Energy and Power Engineering
Vol.5 No.2(2013), Article ID:29415,5 pages DOI:10.4236/epe.2013.52013
Effect of Atmosphere on Volatile Emission Characteristic in Oxy-Fuel Combustion
Electric Power Research Institute of Guangdong Power Grid Corporation, Guangzhou, China
Email: wulecim@sina.com
Received December 6, 2012; revised January 10; 2013; accepted January 22, 2013
Keywords: Oxy-Fuel Combustion; O2/CO2 Atmosphere; Volatile Emission; Gasification Reaction
ABSTRACT
A new type of power supply which was called oxy-fuel combustion power plant was introduced to reduce greenhouse gasses emission. In this paper the volatile emission characteristic of pulverized coal is studied under air atmosphere and oxy-fuel atmosphere. Combustion experiments of Datong bituminous coal were carried out in a wire mesh reactor at heating rates of 1 K/s, 10 K/s and 1000 K/s respectively under air and O2/CO2 atmosphere conditions in order to investigate the volatile emission characteristic. The concentrations of volatile (mainly CO and CH4) emission were on-line measured by infrared gas analyzer. It was indicated that the concentrations of CO and CH4 in O2/CO2 atmosphere were higher than those in air. The direct oxidation of carbon and gasification reaction between carbon and CO2 are the main causes of the increased amount of CO. The higher concentration of CO2 also results in the increased amount of CH4 in O2/CO2 conditions.
1. Introduction
The effect of greenhouse gasses on global climate change has resulted in the development of new technologies with lower emissions that can accommodate capture and sequestration of carbon dioxide [1,2], development of clean energy, smart grid and low-carbon economy has become a common choice for the world. Smart grid will penetrate new types of technologies into power supply side, power grid side and power consumer side to promote the carbon reduction in power systems through coordination of multiple technologies [3-5]. In this paper the specific technical path for a smart grid to achieve low-carbon power system are proposed based on clean production, a new type of power supply called oxy-fuel combustion power plant was introduced. Oxy-fuel combustion and CO2 capture from flue gases is a near-zero emission technology that can be adapted to both new and existing pulverized coal-fired power stations. It can produce a sequestration ready high-CO2-concentration effluent gas, and CO2 can be utilized in depleted oil and gas reservoirs to increase their production or storage in deep ocean and saline aquifers. Oxy-fuel combustion is less expensive for retrofitting existed plant than the other considered options [6- 10].
CO production is an important reaction in the coal combustion process, CO/CO2 product ratio is a major factor that influence the combustion temperature and released energy, CO/CO2 product ratio is also one of the basic research difficulties of coal combustion [11,12]. Carbon can be oxidized either to carbon monoxide or to carbon dioxide, the proportion to which carbon converts to either of the two products on the particle surface is still open to debate. CH4 emission characteristics have an important influence on pulverized coal ignition mechanism as the major component of volatile. Therefore, the generation of CO and CH4 has a major impact on combustion heat release and combustion reactions during pulverized coal combustion process [13].
Experimental and simulation studies have been carried out on CO production in the pulverized coal combustion process under air condition [14,15]. However, the reaction of carbon with oxygen is a continuous process, including heat, low temperature oxidation, ignition and combustion process. The measurement of CO is very difficult since carbon monoxide can be further oxidized in the reactor far from the burning particle. In addition, due to the limitations of the test conditions, previous studies were difficult to achieve precise control of particle heating rate, previous studies were also difficult to study CO/CH4 formation characteristics under different heating rates.
The volatile emission characteristic of pulverized coal is very important for understanding how to switch existing burners from air to oxy-fuel combustion, The volatiles typically carry about 50% of the energy of the fueland in addition to heat release, the volatile oxidation is important for ignition. It is well studied under O2/N2 atmosphere, but under oxy-fuel atmosphere it haven’t been recognized clearly [16,17]. In oxy-fuel combustion pulverized coal burns in O2/CO2 environment with higher CO2 concentration, instead of O2/N2 environment. Because of the high toxicity of CO, it is very important to research whether O2/CO2 combustion lead to more CO emissions. Recently, CO emission characteristics under O2/CO2 combustion and conventional air combustion were studied by many scholars. Zheng et al. [18] did simulation study of CO production in O2/CO2 by FACT, but there is a lack of experimental verification. Wang [19] did combustion experiments using a 3 MW level furnace in the same flame temperature under air and O2/CO2 atmosphere, the results showed that there is no great difference of CO content in the flame and flue gas, but modeling results suggested that the flame CO content increased 5 times in flame under O2/CO2 atmosphere. Woycenko et al. [20] did the experiments at a 2.5 MW combustion furnace of IFRF found that CO content increased a lot in the flame zone, but the CO already burn out completely at the furnace exit, there is no observed significant CO emission. Because of the ignition delay and low peak temperature, when the oxygen content is reduced from 30% to 21%, CO emissions increased from 34 ppmv to 200 ppmv. Glarborg et al. [21] did natural gas combustion experiments, the results showed that even if the excess oxygen exist, the high CO2 concentration in the O2/CO2 combustion at high temperatures can also prevent fuel (CO) was completely oxidized to CO2, its inhibitory effect is most obvious in the fuel-rich area.
The general experiments measured the total amount of the CO in the whole reaction process rather than the instantaneous value, therefore they can not reflect the actual CO formation regularities. Because of the presence of secondary reactions in pulverized coal combustion process, CO can be oxidized to CO2 on the surface of coal particles. It is very difficult to research the basis process of coal combustion in O2/CO2 atmosphere.
In this paper, the unique advantages of the wire mesh reactor were used. As was described in more details below [22], the wire-mesh reactor features a relatively accurate control of particle time-temperature history in the absence of significant secondary reactions of volatiles. The measurement of CO and CH4 formation characteristics during pulverized coal combustion can be very accurate in air and O2/CO2 combustion.
2. Materials and Methods
In our study Datong bituminous coal was used, the proximate and ultimate analyses were given in Table 1.
The details of the experimental process can be found in our recent work [22,23], the combustion experiments were carried out in a wire mesh reactor, 30 mg coal sam ple was distributed between two layers of wire mesh. The wire mesh reactor was heated at a rate of 1 K/s, 10 K/s, 1000 K/s to 1273 K/s separately, while a stream of air (or 21% O2/79% CO2) mixture continually passed through the mesh at 4.0 L/min (measured under ambient conditions) to carry the evolved gas away. The concentrations of volatile (mainly CO and CH4) emission were on-line measured by infrared gas analyzer.
Table 1. Properties of coal used.
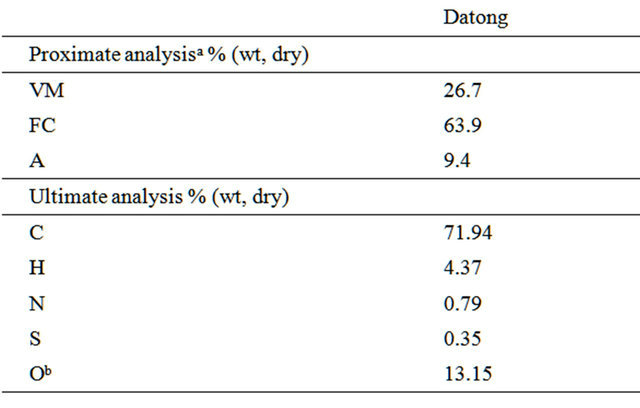
aVM, volatile matter; FC, fixed carbon; A, ash; bBy difference.
3. Results and Discussion
3.1. Effects of Reaction Atmosphere on CO Emission Characteristics
In this paper we studied CO emission characteristics of Datong bituminous coal in air and O2/CO2 atmosphere with a wire mesh reactor heated at a rate of 1 K/s, 10 K/s, 1000 K/s to 1273 K/s separately, results are shown in Figures 1-3.
It can be seen that CO emission concentrations of Datong bituminous coal were higher in O2/CO2 atmosphere than in air atmosphere both under slow heating and fast heating rates. When the reactor was heated at a rate of 1 K/s, the highest concentration of CO in air atmosphere appeared at 863 K, the highest value of CO concentration is 3270 ppm, in O2/CO2 atmosphere the highest value of CO concentration is 4045 ppm as shown in Figure 1. In O2/CO2 atmosphere CO concentrations are generally higher than in traditional air atmosphere, the maximum value is about 23.7% higher. When heated at a rate of 10 K/s, the highest concentration of CO in air atmosphere appeared at 923 K, the highest value of CO concentration is 2464 ppm, in O2/CO2 atmosphere the highest concentration of CO in air atmosphere appeared at 1073 K, the highest value of CO concentration is 7842 ppm which is 3.18 times than that in air atmosphere as shown in Figure 2. When heated at a rate of 1000 K/s, the highest value of CO concentration in air atmosphere is 957.4 ppm, in O2/CO2 atmosphere the highest value of CO concentration is 6023.1 ppm which is 6.3 times than that in
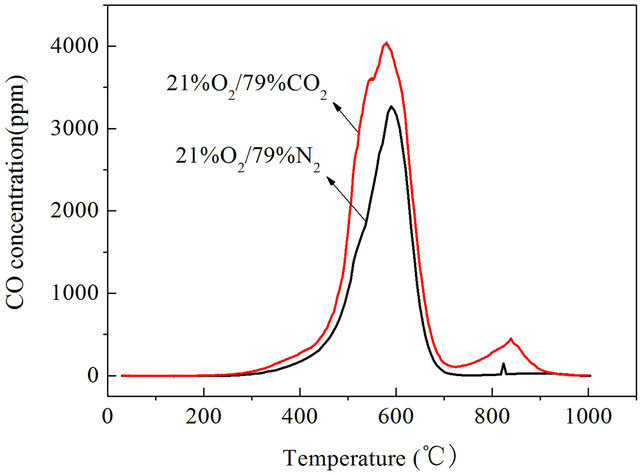
Figure 1. CO emission characteristics with a heating rate of 1 K/s.
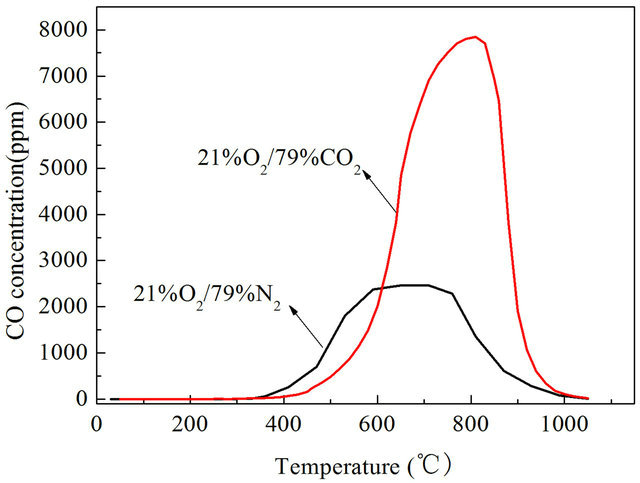
Figure 2. CO emission characteristics with a heating rate of 10 K/s.
air atmosphere as shown in Figure 3.
We found that when the coal was heated at a rate of 10 K/s, the ignition temperature of Datong bituminous coal is 855.99 K (T1) in air atmosphere, the ignition temperature of Datong bituminous coal is 891.73 K (T2) in O2/CO2 atmosphere. That means the temperature corresponds to the maximum CO concentration value is higher than the ignition temperature of pulverized coal, CO concentration continue to rise after coal ignition. In the low-temperature range, CO was produced from volatile emissions and direct oxidation of carbon, CO can be oxidized to CO2 in the presence of oxygen.
CO generate reactions are as follows:
1) Carbon direct oxidation to CO:
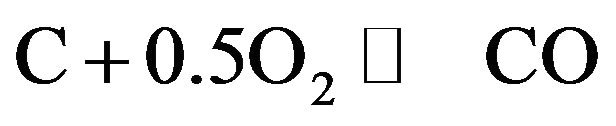 (1)
(1)
2) Because of the existence of the high concentration CO2 gas in O2/CO2 atmosphere and the existence of partly high temperature area, carbon and CO2 gasification gen-

Figure 3. CO emission characteristics with a heating rate of 1000 K/s.
erates CO
 (2)
(2)
3) The gasification reaction of carbon and water
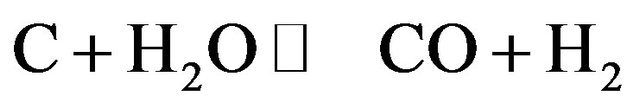 (3)
(3)
4) The reaction of CO2 and H radical
 (4)
(4)
CO2 is not inert but participates in chemical reactions, reactions of CO2 with free radicals may proceed at lower temperatures. The most important step is the reaction with atomic hydrogen (Equation (4)) which is comparatively fast even at medium temperatures. The reactions of CO2 with hydrocarbon radicals, will contribute to CO formation in the near-burner region under oxy-fuel combustion conditions. The higher levels of CO in this region may conceivably increase local problems with corrosion and slag, depending on the burner geometry and strategies for oxygen and flue gas. It would also be expected that the full oxidation of CO to CO2 would occur further downstream in the furnace compared to conventional combustion [24-26]. Reactions between CO2 and CH2 radicals may also attribute to the increase of CO content in the flame zone, but because of the less number of free radicals that reaction would not be expected to be the dominating reaction for the increasing of CO content.
In O2/CO2 combustion of coal the above reaction all exist, the thermal decomposition of CO2 is not the main reason for CO content increases in the flame. In O2/CO2 combustion due to the presence of high concentrations of CO2 and partial area with high temperature, gasification reaction between carbon and carbon dioxide generate CO is more likely to occur than air combustion. Therefore, the direct oxidation of carbon and gasification reaction between carbon and CO2 is the main reason for the increasing of CO content in oxy-fuel combustion.
3.2. Effects of Reaction Atmosphere on CH4 Emission Characteristics
CH4 is an important component of the volatile during pulverized coal combustion and pyrolysis process, the CH4 emission characteristics is very important for understanding the combustion process of pulverized coal in air and O2/CO2 combustion. We studied CH4 emission characteristics of Datong bituminous coal in air and O2/CO2 atmosphere with a wire mesh reactor heated at a rate of 1 K/s, 10 K/s,1000 K/s to 1273 K/s separately, results are shown in Figures 4-6.
It can be found that CH4 emission concentrations of Datong bituminous coal were higher in O2/CO2 atmosphere than in air atmosphere both under slow heating and fast heating rates. When the reactor was heated at rate of 1 K/s, the highest value of CH4 concentration is 75.6 ppm in air atmosphere, in O2/CO2 atmosphere the highest value of CH4 concentration is 107.19 ppm as shown in Figure 4. In O2/CO2 atmosphere CH4 concentrations are generally higher than in traditional air atmosphere, the maximum value is about 41.8% higher. When the reactor was heated at rate of 10 K/s, the highest value of CH4 concentration is 1104 ppm in air atmosphere, in O2/CO2 atmosphere the highest value of CH4 concentration is 1694 ppm which is about 41.8% higher than that under air condition as shown in Figure 5. When heated at a rate of 1000 K/s, the highest value of CH4 concentration in air atmosphere is 318.7 ppm, in O2/CO2 atmosphere the highest value of CH4 concentration is 960.2 ppm which is 3.01 times than that in air atmosphere as shown in Figure 6. So we can say that in O2/CO2 the CH4 emission concentrations were higher than that in air atmosphere.
Coal pyrolysis process is consist of some weak bonds breaking in macromolecules of coal at high temperature, then light gaseous substances, tar, etc release. The weakest depolymerization to generate small molecule chain occurred in early pyrolysis process, the occurrence of medium temperature cross-linking generating CH4. Another process in early pyrolysis is the functional group decomposition generating CH4, CO, H2 and other gases. Reaction atmosphere affect coal pyrolysis process, atmosphere has an impact on pyrolysis products through secondary reactions. In O2/CO2 combustion, the presence of high concentrations of CO2 promotes the formation of CH4, therefore leading to the increase of CH4 concentration in oxy-fuel combustion.
4. Conclusions
Combustion experiments of Datong bituminous coal were carried out in a wire-mesh reactor at heating rates of 1 K/s, 10 K/s and 1000 K/s respectively under air and O2/ CO2 atmosphere conditions. The concentrations of CO and CH4 emission from the coal were on-line measured
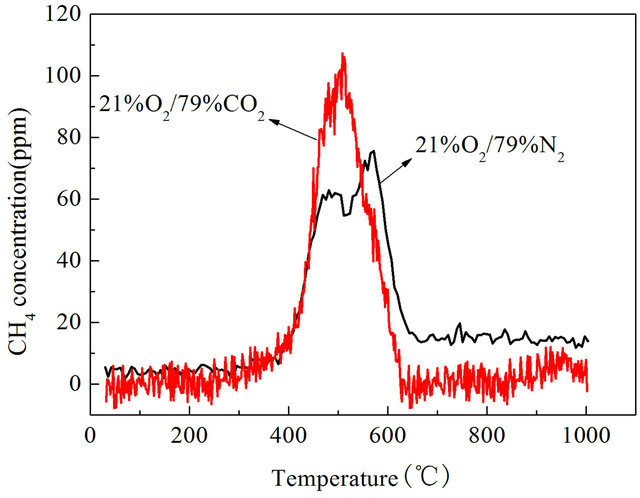
Figure 4. CH4 emission characteristics with a heating rate of 1 K/s.
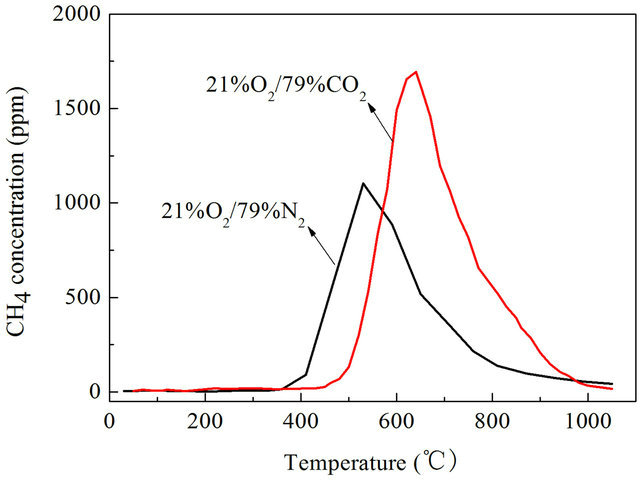
Figure 5. CH4 emission characteristics with a heating rate of 10 K/s.
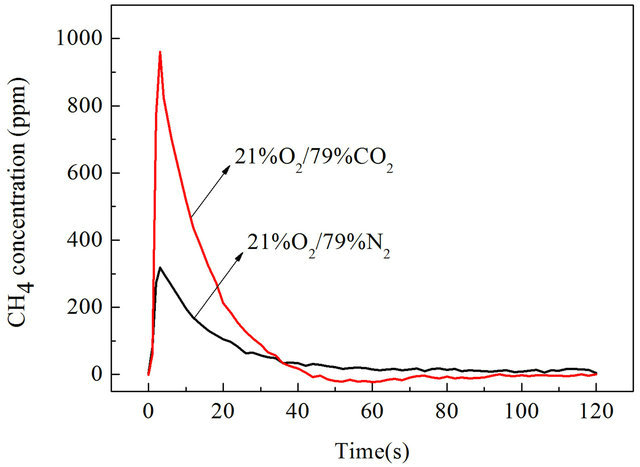
Figure 6. CH4 emission characteristics with a heating rate of 1000 K/s.
by infrared gas anlyzer. The following main conclusions can be drawn: 1) the concentrations of CO and CH4 in O2/CO2 atmosphere were higher than those in air. The direct oxidation of carbon and gasification reaction between carbon and CO2 are the main causes of the increased amount of CO; 2) the higher concentration of CO2 also results in the increased amount of CH4 in O2/ CO2 conditions.
REFERENCES
- S. Gunter, A. M. Leema, S. Uwe and M. Jorg, “Oxy-Fuel Coal Combustion—A Review of the Current State of the Art,” International Journal of Greenhouse Gas Control, Vol. 5, No. S1, 2011, pp. 16-35. doi:10.1016/j.ijggc.2011.05.020
- F. W. Terry, “Combustion Processes for Carbon Capture,” Proceedings of the Combustion Institute, Vol. 31, No. 1, 2007, pp. 31-47. doi:10.1016/j.proci.2006.08.123
- Z. Liu, “Build Strong Smart Grid as Pillar of Sound and Rapid Development,” Power System and Clean Energy, Vol. 25, No. 9, 2009, pp. 1-3. (in Chinese)
- EPRI, “Power Delivery System and Electricity Markets of the Future,” Palo Alto, CA, EPRI, 2003.
- M. Amin and P. F. Schewe, “Preventing Blackouts: Building a Smarter Power GRID,” Scientific American, 2008, pp. 60-67.
- B. T. Maja, B. Jacob, A. J. Peter, G. Peter, D. J. Anker, “Oxy-Fuel Combustion of Solid Fuels,” Progress in Energy and Combustion Science, Vol. 36, No. 5, 2010, pp. 581-625. doi:10.1016/j.pecs.2010.02.001
- B. J. P. Buhre, L. K. Elliott, C. D. Sheng, R. P. Gupta and T. F. Wall, “Oxy-Fuel Combustion Technology for Coal- Fired Power Generation,” Progress in Energy and Combustion Science, Vol. 31, No. 4, 2005, pp. 283-307. doi:10.1016/j.pecs.2005.07.001
- D. Singh, E. Croiset, P. L. Douglas and M. A. Douglas, “Techno-Economic Study of CO2 Capture from an Existing Coal-Fired Power Plant: MEA Scrubbing vs O2/CO2 Recycle Combustion,” Energy Conversion and Management, Vol. 44, No. 19, 2003, pp. 3073-3091. doi:10.1016/S0196-8904(03)00040-2
- M. Alejandro and R. S. Christopher, “Ignition and Devolatilization of Pulverized Bituminous Coal Particles during Oxygen/Carbon Dioxide Coal Combustion,” Proceedings of the Combustion Institute, Vol. 31, No. 2, 2007, pp. 1905-1912. doi:10.1016/j.proci.2006.08.102
- A. B. Paula and A. Yiannis, “Single-Coal-Particle Combustion in O2/N2 and O2/CO2 Environments,” Combustion and Flame, Vol. 153, 2008, pp. 270-287.
- T. Zeng and W. Fu, “The Ratio CO/CO2 of Oxidation on a Burning Carbon Surface,” Combustion and Flame, Vol. 107, No. 3, 1996, pp. 197-210. doi:10.1016/S0010-2180(96)00071-5
- M. Faraday and C. Lyell, “Explosions in Coal Mines,” Philosophical Magazine, Vol. 26, 1845, pp. 16-35.
- K. Cen, Q. Yao and Z. Luo, “Advanced Combustion,” Zhejiang University Press, Hangzhou, 2002, pp. 277-284.
- J. Arthur, “Reactions between Carbon and Oxygen,” Transactions of the Faraday Society, Vol. 47, 1951, pp. 164-178. doi:10.1039/tf9514700164
- L. Tognotti, J. Longwell and A. Sarofim, “The Products of the High Temperature Oxidation of a Single Char Particle in an Electrodynamic Balance,” Proceedings of the Combustion Institute, Vol. 23, No. 1, 1991, pp. 1207- 1213.
- N. Kimura, K. Omata, T. Kiga, S. Takano and S. Shikisima, “Characteristics of Pulverized Coal Combustion in O2/CO2 Mixtures for CO2 Recovery,” Energy Conversion and Management, Vol. 36, No. 6-9, 1995, pp. 805-808. doi:10.1016/0196-8904(95)00126-X
- K. Renu, K. Liza and F.Terry, “Differences in Reactivity of Pulverised Coal in Air (O2/N2) and Oxy-Fuel (O2/CO2) Conditions,” Fuel Processing Technology, Vol. 90, No. 6, 2009, pp. 797-802. doi:10.1016/j.fuproc.2009.02.009
- L. Zheng and E. Furimsky, “Assessment of Coal Combustion in O2/CO2 by Equilibrium Calculations,” Fuel Processing Technology, Vol. 81, No. 1, 2003, pp. 23-34. doi:10.1016/S0378-3820(02)00250-3
- C. Wang, G. Berry and K. Chang, “Combustion of Pulverized Coal Using Waste Carbon Dioxide and Oxygen,” Combustion and Flame, Vol. 72, No. 3, 1988, pp. 301- 310. doi:10.1016/0010-2180(88)90129-0
- D. Woycenko, K. Vande and P. Roberts, “Combustion of Pulverized Coal in a Mixture of Oxygen and Recycled Flue Gas,” European Commission Journal of Clean Coal Technology Program, Vol. 1, 1995, pp. 92-99.
- P. Glarborg and L. Bentzen, “Chemical Effects of a High CO2 Concentration in Oxy-Fuel Combustion of Methane,” Energy & Fuels, Vol. 22, No. 1, 2008, pp. 291-296. doi:10.1021/ef7005854
- Y. Qiao, L. Zhang and E. Binner, “An Investigation of the Causes of the Difference in Coal Particle Ignition Temperature between Combustion in Air and in O2/CO2,” Fuel, Vol. 89, No. 11, 2011, pp. 3381-3387. doi:10.1016/j.fuel.2010.05.037
- L. Wu, Y. Qiao, B. Gui and M. Xu, “Effects of Chemical Forms of Alkali and Alkaline Earth Metallic Species on the Char Ignition Temperature of a Loy Yang Coal under O2/N2 Atmosphere,” Energy & Fuels, Vol. 26, No. 1, 2012, pp. 112-117. doi:10.1021/ef2011386
- F. Liu, H. Guo and G. Smallwood, “The Chemical Effect of CO2 Replacement of N2 in Air on the Burning Velocity of CH4 and H2 Premixed Flames,” Combustion & Flame, Vol. 133, No. 4, 2003, pp. 495-497.
- F. Liu, H. Guo, G. Smallwood and O. Gulder, “The Chemical Effects of Carbon Dioxide as an Additive in an Ethylene Diffusion Flame: Implications for Soot and NOx Formation,” Combustion & Flame, Vol. 125, No. 1-2, 2001, pp. 778-787. doi:10.1016/S0010-2180(00)00241-8
- A. Masri, R. Dibble and R. Barlow, “Chemical Kinetic Effects in Nonpremixed Flames of H2/CO2 Fuel,” Combustion & Flame, Vol. 91, No. 3-4, 1992, pp. 285-309. doi:10.1016/0010-2180(92)90059-X

