Green and Sustainable Chemistry
Vol.07 No.03(2017), Article ID:78185,10 pages
10.4236/gsc.2017.73015
Production of Bio-Phenols for Industrial Application: Scale-up of the Base-Catalyzed Depolymerization of Lignin
Björn Rößiger, Robert Röver, Gerd Unkelbach, Daniela Pufky-Heinrich
Fraunhofer Center for Chemical-Biotechnological Processes (CBP), Leuna, Germany

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 4, 2017; Accepted: August 1, 2017; Published: August 4, 2017
ABSTRACT
Objective of this study was the investigation on the up-scaling of base-cata- lyzed depolymerization (BCD) of lignin to pilot plant dimension. The cleavage process was carried out in dilute alkaline solution at temperatures up to 340˚C and a pressure of 25 MPa in a continuously operated tubular flow reactor with throughputs up to 20 kg/h. Investigations included the proof of the feasibility of the scale-up as well as a parameter study on the cleavage of hardwood Organosolv lignin and softwood Kraft lignin within the established pilot plant. Yields and molecular compositions of the isolated product fractions BCD-oil (liquid phenolic fraction) and BCD-oligomers (solid phenolic fraction) are similar to those described in technical lab scale, showing a good scalability. Here, BCD-oils rich in phenolic monomers such as guaiacol, catechol and/or syringol were obtained with a content of up to 13.3 wt% and 14.5 wt% from Organosolv lignin and Kraft lignin, respectively. Formation of BCD- oligomers strongly depends on temperature and residence times within the reactor.
Keywords:
Lignin, Base-Catalyzed Depolymerization, Phenolic Monomers, Phenolic Oligomers

1. Introduction
The valorization of lignin is one of the most important challenges for the development of cost-effective biorefinery processes based on lignocellulosic biomass. Herein, the alkylphenolic structure of the lignin molecule can be catalytically transferred into low molecular weight compounds such as phenols, alkylphenols and phenol resins and replacing those obtained from fossil resources [1] [2] . The products are highly promising fuels or fuel additives [3] [4] , antifungal components [5] or for the preparation of polyols and polyurethane resins [6] . However, the natural complexity and high stability of lignin bonds make lignin depolymerization still a challenging task. The process of base-catalyzed depolymerization (BCD) of lignin results in hydrolysis of the aryl-aryl-ether bonds and the aryl- alkyl-ether bonds in the lignin macromolecule and thereby in the production of monomers as well as dimeric and oligomeric alkyl-functionalized phenolic compounds [7] [8] [9] . The BCD process is carried out in aqueous or alcoholic systems at temperatures of up to 350˚C, pressures up to 25 MPa and mainly at short residence times (<1 h).
Intensive research on the BCD of several types of lignin has been performed and optimized at laboratory and technical lab scale. From this it is evident that a proper selection of the reaction parameters influences the process of lignin depolymerization and subsequent repolymerization of unsaturated decomposition products. Char formation caused by repolymerization can be minimized at continuous operation of the BCD process due to a precise setting of reaction parameters (temperature, residence time) and engineering design of the reactor (e.g. heating and cooling rates, residence time distribution, material design). Dedicated reactor systems have been described at laboratory and technical lab scale [10] [11] and also at a more upscale and direct approach [12] [13] . However, a further development towards an industrialization of the process with demonstration of the procedure in industrial relevant environment on the pilot scale (technology readiness level TLR 6) has not been shown so far. This development is mandatory for investigation of efficient downstream processing. Sustainable processes for phenol separation and purification have to be found giving platform and fine chemicals in high quality for further application. Until now, the down streaming of hydrothermal conversion processing of lignin has been hardly investigated [14] .
Aim of this study was to evaluate a pilot scale flow reactor system for BCD with different kinds of lignin and thus, to validate technical lab scale design and data [10] . Processability of the cleavage process in industrial relevant environment is the first step towards a process design that is material and energy efficient for integration as a technology module in a lignocellulosic biorefinery.
2. Materials and Methods
2.1. Materials
Two different lignins were investigated for their suitability for pilot plant BCD and separability of cleavage products: beech wood Organosolv lignin (Fraunhofer CBP) and spruce wood Kraft lignin (Domtar BioChoiceTM). Gel permeation chromatography (GPC) showed number average molecular weights and the weight average molecular weights MN/MW of 1006/2757 g/mol and 1488/10,590 g/mol, respectively. The moisture contents of the lignins were 3.1 wt% (Organosolv lignin) and 33.8 wt% (Kraft lignin).
The Organosolv lignin feed solutions were prepared dissolving lignin (2.5, 5.0, 10.0 wt% rel. dry mass) in diluted alkaline solution (2.5 wt% sodium hydroxide). The Kraft lignin feed solutions were prepared with a constant lignin: sodium hydroxide ratio of 1:1 (2.5, 5.0, 7.5 wt% rel. dry mass Kraft lignin/sodium hydroxide).
2.2. Base-Catalyzed Depolymerization
BCD was carried out in a 2150 mm long, 40 mm internal diameter flow reactor made of Inconel 625 high-grade steel. Figure 1 shows a schematic flow sheet of the pilot plant. The prepared alkaline lignin solution was kept stirred at room temperature in a 300 l mixing vessel and continuously fed to the pilot plant. The reactor was slowly pressurized to 25 MPa and heated to desired temperature using deionized water. Once the reactor reached steady-state, the feed was switched to the alkaline lignin solution. The mass flow rate can be varied in the range of 5 to 20 kg/h by a high-pressure pump and an automatic adjustable pressure retention valve. The residence time in the reactor was calculated in the range of 450 to 900 s assuming ideal plug flow. The density of the reaction mixture, which affects the residence time, was calculated depending on the reaction temperature and the feed composition (lignin or sodium hydroxide concentration). At the reactor outlet, the reaction product passed through a double pipe heat exchanger preheating the feed solution to approximately 200˚C and cooling down the reactor outlet to approximately 60˚C.
After heat recovery, the reaction product mixture was water-cooled by a double pipe heat exchanger and depressurized to ambient conditions. Gaseous reaction products were treated by thermal post-incineration. Liquid reaction products were continuously collected in an intermediate bulk container. An inline product-
Figure 1. Schematic flow sheet of the depolymerization pilot plant.
sampling pipeline installed in front of the vapour-liquid separator receiving samples for recovery of the obtained product fractions.
2.3. Separation and Purification of Reaction Products
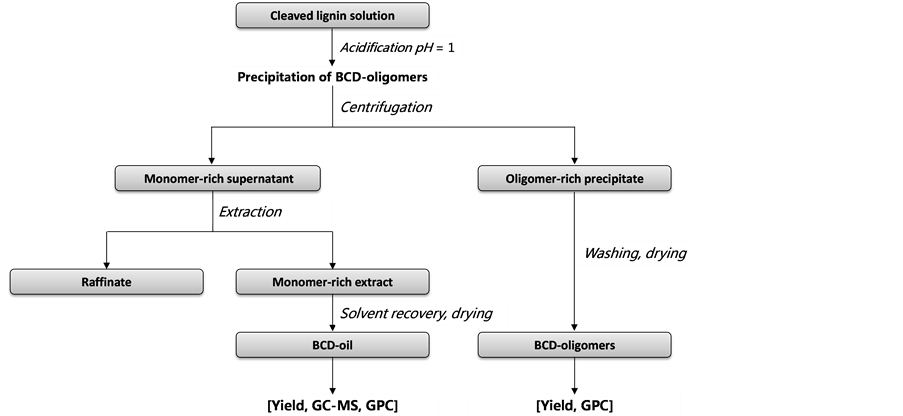
The separation and purification of the cleavage solution was performed in lab scale for analytical purposes from product-sampling during parameter studies at pilot scale depolymerization of lignin. An appropriate protocol was implemented on the basis of a previous publication on technical lab scale depolymerization [10] . Figure 2 shows a summary of the separation and purification procedure and the analytical methods that were applied on the obtained liquid and solid product fractions (BCD-oil, BCD-oligomers). The gaseous reaction products formed during the reaction were not analysed. At first, 500 g of the cleavage reaction product mixture was acidified to pH 1 using hydrochloric acid (37 wt%) to precipitate BCD-oligomers, modified lignin and condensed repolymerization products. The suspension was separated by centrifugation operating at 149 rad/s, 20˚C for 15 minutes. The centrifugation gave a transparent, light yellow to dark orange monomer-rich supernatant and a brown to black oligomer-rich solid precipitate. After decantation, the precipitate was washed with 500 ml of deionized water for salt removal and centrifuged again at the same operation conditions.
The purified precipitate was dried in air at 105˚C to constant weight and kept as BCD-oligomers for further analysis. The monomer-rich supernatants were combined and extracted tree times with 175 ml of methyl isobutyl ketone MIBK (25, 50, 100 ml). The organic phases of each extraction were combined and dried with anhydrous sodium sulphate. The drying agent was washed with 25 ml MIBK. After filtration, the organic solvent was removed from the extract by vacuum-evaporation and the obtained BCD-oil was vacuum-dried to constant weight at 40˚C.
Figure 2. Summary of the separation and purification protocol and analytical characterisation methods.
2.4. Analytical Characterization of Reaction Products
The yields of the obtained product fractions were calculated using Equation (1) and Equation (2).
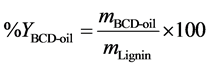 (1)
(1)
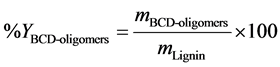 (2)
(2)
where, 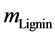 is the mass of lignin in 500 g reaction product mixture,
is the mass of lignin in 500 g reaction product mixture, 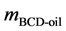 is the dry mass of extracted BCD-oil and
is the dry mass of extracted BCD-oil and 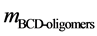 is the dry mass of precipitated BCD-oligomers.
is the dry mass of precipitated BCD-oligomers.
The number average molecular weights MN and the weight average molecular weights MW of both fractions were determined by gel permeation chromatography (GPC) using a HPLC series 1260 (Agilent Technologies), equipped with GPC- columns (Appli Chrom ABOA DMSO-Phil-P Pore 250 & 350) and a refractive index detector.
The concentration of the phenolic monomers guaiacol, catechol and syringol in the BCD-oil were quantified by gas chromatography-mass spectrometry (Agilent GC 7890A and Agilent MSD 5975C) equipped with a HP-MS5 capillary column (length 30 m, inner diameter 0.25 mm, film thickness 0.33 µm). 1-Me- thylnaphalene was used as internal calibration standard.
3. Results and Discussion
The feasibility of base-catalyzed depolymerization (BCD) using a pilot scale flow reactor was demonstrated for cleaving Organosolv lignin and Kraft lignin. Parametric studies were performed on the catalytic depolymerization in order to evaluate the influence of the process parameters (temperature, residence time, lignin and sodium hydroxide concentration) on the isolated yields and the molecular composition of the obtained product fractions (BCD-oil, BCD-oligo- mers).
The aim of this investigation was to identify operating parameters resulting in high product yield and depolymerization degree, which means low molecular weights of BCD-oligomers and high monomer concentration in the BCD-oil. Simultaneously, the results should be compared with the data from technical lab scale experiments for scalability proofing [10] .
3.1. Effect of Temperature and Residence Time
It is observed that BCD-oil formation increases and BCD-oligomer formation decreases with higher process intensity. Although temperature has the strongest influence on the yields and the degree of conversion, the impact of residence time constantly raised with increasing temperature. Figure 3 shows the effects of temperature and residence time on the yields and the molecular weights of the obtained BCD-fractions and the monomer concentrations in BCD-oils. For this, a conversion of 2.5 wt% lignin dissolved in 2.5 wt% NaOH solution was investigated.
Figure 3. Effects of temperature (250˚C, 275˚C, 300˚C, 340˚C) and residence time (450, 600, 750 s) on (1) The yields of BCD- oil and BCD-oligomers (wt% lignin) (top left); (2) The monomer concentration in BCD-oil (wt% oil) (top right); and (3) The number/weight average molecular weights MN/MW of BCD-oil and BCD-oligomers (below) (MN/MW, Organosolv lignin = 1006/2757 g/mol, MN/MW,Kraft lignin = 1488/10,590 g/mol) (lignin concentration: 2.5 wt%, sodium hydroxide concentration: 2.5 wt%).
With Organosolv lignin, the highest BCD-oil yield (13.3 wt%) and the lowest BCD-oligomer yield (46.6 wt%) was reached at 340˚C and a residence time of 750 s. This BCD-oil consisted of 1 wt% guaiacol and 36 wt% catechol. Due to demethoxylation reactions no syringol was quantifiable at these strict process conditions. The same trend was noted at the cleavage of sulphur-containing Kraft lignin. The highest BCD-oil yield (14.5 wt%) and lowest BCD-oligomer yield (58.8 wt%) was obtained at the strictest process conditions (300˚C, 750 s) applied on Kraft lignin. Here, the BCD-oil contained 9 wt% guaiacol and 24 wt% catechol.
Similarly, a significant influence of process parameters on the molecular weights of the cleavage products was observed. In general, the number average molecular weights MN of the BCD-oligomers were significant lower compared to the initial lignin due to cleavage of the ether-bonds within the lignin molecule and thus, formation of oligomeric lignin structures. The lowest molecular weight of Organosolv BCD-oligomers (MN/MW 675/1372 g/mol) was determined at 250˚C and residence time of 600 s. At high temperatures about 300˚C a repolymerization of the BCD-oligomers, especially from Organosolv lignin, to high molecular weight products (MN/MW 1228/4377 g/mol at 340˚C, 750 s) was observed.
In order to avoid repolymerization, the temperature was limited to 300˚C in the Kraft lignin depolymerization studies. For that reason and in contrast to Organosolv lignin depolymerization, no high molecular weight products were proved using Kraft lignin. The molecular weights of Kraft BCD-oligomers constantly decreased with increasing process intensity.
It can be summarized that with respect to the monomer concentration in BCD-oil, an increase in temperature from 250˚C to 340˚C favoured the formation of catechol and reduced the amounts of guaiacol and syringol in the BCD- oil. In comparison to this temperature effect the residence time has a subsidiary effect. An increase in the retention time of 450 s to 750 s at 300˚C enhanced the formation of catechol and reduced the amounts of guaiacol and syringol.
Higher yields of cleavage products for both types of lignin as well as no repolymerization were found during technical lab scale experiments [10] . This might be due to the differences in reactor design, which causes deviations in flow pattern and residence time distribution. The influence of these transport phenomena on yield, selectivity and product quality will be quantitatively investigated in further pilot scale experiments.
3.2. Effect of Lignin and Sodium Hydroxide Concentration
Besides the influence of temperature and residence time, the feedstock lignin concentration was varied between 2.5 wt% and 10 wt%. The effects on the yields, the molecular weights and the monomer concentrations in BCD-oil were investigated at constant process parameters (300˚C, 600 s, 2.5 wt% NaOH). The results are summarized at Table 1.
With increasing lignin concentration (2.5 wt% to 10.0 wt% Organosolv lignin), the BCD-oil yield drops from 11.2 wt% to 6.3 wt% applying a constant NaOH concentration of 2.5 wt%. The significant decrease in BCD-oil yield and the simultaneous increase in the molecular weights and yields of oligomeric products with higher lignin concentration, are pointing to an incomplete deprotonation of the lignin hydroxyl groups due to a too low NaOH concentration. For this reason, both the lignin and the NaOH concentration were increased in the experiments using Kraft lignin. Finally, constant lignin: sodium hydroxide ratio leads to constant product yields over the entire concentration range. Nearly constant yields and molecular weights of BCD-oil (12.1 to 13.4 wt%, MN/MW = 163/294 g/mol to 168/297 g/mol) and BCD-oligomers (57.3 to 63.4 wt%, MN/MW = 606/1442 g/mol to 697/1660 g/mol) were found within the entire concentration range.
Table 1. Effects of lignin concentration (2.5, 5.0, 7.5, 10.0 wt%) and sodium hydroxide concentration (2.5, 5.0, 7.5 wt%) on (1) The yields of BCD-oil and BCD-oligomers (wt% lignin); (2) The monomer concentration in BCD-oil (wt% oil); and (3) The number/weight average molecular weights of BCD-oil and BCD-oligomers (MN/MW,Organosolv lignin = 1006/2757 g/mol, MN/MW,Kraft lignin = 1488/10590 g/mol) (temperature: 300˚C, residence time: 600 s).
4. Conclusions
Bio-phenolic compounds are promising candidates for industrial application, e.g., as precursors for fine chemicals or as monomers for biopolymer production. The provision of sufficient sample quantities in reproducible, high product quality is prerequisite for the implementation of these technologies in biorefinery concepts. Therefore the design of a continuous process in industry-relevant environment is the first step for a successful implementation.
The feasibility of BCD of lignin using a pilot scale flow reactor system was successfully demonstrated. Parameter studies were performed to evaluate the influence of the process parameters temperature, residence time, lignin and sodium hydroxide concentration on the isolated yields and the molecular composition of the recovered product fractions BCD-oil and BCD-oligomers. Yields of up to 13.3 wt% and 14.5 wt% for BCD-oils from Organosolv lignin and Kraft lignin were obtained. These results confirm the trends from published lab scale depolymerization studies utilizing a comparable reactor set-up [10] .
Higher process intensity results in higher depolymerization degree and thus, in growing formation of monomeric and dimeric phenolic products and also of gaseous and liquid degradation products from lignin, like methanol and acetic acid. From the results, a sudden decrease in product yield of BCD-oil and BCD- oligomers can be assumed above 300˚C reaction temperature and prolonged retention times (≥600 s) accompanied with an increase rise in repolymerization products. A continuous BCD is run up to 10 wt% of lignin under the prerequisite that repolymerization is prevented and the pH value is kept constant by adding correspondence equivalents of base.
5. Outlook
Further scientific investigation of the catalytic cleavage of lignin will be performed in order to gain a deeper understanding of the reaction mechanism, to define product specifications of phenol derivatives and to deliver an efficient number of process and engineering data for further scale-up. Moreover, development and optimization of downstream and purification methods for the selective separation of the desired products is essential and will be carried out. In order to increase the technology readiness level of the BCD process and thus, to develop an industry relevant process an overall approach regarding material and energy efficiency has to be established as well as its technical feasibility and implementation examined.
Acknowledgements
The authors gratefully thank and acknowledge Moritz Leschinsky, PhD (Fraunhofer CBP) and for the Organosolv lignin supply and Detlef Schmiedl, PhD (Fraunhofer ICT) for providing detailed data from technical lab scale screening experiments.
Moreover, we would like to acknowledge the Federal Ministry of Food and Agriculture (BMEL) and the “Fachagentur Nachwachsende Rohstoffee. V.” (FNR) for the financial support of the project “Lignoplast” (support code 22014212).
Cite this paper
Rößiger, B., Röver, R., Unkelbach, G. and Pufky-Heinrich, D. (2017) Production of Bio-Phenols for Industrial Application: Scale-Up of the Base- Catalyzed Depolymerization of Lignin. Green and Sustainable Chemistry, 7, 193-202. https://doi.org/10.4236/gsc.2017.73015
References
- 1. Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L. and Weckhuysen, B.M. (2010) The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chemical Reviews, 110, 3552-3599. https://doi.org/10.1021/cr900354u
- 2. Graglia, M., Kanna, N. and Esposito, D. (2015) Lignin Refinery: Towards the Preparation of Renewable Aromatic Building Blocks. ChemBioEng Reviews, 2, 377-392.
- 3. Li, C., Zhao, X., Wang, A., Huber, G.W. and Zhang, T. (2015) Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chemical Reviews, 115, 11559-11624. https://doi.org/10.1021/acs.chemrev.5b00155
- 4. Xu, C., Arancon, R.A., Labidi, J., and Luque, R. (2014) Lignin Depolymerisation Strategies: Towards Valuable Chemicals and Fuels. Chemical Society Reviews, 43, 7485-7500. https://doi.org/10.1039/C4CS00235K
- 5. Dos Santos, P.S.B., Erdocia, X., Gatto, D.A. and Labidi, J. (2016) Bio-Oil from Base-Catalyzed Depolymerization of Organosolv Lignin as an Antifungal Agent for Wood. Wood Science and Technology, 50, 599-615. https://doi.org/10.1007/s00226-015-0795-8
- 6. Mahmood, N., Yuan, Z., Schmidt, J. and Xu, C.(2016) Depolymerization of Lignins and Their Applications for the Preparation of Polyols and Rigid Polyurethane Foams: A Review. Renewable and Sustainable Energy Reviews, 60, 317-329.
- 7. Schmiedl, D., Böringer, S., Schweppe, R. and del Río, J.C. (2014) Kraft Lignin Depolymerisation by Based Catalysed Degradation (BCB)—The Effect of Process Parameters on Conversion Degree and Structural Features of BCD-Fractions. 13th European Workshop on Lignocellulosics and Pulp, Seville.
- 8. Zhang, Y., Ye, Y.Y., Fan, J. and Chang, J. (2013) Selective Production of Phenol, Guaiacol and 2,6-Dimethoxyphenol by Alkaline Hydrothermal Conversion of Lignin. Journal of Biobased Materials and Bioenergy, 7, 696-701. https://doi.org/10.1166/jbmb.2013.1397
- 9. Katahira, R., Mittal, A., McKinney, K., Chen, X. Tucker, M.P., Johnson, D.K. and Beckham, G.T. (2016) Base-Catalyzed Depolymerization of Biorefinery Lignins. ACS Sustainable Chemistry & Engineering, 4, 1474-1486. https://doi.org/10.1021/acssuschemeng.5b01451
- 10. Schmiedl, D., Endisch, S., Pindel, E., Rückert, D., Reinhardt, S., Unkelbach, G. and Schweppe, R. (2012) Base Catalyzed Degradation of Lignin for the Generation of Oxy-Aromatic Compounds—Possibilities and Challenges. Erdöl Erdgas Kohle, 128, 357-363.
- 11. Roberts, V.M., Stein, V., Reiner, T., Lemonidou, A., Li, X. and Lercher, J.A. (2011) Towards Quantitative Catalytic Lignin Depolymerization. Chemistry—A European Journal, 17, 5939-5948. https://doi.org/10.1002/chem.201002438
- 12. Nguyen, T.D.H., Maschietti, M., Belkheiri, T., ömand, L.-E., Theliander, H., Vamling, L., Olausson, L. and Andersson, S.-I. (2014) Catalytic Depolymerisation and Conversion of Kraft Lignin into Liquid Products Using near-Critical Water. Journal of Supercritical Fluids, 86, 67-75.
- 13. Beauchet, R., Monteil-Rivera, F. and Lavoie, J.M. (2012) Conversion of Lignin to Aromatic-Based Chemicals (L-Chems) and Biofuels (L-Fuels). Bioresource Technology, 121, 328-334.
- 14. Schuler, J., Hornung, U., Kruse, A., Dahmen, N. and Sauer, J. (2017) Hydrothermal Liquefaction of Lignin. Journal of Biomaterials and Nanobiotechnology, 8, 96-108. https://doi.org/10.4236/jbnb.2017.81007