Electrolytic, Photoelectrolytic and Photochemical Solar-to-Hydrogen Production Technologies. International Journal of Hydrogen Energy, 30, 1-15.
- Ramadan, A., El-Shenawy, E., Dayem, A.A. and Eid, S.A. (2008) Optimization of PV-Hydrogen Electrolyzes System. 3rd International Conference on Engineering Science & Technology, Cairo.
- Chennouf, N., Settou, N., Negrou, B., Bouziane, K. and Dokkar, B. (2012) Experimental Study of Solar Hydrogen Pro- duction Performance by Water Electrolysis in the South of Algeria. Energy Procedia, 18, 1280-1288. http://dx.doi.org/10.1016/j.egypro.2012.05.145
- See, D.M. and White, R.E. (1997) Temperature and Concentration Dependence of the Specific Conductivity of Con- centrated Solutions of Potassium Hydroxide. Journal of Chemical & Engineering Data, 42, 1266-1268. http://dx.doi.org/10.1021/je970140x
- Fatouh, M., Shedid, M.H. and Elshokary, S. (2013) Effect of Operating and Geometric Parameters on Hydrogen Production from an Alkali Electrolyzer. International Journal on Power Engineering and Energy (IJPEE), 4, 395-399.
- Ibrahim, A. (2011) Analysis of Electrical Characteristics of Photovoltaic Single Crystal Silicon Solar Cells at Outdoor Measurements. Smart Grid and Renewable Energy, 2, 169-175. http://dx.doi.org/10.4236/sgre.2011.22020
- Da Silva, E.P., Marin Neto, A.J., Ferreira, P.F.P., Camargo, J.C., Apolinário, F.R. and Pinto, C.S. (2005) Analysis of Hydrogen Production from Combined Photovoltaics, Wind Energy and Secondary Hydroelectricity Supply in Brazil. Solar Energy, 78, 670-677. http://dx.doi.org/10.1016/j.solener.2004.10.011
- Zeng, K. and Zhang, D. (2010) Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Progress in Energy and Combustion Science, 36, 307-326.
- Lenntech (2014) Composition of Seawater. http://www.lenntech.com/composition-seawater.htm
NOTES
*Corresponding author.














