World Journal of AIDS
Vol. 2 No. 1 (2012) , Article ID: 18016 , 11 pages DOI:10.4236/wja.2012.21002
Increased Activity of NK Cells and Plasmacytoid Dendritic Cells in HIV-Exposed Seronegative (ESN) Individuals*
![]()
1Departments of Immunopathology, Post Graduate Institute of Medical Education and Research, Chandigarh, India; 2Clinic for Immunology and Rheumatology, Hannover Medical School, Hannover, Germany; 3Internal Medicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India; 4Division of Arthritis and Rheumatic Disease, Oregon Health and Science University, Portland and Hillsboro, USA.
Email: #skarora_in@yahoo.com
Received October 19th, 2011; revised November 17th, 2011; accepted November 30th, 2011
Keywords: HIV Exposed Seronegative; Innate Immune Responses; NK Cells; Plasmacytoid Dendritic Cells
ABSTRACT
The mechanisms involved in resistance to HIV-1 infection, especially the role of innate immune response, have not been thoroughly explored in individuals who are repeatedly exposed to HIV-1, but do not get the infection, termed as Exposed sero-negative or ESN. Frequency and activation state of natural killer (NK) cells and plasmacytoid dendritic cells (pDC) in ESNs from North India were compared with those in recently infected HIV positives (RHIV), chronically infected HIV positives (HIV+) and healthy controls (HC). The activation state of NK cells in terms of cytokine response (IFNγ & TNFα) was significantly higher in ESNs compared to the healthy controls, recently infected HIV+ and chronically infected HIV+. Although the number of circulating pDC in different study groups was not significantly different, yet these cells seem to have significantly higher activation state in terms of IFNα production (ex-vivo in response to CpG ODN) in ESNs when compared with other groups. Increased activation status of NK cells and pDC in Exposed but Seronegative individuals indicates their continuous stimulation with HIV antigens due to regular exposure with infected partners and which might be imparting resistance to viral infection in these individuals.
1. Introduction
Over almost 3 decades since it was first identified, the HIV/AIDS epidemic has already caused an estimated 25 million deaths worldwide [1]. We do understand that not all individuals who are repeatedly exposed to HIV-1 show evidence of infection, sero-conversion and development of disease. Quite apart from those who seroconvert but progress slowly to AIDS (i.e., slow progressors, long-term non-progressors, elite controllers), this rare category of exposed seronegative (ESN) individuals, either resist infection or harbour extremely low levels of virus. The correlates of protection that confer this unique status to a minority of HIV-exposed individuals remain a subject of intense interest, as no single genetic or immunologic parameter has yet been able to fully explain this phenomenon. However, studying the disease profile in these individuals in more details may provide invaluable information that will aid in the design of newer vaccines and therapeutic approaches [2,3].
The role of innate immunity in the modulation of susceptibility to HIV infection is unclear and only a few recent reports describe a possible role for this arm of the immune response [3-5]. We had earlier reported that Human immunodeficiency virus (HIV) gag antigen-specific T-helper and granule-dependent CD8 T-cell have enhanced activities in exposed but uninfected (ESN) heterosexual partners of HIV type 1-infected individuals in North India [6]. Further we tried to explore the association of gene polymorphisms in CCR5, CCR2, CX3CR1, SDF-1 and RANTES to protection in these individuals [7], but the role of cellular innate immune components remained unexplored. The innate immune system is composed of a wide variety of cellular components including NK cells and dendritic cells which play a significant role in preventing the infection by pathogens. Natural killer (NK) cells, which account for about 10% of peripheral blood lymphocytes, are very effective in producing cytokines like IFNγ, TNFα and GM-CSF which are important regulators of immune responses and haematopoiesis and link the innate to the adaptive immune response through a bidirectional cross-talk with dendritic cells [8,9]. Although low numbers of NK cells have been shown to be associated with rapid progression to AIDS in HIV-infected individuals [10], their role in protection against the establishment of HIV-1 infection remains to be fully elucidated.
The dendritic cells participate in the innate immune response against pathogens by producing cytokines IL-12, IL-6 and IFNa thereby initiating the adaptive immune response [11]. Natural IFNα producing cells (IPCs), now called plasmacytoid dendritic cells (pDCs), are the major source of type-I interferons in antiviral innate immune responses [12,13]. The normal production of type-I interferons in HIV-infected subjects even with low CD4+ cell numbers appears to protect individuals from disease [14]. Loss of circulating pDCs correlates with a high HIV viral load and the occurrence of opportunistic infections and Kaposi’s Sarcoma [15]. Moreover the LTNP patients, despite being able to avoid progression to AIDS for an extended period of time, are indeed infected, while the ESN individuals are not infected at the first place. Thus the identification of the immune correlates in ESN individuals could clarify how to design preventive vaccine and newer therapeutic approaches. Accordingly the present study was designed with the aim to evaluate the status and possible role of innate immune responses in terms of NK cells and pDCs in HIV-1 exposed seronegative (ESN) individuals.
2. Patients & Methods
2.1. Study Population and Study Design
The study subjects were recruited from the Integrated Counselling and Testing Centre (ICTC) for HIV in a tertiary care hospital in North India. Twenty ESN individuals were enrolled in this study. All participants had a history of prolonged unprotected penetrative sexual exposure with an HIV-1 infected partner, which was defined as sexual intercourse at least twice per week for a period of 4 months in the last 2 years [16]. Only one ESN was involved in homosexual activity with multiple partners while all others were cases of heterosexual exposure with a single partner (sero-discordant couples). None of the sexual partners of ESNs was receiving ART. Twenty chronically HIV-1-infected, therapy-naïve individuals with no opportunistic infection were also enrolled. As unexposed controls, 20 HIV-seronegative, apparently healthy individuals, with no known risk factors for HIV-1 infection were recruited as healthy control (HC) group. Lastly, as a better control for comparison with ESNs, a group of 10 recently infected (infected within last 5 - 6 months) HIV positive individuals was also enrolled for the study. Only those chronically infected HIV+ and individuals with recent HIV infection were enrolled who had acquired the infection through sexual route in order to match their risk factors with those of ESNs. This study was approved by the Institutional Ethics Committee (IEC), and a clear explanation of the objectives and the implications of the results were given to each participant; subsequently, an informed consent was obtained.
2.2. Sampling
Peripheral blood was collected from each individual either in a K2 EDTA or sodium heparin vacutainer® vial (BD Biosciences, USA) for immunophenotyping or in vitro stimulation assay respectively. One mL of EDTA blood was used for phenotypic analysis of NK cells, pDCs, CD4 cell counts, TLC (total leukocyte count) and DLC (differential leukocyte count). Plasma was separated from the remaining EDTA blood for the determination of HIV viral load. The leukocyte rich buffy coat was used for DNA and RNA extraction using commercially available kits (Qiagen, Germany). Total RNA isolated from EDTA blood was immediately reverse transcribed to cDNA using the RevertAid™ First Strand Synthesis Kit (Fermentas, Canada) and stored at –20˚C till further use. Heparinized blood was used for isolation of PBMC using Ficoll-Hypaque (Histopaque®, Sigma-Aldrich, USA) density gradient centrifugation. Purified lymphocytes at the interphase were collected and cell viability was determined by Trypan blue dye exclusion. The cells were resuspended at a concentration of 1 × 106 cells/mL in RPMI 1640 with 10% FCS (Sigma-Aldrich), 10 mL/L antibiotic-antimycotic solution (Sigma-Aldrich) to be further used for the functional analysis of both the NK cells and the pDCs.
2.3. Confirmation of the Absence of HIV Proviral DNA in ESNs by Nested PCR
Absence of proviral DNA of HIV-1 was confirmed in each ESN subject using an in-house developed nestedPCR assay. Extracted DNA was used in the first PCR to amplify a 768 bp fragment of pol gene using primers POL Pr-1 [5’-TTC CCA TTA GTC CTA TTG AAA CTG T-3’] and POL Pr-2 [5’-TCA TTG ACA GTC CAG CTA TCC TTT T-3’] [17]. The amplified PCR product was then used as template in the second PCR to amplify a 540 bp internal sequence using nested primers: Pr-F1 [5’-GCC TGA AAA TCC ATA TAA CAC TCC-3’] and Pr-F2 [5’-CCA TCC AAA GAA ATG GAG GTT C-3’]. DNA extracted from the whole blood of normal healthy individuals was used as negative control in the PCR reaction. For positive control, a full-length infectious clone of HIV-1 subtype-B cloned in pUC-18 plasmid DNA (pNL4-3) (gifted by Dr. Shahid Jameel, ICGEB, New Delhi) was used. DNA from a HIV serology positive individual was included as a positive control.
2.4. Confirmation of Absence of CCR5 (32 Mutation by PCR
Presence of CCR5D32 mutation (associated with resistance to HIV and slow progression to AIDS) was excluded among ESNs by PCR as described by Barker et al. [18]. Primers CCR5-S [5’-TTA AAA GCC AGG ACG GTC AC-3’] and CCR5-AS [5’-GAC CAG CCC CAA GAT GAC TA-3’], which would amplify a fragment of either 204 or 172 bp, corresponding respectively to wild type and 32 bp-deleted CCR5 alleles were used. PCR products were resolved on a 2% agarose gel and visualised on a UV-transilluminator gel-doc.
2.5. Identification of Recently Infected HIV Positives
Newly diagnosed HIV positive individuals at the Integrated Counselling and Testing Centre were initially screened for recent infection (5 - 6 months) on the basis of Post-HIV test counselling. The individuals shortlisted on the basis of a recent sexual/matrimonial alliance with a known HIV positive were confirmed for recent seroconversion using Calypte® HIV-1 BED Incidence EIA (Calypte Biomedical Corporation, USA). It is an in vitro quantitative enzyme immunoassay for the determination of the proportion of HIV-1 specific IgG in blood samples (including serum and plasma) with respect to total IgG as an aid in determining the elapsed time since HIV-1 infection occurred. A threshold cut-off based on a calibrator specimen determines the classification of recent seroconversion. Standard protocol as described in the kit insert was followed.
2.6. CD4 Counts and Plasma HIV Viral Load
Absolute CD4 lymphocyte counts were determined by flow-cytometry using BD TritestTM CD3 FITC/CD4 PE/ CD45 PerCP with BD TrucountTM tubes as per manufacturer’s protocol (BD Biosciences, USA). The sample was acquired on the BD FACSCaliburTM (BD Bioscience, USA) flow cytometer and analysed using the BD Multiset™ software.
HIV viral load in the plasma samples was quantified using the COBAS AMPLICOR HIV-1 MONITOR Test, version 1.5 (v1.5) (Roche Diagnostics, Switzerland).
2.7. Enumeration of NK Cells and Plasmacytoid DCs by Immunophenotyping
Fluorochrome-labeled monoclonal antibodies (mAbs) CD3-PECy5, CD16-PE, CD56-PE, CD4-PE, CD11cPECy5, lineage cocktail (a mixture of anti CD3, CD14, CD16, CD19, CD20, CD56)-FITC and the corresponding isotype control antibodies were all from BD-Pharmingen (USA). Frequency and phenotype of NK cells (defined as CD3–/CD16+/CD56+) and pDCs (defined as CD4+/ Lineage–/CD11c–) [13 ] was determined by three colour flow-cytometry.
For cell surface staining, 100 µl of EDTA anti-coagulated whole blood was incubated with the appropriate corresponding fluorochrome labelled mAb for 20 min at room temperature in the dark. The erythrocytes were lysed, washed with 2 ml of cold PBS and were fixed with 250 µl of 2% formaldehyde. For all experiments, appropriate isotype-matched control antibodies were also included. Flow cytometry was performed using the BD FACSCaliburTM instrument and the data analyzed with CellQuest ProTM software. The absolute number of the different leukocyte subpopulations was calculated on the basis of manually determined total and differential peripheral blood cell counts.
2.8. Intracellular Staining for IFNγ and TNFα in NK Cells
Intracellular staining for IFNγ and TNFα in NK Cells was carried out as described elsewhere [9]. Prior to intracellular staining for cytokine production, cells were stimulated with PMA (50 ng/mL) and ionomycin (1 µg/ mL, Sigma-Aldrich, USA). Briefly, cells were cultured in the presence of Brefeldin A (Sigma-Aldrich, USA) and were surface stained with CD3, CD16 and CD56 antibodies. After fixation with 2% paraformaldehyde, and permeabilisation with 0.1% saponin (Sigma-Aldrich, USA) in PBS, cells were stained with antibodies directed against the appropriate cytokines (IFNγ-FITC, TNFα- FITC). The cells were acquired on the flowcytometer and analyzed as described above.
2.9. IFNα and Inflammatory Cytokine Estimation after Stimulation with CpG-ODN
One million PBMCs from each subject were cultured with or without 10 µg/ml of A-Class CpG ODN 2216 (5’-GGGGGACGATCGTCGGGGGG-3’) (Sigma Aldrich, USA), for 18 hrs at 37˚C and 5% CO2. IFNα levels were assessed in culture supernatants using a comercially available ELISA kit (Bender Medsystems, Austria) as per manufacturer’s protocol. Levels of inflammatory cytokines i.e. IL-6, IL-8, TNF and IL-12p70 were assessed in culture supernatants by flow cytometry using the BDTM Cytometric Bead Array (CBA) Human Inflammation Kit (BD Biosciences, USA) according to manufacturer’s protocol.
2.10. IRF7 Gene Expression by Real Time PCR
Relative quantification of IRF7 gene expression was done by Real Time PCR using SYBR Green chemistry. The expression of IRF7 gene was normalized to the house-keeping gene GAPDH using the primers: IRF7-F: 5’-TGG TCC TGG TGA AGC TGG AA-3’, IRF7-R: 5’-GAT GTC GTC ATA GAG GCT GTT GG-3’, GAPDH-F: 5’-GAA GGT GAA GGT CGG AGT-3’ and GAPDH-R: 5’-GAA GGT GAA GGT CGG AGT-3’.
2.11. Statistical Analysis
The statistical analysis was carried out using Graph Pad Prism v5.00 software (Graph Pad Software Inc., USA) and the level of significance was set at p < 0.05. Multiple comparisons among different groups were performed by analysis of variance (ANOVA). Correlation analysis was done using the non-parametric Spearman’s Rank Correlation Coefficient Analysis.
3. Results
3.1. Characteristics of Study Population
All individuals included in this study were from the North Indian states. The demographic profile of individuals in different study groups is summarized in Table 1. None of the subjects presented clinical evidence of any other infectious disease at the moment of blood sampling. The recently infected HIV+ individuals (RHIV) were from a significantly younger age group (23.50 ± 3.03 years) as compared to the other study groups (All > 30 years). The HC, ESN and RHIV study groups had almost equal distribution of individuals from both sexes. However, the chronic HIV positives (HIV+) were predominantly males (Table 1). All ESNs were negative for HIV-1 infection by serology as well as by DNA PCR assay. Additionally, none of the ESNs were positive for the presence of CCR5 D32 mutation. The mean CD4 cell count in ESNs was similar to that of HCs but was found to be significantly higher in comparison to RHIV (p < 0.005) and ART naïve HIV+ group (p < 0.001) (Table 1).
3.2. Frequency of NK Cells and pDCs in Peripheral Blood
The frequency and absolute counts of NK cells (Figure 1(a)) and Plasmacytoid DCs (Figure 1(b)) was determined in the peripheral blood by flow cytometry. NK cell percentage (data not shown) as well as absolute count in ESNs were found to be similar to HC individuals but significantly higher when compared with RHIV (p < 0.005 and p < 0.05 respectively) or chronically infected HIV+ individuals (p < 0.005 and p < 0.001 respectively), (Figure 1(c)). Although there was no significant difference in terms of the percentage of circulating pDCs in peripheral blood of different groups, the absolute count of pDCs was significantly higher in ESNs when compared with the chronic HIV+ individuals (Figure 1(d); p < 0.05). The Spearman’s correlation analysis revealed no significant correlation between NK cell numbers and CD4 counts in both RHIV and HIV+ study groups (Figure 1(e)). On the other hand a significant positive correlation was observed between the CD4 cell counts and absolute pDC numbers (Figure 1(f), p < 0.005) in the peripheral blood of both the study groups.
3.3. Cytotoxic Potential of NK Cells
The percentage of specific lysis in case of ESNs was found to be similar to HC subjects but significantly higher when compared to the RHIV or the HIV+ groups (Figure 2(a), p < 0.001 for both). The increased cytotoxicity in case of ESNs indicates the activated state of NK cells among these individuals. Spearman’s correlation analysis revealed a significant inverse relationship between CD4 cell counts and NK cell cytotoxicity in case of RHIV (Figure 2(b); Spearman’s ρ = –0.7939, p < 0.05). An inverse correlation trend was also observed in case of ART naïve chronic HIV+ group, but the results were not statistically significant (Figure 2(b)).
3.4. Production of IFNγ and TNFα by NK Cells
The percentages of CD3–/CD16+/CD56+ NK cells that expressed IFNγ (Figure 2(c)) or TNFα (Figure 2(e)) after PMA Ionomycin stimulation were found to be significantly higher in ESNs when compared with the RHIV group (p < 0.001), the chronically infected HIV+ group individuals (p < 0.05) or the healthy controls (p < 0.05).
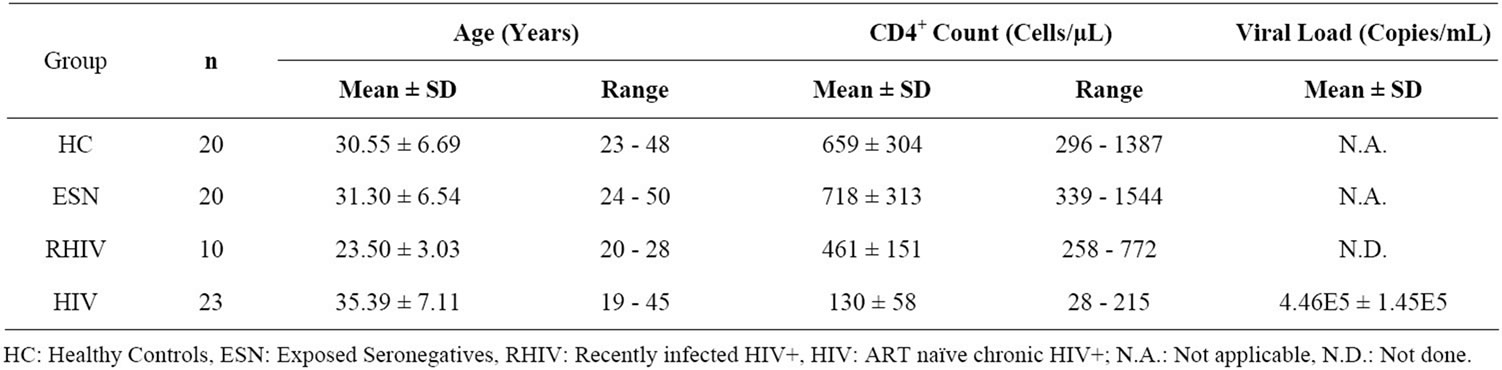
Table 1. Demographic profile of individuals in different study groups.
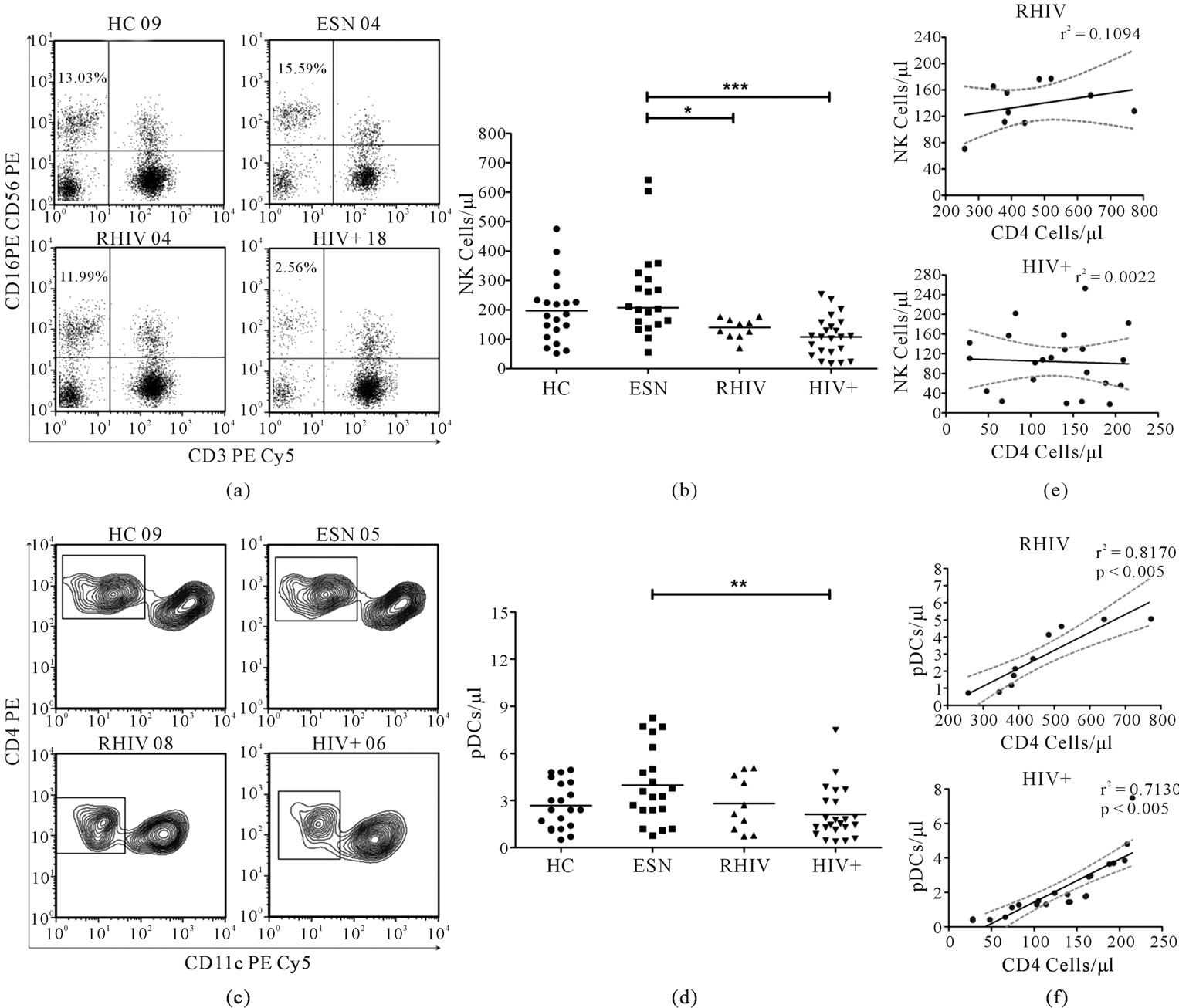
Figure 1. Absolute number of NK cells and pDCs in peripheral blood of individuals in different study groups (a) NK cells were identified as CD3–/CD16+/CD56+ after gating the lymphocytes on the basis of forward and side scatter. Representative dot plots show the NK cells in the upper left quadrant in different study groups; (b) Absolute count of NK cells per micro litre of blood for each individual in different study groups. Each point represents one individual and the horizontal line represents the mean. Significant differences are indicated on the top (ANOVA Dunnett’s multiple comparison test; *p < 0.05, ***p < 0.001); (c) pDCs were identified, after gating Lineage (CD3/CD14/CD16/CD19/CD20/CD56) negative mononuclear cells (plots not shown), as being CD4+/CD11c-. Representative contour plots show the gated pDCs in different study groups; (d) Absolute count of pDCs per micro litre of blood, determined on the basis of frequency and absolute blood counts. Each point epresents one individual and the horizontal line epresents the mean. Significant differences are indicated on the top (ANOVA Dunnett’s multiple comparison test; **p < 0.01); (e) Linear regression analysis carried out and the best fit line with 95% confidence band plotted along CD4 counts vs absolute NK cells/µL blood and (f) CD4 counts vs absolute pDC/µL blood in order to find if any correlation existed. r2 and significant p values are indicated.
Spearman’s correlation analysis however revealed no significant correlation between percentage of IFNγ producing NK cells and CD4 cell counts in the patient groups (Figure 2(d)) or CD4 counts vs % TNFα positive NK cells (Figure 2(f)). These findings suggest that the enhanced capability of NK cells from ESNs to produce IFNγ and TNFα could be associated with natural resistance to HIV-1 infection in these individuals, which need to be demonstrated through a further study on larger number of individuals.
3.5. IFNα and Inflammatory Cytokine Production by pDC after Ex-Vivo Stimulation with CpG-ODN
The ability of circulating PBMC (predominantly pDC) to
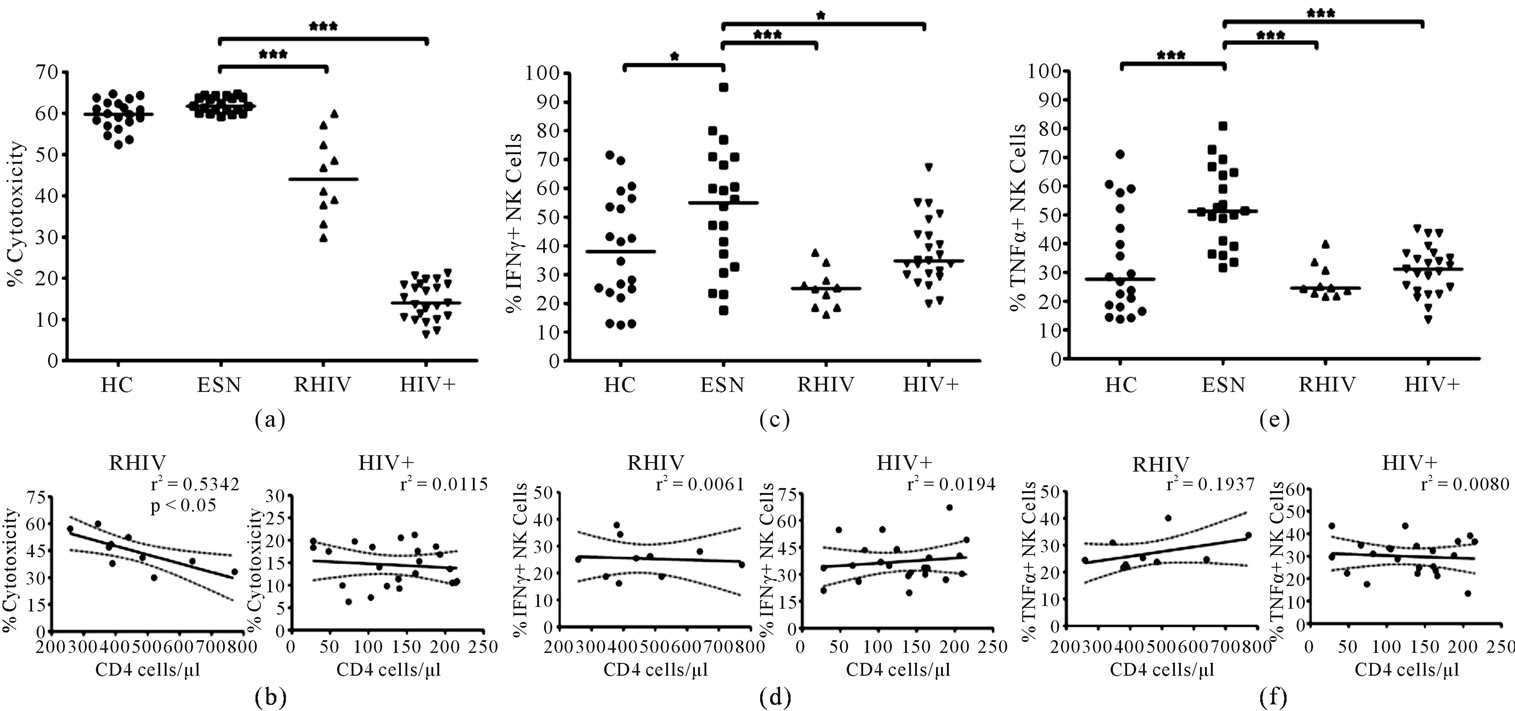
Figure 2. Functional analysis of NK cells in terms of cytotoxicity and cytokine production in different study groups (a) NK cell cytotoxicity assessed against K562 cell line using a commercially available LDH enzyme release assay. Individual values of % cytotoxicity in each study group represented in the scatter plot. The horizontal line represents the mean and significant difference indicated on the top (ANOVA Dunnett’s multiple comparison test; ***p < 0.001); (b) Linear regression analysis carried out to find correlation between NK cell cytotoxicity and the CD4 cell counts and best fit line with 95% confidence band plotted. r2 and significant p values are indicated; (c) Intracellular levels of IFNγ and (d) Correlation between CD4 counts and intracellular IFNγ and TNFα levels assessed by linear regression analysis and best fit line with 95% confidence band plotted along CD4 counts vs % IFNγ positive NK cells and (e) TNFα in NK cells assessed after stimulating the PBMC with PMA and Ionomycin. The points represent individual values in different study groups with the horizontal line representing the mean and significant differences indicated on the top (ANOVA Dunnett’s multiple comparison test; *p < 0.05, ***p < 0.001) and (f) CD4 counts vs % TNFα positive NK cells. r2 and significant p values are indicated.
produce IFNα and inflammatory cytokines ex vivo in response to stimulation with pDC specific stimulant CpG ODN was determined in the study groups. The cells from ESNs produced significantly higher amount of IFNα in comparison to the RHIV (Figure 3, p < 0.001), the HIV+ group (p < 0.001) as well as the healthy controls (p < 0.001). The levels of inflammatory cytokines IL6, IL8 in culture supernatants of CpG induced PBMCs from ESNs were less but not significantly different from those of RHIV, HIV+ and the control HC group (Figure 3). However, the levels of TNFα were found to be significantly lower in the ESN group when compared with HIV+ (p < 0.05) but not when compared with RHIV and HC groups. Significantly higher levels of IFNα in ESNs compared to the healthy controls and HIV positive study groups, indicate the activated state of circulating pDC in these individuals.
3.6. Interferon Regulatory Factor-7 (IRF-7) Gene Expression
The expression of IRF-7, major transcription factor controlling the expression of IFNα in plasmacytoid DCs (19) as evaluated by real-time PCR, was found to be significantly higher in case of chronic HIV positives as compared to ESNs when the data was normalized to GAPDH (Figure 4; p < 0.005). Although the expression of IRF7 was higher in ESNs compared to the RHIV and the HC groups the difference was not statistically significant.
4. Discussion
Several genetic and adaptive immune mechanisms of natural resistance have been reported in both ESN and long-term nonprogressors (LTNP) [18 -24]. However, these mechanisms only partially explain the phenomenon of natural resistance observed in some individuals exposed to HIV-1. To date, only a genetic factor, the homozygous Δ32 mutation in the CCR5 gene, has been shown to confer a high degree of resistance to HIV-1 infection. However, this genetic trait explains why the ESN individuals remain uninfected in only 2% - 4% of this group, thereby strongly suggesting that other, yet unexplored, mechanisms mediate this resistance [25]. Although rare in Asian population [26], the presence of CCR5 Δ32 mutation was ruled out with none of the ESNs recruited in our study found to be carrying this mutation.
There have been some reports highlighting the role of soluble factors, such as type I IFNs, chemokines and α and β-defensins produced by cells of the innate immune
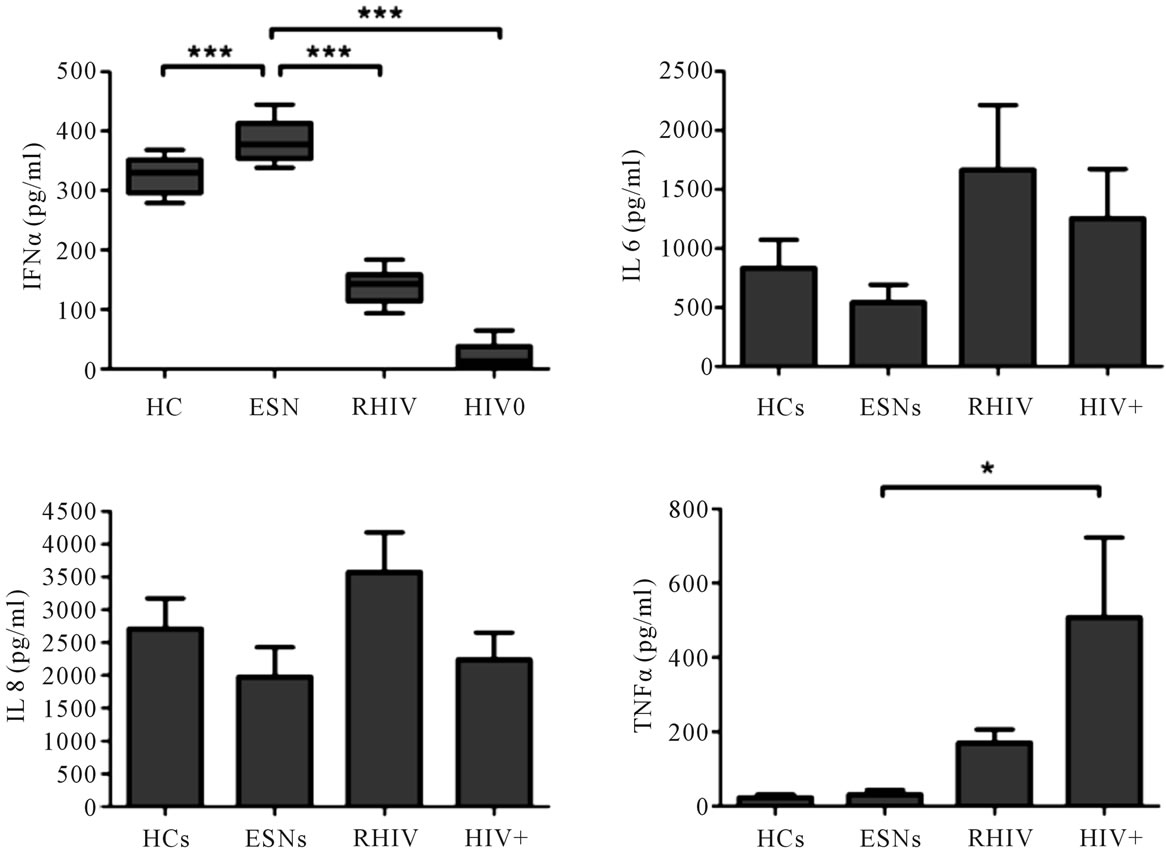
Figure 3. Cytokine levels in culture supernatants after incubation of PBMCs with/without CpG ODN 2216 for 18 hrs. Cytokine levels were assessed using ELISA (IFNα) and flow cytometry (CBA, Human Inflammatory kit, BD Pharmingen). IFNα levels are represented as Box and Whisker plots. Mean ± SEM values of inflammatory cytokines are represented after subtracting the values of unstimulated controls. Data was subjected to ANOVA followed by Dunnett’s multiple comparison test with significant values indicated on the top. (*p < 0.05, ***p < 0.001). CpG ODN: oligodeoxy nucleotides with CpG motifs; PBMC: peripheral blood mononuclear cells.
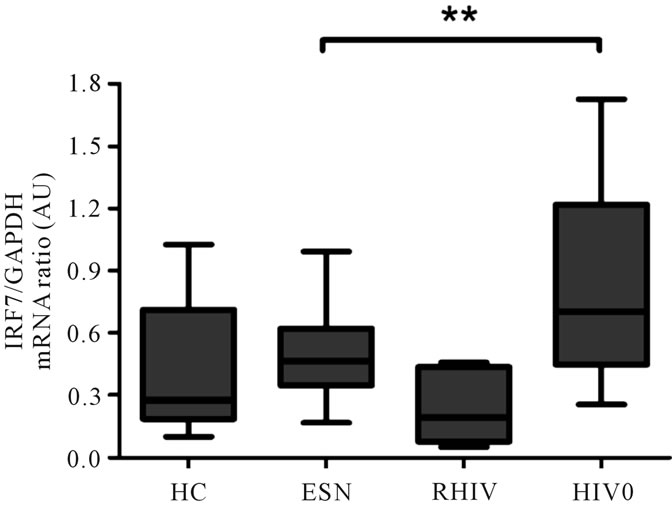
Figure 4. mRNA expression of Interferon regulatory factor 7 (IRF 7) was examined in different study groups by realtime RT-PCR and normalized to the housekeeping gene GAPDH. Data expressed as box and whisker plots with the median and significant values are indicated on the top (**p < 0.005).
system, as protective mechanisms during HIV-1 exposure [27,28]. In order to explore new mechanisms associated with the phenomenon of natural resistance to HIV-1 infection, we evaluated the frequency and functional response of NK cells and plasmacytoid DCs, which are known to have direct effector functions as well as play a role in the modulation of the adaptive immune response.
To the best of our knowledge, ours is the first study to have recruited a group of recently infected HIV positives (5 - 6 months) as a control group along with chronically infected HIV positive individuals to compare the findings from ESNs. Most of the reports on ESNs so far, have compared them with chronically infected HIV positives [18,25,29] or healthy controls only [4], which may not actually present the true picture considering the long term effects of HIV infection on the immune system The reduced CD4 cell count during progressive stages of disease as observed in our study is a hallmark of HIV infection. Individuals who maintain the CD4 count due to many different mechanisms which may be intrinsic to the individual, are resistant to disease progression as seen in LTNPs. Somewhat similar mechanisms may be playing role in the ESN group of individuals where the CD4 count was found to be significantly higher as compared to the HIV positive groups in our study which is in line with an earlier report from Fowke et al., [30] who have reported higher CD4 counts in a cohort of HIV resistant commercial sex workers from Nairobi and have also demonstrated the presence of HIV-specific T-helper (Th) cell responses.
The NK cells are critical in the early control of various viral infections and work in synergy with CD8+ T cells to clear infection. Work in the herpes simplex virus type 1 (HSV-1) model has demonstrated that NK cells are the largest fraction of the lymphocyte infiltrate during the first 1 - 5 days of infection but decline as CD8+ T cell numbers expand by day 5 of infection [31]. The analysis of the total CD3–CD16+CD56+ NK cell population in the peripheral blood in our study groups revealed a significantly higher percentage and absolute count in the ESN group as compared to the recently and chronically infected HIV positives. Although the NK cell frequency in ESNs was also higher compared to the uninfected controls, the difference was not statistically significant. NK cell counts were found to be similar in the RHIV and chronic HIV positive groups. A possible explanation to this observation lies in the emergence of a novel dysfunctional subset of NK cells, the CD3 neg CD56 neg CD16 pos NK cells [32 ]. This subset becomes more prominent in individuals with active viral replication at the expense of the two other subsets of NK cells, resulting in an overall stable number of NK cells over the course of HIV infection. No significant correlation between NK cell numbers and the clinical stage of the disease in HIV positive subjects (in terms of CD4 counts) was observed in our study although a positive correlation between the two has earlier been reported in a few studies [33,34]. Elevated NK cell activity was observed in ESNs in terms of significantly higher IFNγ and TNFα production as compared to the uninfected controls, recently and chronically infected HIV positives. The increased production of cytokines by activated NK cells in ESNs may play a role in anti-HIV-1 defence. Both IFNγ and TNFα modulate HIV-1 infection in vitro and can suppress viral replication in certain conditions [35]. IFNγ is known to have direct anti-HIV-1 activity, mainly mediated through antagonism of tat-induced LTR transactivation [36] and has been previously proposed as one of the possible mechanisms responsible for resistance to HIV-1 infection [37]. Our results are in concordance with a study in a cohort of Vietnamese IDUs [29] in which NK cells from the exposed but uninfected individuals, produced considerably more of the chemokines CCL3, CCL4 and CCL5 as well as cytokines IFNγ and TNFα than did NK cells from HIV positive individuals and HIV-negative volunteers. Increased IFNγ and TNFα production by ESNs in our study, as a possible correlate of protection from HIV, is also supported by Montoya et al. [25 ] who have reported similar results in an ESN cohort. These findings support the hypothesis that IFNγ and TNFα production by NK cells contributes to protection from acquiring HIV infection. NK cell cytotoxicity was also found to be significantly higher in ESNs in our study compared to the recently and chronically infected HIV positives. However, the difference was not significant when compared with uninfected controls. NK cell cytolytic activity has been shown to be higher in Vietnamese EU IDUs than in healthy unexposed individuals or in IDUs who eventually underwent seroconversion [29]. More recent studies have demonstrated either reduced or enhanced cytolytic activity of NK cells in HIV-1 infection. [38,39]. Continuous surveillance of virally infected cells by NK cells and increased NK cell activity of virally infected cells despite reduced numbers in viremic HIV positives has been shown using CD107a expression which is a recently established marker for NK cytotoxicity [40]. As a result of these conflicting data, the role of NK cell cytotoxicity in conferring resistance to HIV infection calls for further evaluation.
In our study we found a significantly higher pDC number in ESNs compared to the chronically infected HIV positives, but not significantly different compared to the control (HC & RHIV) groups. IFNα exhibits potent antiviral activity as it regulates the responses of numerous cell subsets involved in both innate and adaptive immune responses against viral pathogens [41 ]. The engagement of TLR7 and TLR9 by PAMPs activates pDCs to rapidly produce high levels of type 1 IFNs and moderate amounts of inflammatory cytokines, including TNFα and IL-6 [42 ]. Our results indicate a possible role of IFNα in conferring protection to ESNs from getting infected with HIV as we observed significantly higher production of IFNα in ESNs compared to the other study groups and uninfected controls. Interestingly, a high frequency of plasmacytoid dendritic cells (pDC) and increased production of IFN-α in response to viral infection have earlier been reported in the blood of long-term nonprogressors and long-term survivors [13]. The presence of higher number of males in our chronically infected HIV positive study group could have influenced our assessment of IFNα levels as it has recently been reported that pDCs derived from women produce significantly more interferon-α (IFN-α) in response to HIV-1-encoded TLR7 ligands [43 ]. Among the ART naïve chronically infected HIV positives in our study the mean IFNα levels in females were found to be higher than males but the difference was not significant. IFNα production has been shown to be inversely correlated with HIV disease status in several studies [12,44] but not in all including our study, probably because multiple parameters are involved in HIV replication control.
Interferon regulatory factor 7 (IRF7) has been indicated to be the master regulator of IFNα production in pDC signal transduction pathway [18]. In our study, although the ESNs had a higher expression of this transcription factor compared to the recently infected HIV positives or the HIV uninfected healthy controls, the difference was not statistically significant. Significantly, a higher level of IRF7 RNA in ART naïve viremic subjects is in discrepancy with lower IFNα production by these subjects. There is some recent evidence which suggests that IFNα production is high in vivo in HIV infected progressors but a combination of feedback inhibition and prior direct activation through TLRs by HIV RNA leads to diminished ex-vivo IFNα production upon exposure to CpG-A or HIV particles [45 ]. While the IRF7 gene expression was assessed in terms of its mRNA abundance in the peripheral blood of the subjects, the IFNα levels were based on an in vitro stimulation assay, the two parameters could not be correlated and may be the reason of this discrepancy. Retention of PDC function in EUIDUs without an increase in PDC numbers as reported by Tomescu et al. [4] suggests that exposure route may also play an important role and calls for further evaluation.
5. Acknowledgements and Funding
The research was funded by Indian Council of Medical Research (Ministry of Health and Family Welfare, Govt. of India), under the memorandum of agreement with BMBF, Germany to SKA and RES.
6. Authors’ Contributions
AS carried out the flow cytometry assays, immunoassays, molecular experiments and initial draft of the manuscript. RJ and RES participated in the design of the study and provided initial help with the Real Time PCR experiments. AW participated in the recruitment of the study subjects in the clinic. SKA as PI conceived and designed the study and arranged funds and lab facilities. He also critically edited the manuscript for final submission. All authors read and approved the final manuscript.
REFERENCES
- UNAIDS, WHO, “AIDS epidemic update December,” The Joint United Nations Programme on HIV and AIDS, Geneva 2010. http://data.unaids.org/pub/report/2010/jc1700_epi_update_2010_en.pdf
- B. L. Shacklett, “HIV-Exposed, Persistently Seronegative Individuals: An Unsolved Puzzle?” The HIV/AIDS Newsletter, Vol. 1, No. 2, 2008, pp. 1-3.
- M. Miyazawa, L. Lopalco, F. Mazzotta, Lo. S. Caputo, F. Veas and M. Clerici, “The Immunologic Advantage’ of HIV-Exposed Seronegative Individuals,” AIDS, Vol. 23, No. 12, 2009, pp. 161-175. doi:10.1097/QAD.0b013e3283196a80
- C. Tomescu, F. M. Duh, M. A. Lanier, A. Kapalko, K. C. Mounzer and M. P. Martin, “Increased Plasmacytoid Dendritic Cell Maturation and Natural Killer Cell Activation in HIV-1 Exposed, Uninfected Intra-Venous Drug Users,” AIDS, Vol. 24, No. 14, 2010, pp. 2151-2160. doi:10.1097/QAD.0b013e32833dfc20
- S. Ravet, D. Scott-Algara, E. Bonnet, H. K. Tran, T. Tran and N. Nguyen, “Distinctive NK-Cell Receptor Repertoires Sustain High-Level Constitutive NK-Cell Activation in HIV-Exposed Uninfected Individuals,” Blood, Vol. 109, No. 10, 2007, pp. 4296-4305. doi:10.1182/blood-2006-08-040238
- S. Pallikkuth, A. Wanchu, A. Bhatnagar, R. K. Sachdeva and M. Sharma, “Human Immunodeficiency Virus (HIV) Gag Antigen-Specific T-Helper and Granule-Dependent CD8 T-Cell Activities in Exposed but Uninfected Heterosexual Partners of HIV Type 1-Infected Individuals in North India,” Clinical Vaccine Immunology, Vol. 14, No. 9, 2007, pp. 1196-1202.
- P. Suresh, A. Wanchu, R. K. Sachdeva and A. Bhatnagar, “Gene Polymorphisms in CCR5, CCR2, CX3CR1, SDF-1 and RANTES in Exposed but Uninfected Partners of HIV-1 Infected Individuals in North India,” Journal of Clinical Immunology, Vol. 26, No. 5, 2006, pp. 476-484. doi:10.1007/s10875-006-9036-0
- A. Iannello, O. Debbeche, S. Samarani and A. Ahmad, “Antiviral NK Cell Responses in HIV Infection: II. Viral Strategies for Evasion and Lessons for Immunotherapy and Vaccination,” Journal of Leukocyte Biology, Vol. 84, No. 1, 2008, pp. 27-49. doi:10.1189/jlb.0907649
- R. Jacobs, K. Weber, K. Wendt, H. Heiken and R. E. Schmidt, “Altered Coexpression of Lectin-Like Receptors CD94 and CD161 on NK and T Cells in HIV Patients,” Journal of Clinical Immunology, Vol. 24, No. 3, 2004, pp. 281-286. doi:10.1023/B:JOCI.0000025449.16468.6f
- H. Bruunsgaard, C. Pedersen, P. Skinhoj and B. K. Pedersen, “Clinical Progression of HIV Infection: Role of NK Cells,” Scandinavian Journal of Immunology, Vol. 46, No. 1, 1997, pp. 91-95. doi:10.1046/j.1365-3083.1997.d01-98.x
- L. Azzoni, R. M. Rutstein, J. Chehimi, M. A. Farabaugh, A. Nowmos and L. A. Montaner, “Dendritic and Natural Killer Cell Subsets Associated with Stable or Declining CD4+ Cell Counts in Treated HIV-1—Infected Children,” Journal of Infectious Diseases, Vol. 191, No. 9, 2005, pp. 1451-1459. doi:10.1086/429300
- V. Soumelis, I. Scott, F. Gheyas, D. Bouhour, G. Cozon and L. Cotte, “Depletion of Circulating Natural Type 1 Interferon-Producing Cells in HIV-Infected AIDS Patients,” Blood, Vol. 98, 2001, pp. 906-912. doi:10.1182/blood.V98.4.906
- M. Muller-Trutwin and A. Hosmalin, “Role for Plasmacytoid Dendritic Cells in Anti-HIV Innate Immunity,” Immunology and Cell Biology, Vol. 83, No. 4, 2005, pp. 578-583. doi:10.1111/j.1440-1711.2005.01394.x
- V. Soumelis, I. Scott, Y. J. Liu and J. Levy, “Natural Type 1 Interferon Producing Cells in HIV Infection,” Human Immunology, Vol. 63, No. 12, 2002, PP. 1206-1212. doi:10.1016/S0198-8859(02)00760-7
- M. Colonna, G. Trinchieri and Y. J. Liu, “Plasmacytoid Dendritic Cells in Immunity,” Nature Immunology, Vol. 5, No. 12, 2004, pp. 1219-1226. doi:10.1038/ni1141
- W. A. Goh, J. Markee, R. E. Akridge, M. Meldorf, L. Musey and T. Karchmer “Protection against Human Immunodeficiency Virus Type 1 Infection in Persons with Repeated Exposure: Evidence for T Cell Immunity in the Absence of Inherited CCR5 Coreceptor Defects,” Journal of Infectious Diseases, Vol. 179, No. 3, 1999, pp. 548-557. doi:10.1086/314632
- N. Sachdeva, S. Sehgal and S. K. Arora, “Frequency of Drug-Resistant Variants of HIV-1 Coexistent with WildType in Treatment-Naive Patients of India,” Medscap General Medicine, Vol. 7, No. 3, 2005, p. 68.
- E. Barker, C. E. Mackewicz, G. Reyes-Teran, A. Sato, S. A. Stranford and S. H. Fujimura, “Virological and Immunological Features of Long-Term Human Immunodeficiency Virus-Infected Individuals Who Have Remained Asymptomatic Compared with Those Who Have Progressed to Acquired Immunodeficiency Syndrome,” Blood, Vol. 92, No. 9, 1998, pp. 3105-3114.
- K. Honda, H. Yanai, H. Negishi, M. Asagiri, M. Sato and T. Mizutani, “IRF-7 is the Master Regulator of Type-I Interferon-Dependent Immune Responses,” Nature, Vol. 434, No. 7034, 2005, pp. 772-777. doi:10.1038/nature03464
- Y. Huang, W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang and T. He, “The Role of a Mutant CCR5 Allele in HIV-1 Transmission and Disease Progression,” Nature Medicine, Vol. 2, No. 11, 1996, pp. 1240-1243. doi:10.1038/nm1196-1240
- Beretta, S. H. Weiss, G. Rappocciolo, R. Mayur, C. D. Santis and J. Quirinale, “Human Immunodeficiency Virus Type 1 (HIV-1)-Seronegative Injection Drug Users at Risk for HIV Exposure Have Antibodies to HLA Class I Antigens and T Cells Specific for HIV Envelope,” Journal of Infectious Diseases, Vol. 173, No. 2, 1996, pp. 472-476. doi:10.1093/infdis/173.2.472
- S. Mazzoli, D. Trabattoni, S. L. Caputo, S. Piconi, C. Ble, F. Meacci, “HIV-Specific Mucosal and Cellular Immunity in HIV-Seronegative Partners of HIV-Seropositive Individuals,” Nature Medicine, Vol. 3, No. 11, 1997, pp. 1250-1257. doi:10.1038/nm1197-1250
- L. A. Pinto, J. Sullivan, J. A. Berzofsky, M. Clerici, H. A. Kessler and A. L. Landay, “ENV-Specific Cytotoxic T Lymphocyte Responses in HIV Seronegative Health Care Workers Occupationally Exposed to HIV-Contaminated Body Fluids,” Journal of Clinical Investigation, Vol. 96, No. 2, 1995, pp. 867-876. doi:10.1172/JCI118133
- L. X. Truong, T. T. Luong, D. Scott-Algara, P. Versmisse, A. David and D. Perez-Bercoff, “CD4 Cell and CD8 Cell-Mediated Resistance to HIV-1 Infection in Exposed Uninfected Intravascular Drug Users in Vietnam,” AIDS, Vol. 17, No. 10, 2003, pp. 1425-1434. doi:10.1097/00002030-200307040-00002
- C. J. Montoya, P. A. Velilla, C. Chougnet, A. L. Landay and M. T. Rugeles, “Increased IFN-Gamma Production by NK and CD3+/CD56+ Cells in Sexually HIV-1Exposed but Uninfected Individuals,” Clinical Immunology, Vol. 120, No. 2, 2006, pp. 138-146. doi:10.1016/j.clim.2006.02.008
- G. Kaur, P. Singh, C. C. Rapthap, N. Kumar, M. Vajpayee and S. K. Sharma, “Polymorphism in the CCR5 Gene Promoter and HIV-1 Infection in North Indians,” Human Immunology, Vol. 68, No. 5, 2007, pp. 454-461. doi:10.1016/j.humimm.2007.01.016
- J. A. Levy, I. Scott and C. Mackewicz, “Protection from HIV/AIDS: The Importance of Innate Immunity,” Clinical Immunology, Vol. 108, No. 3, 2003, pp. 167-174. doi:10.1016/S1521-6616(03)00178-5
- D. Trabattoni, S. L. Caputo, G. Maffeis, F. Vichi, M. Biasin and P. Pierotti, “Human Alpha Defensin in HIV-Exposed but Uninfected Individuals,” Journal of Acquired Immune Deficiency Syndromes, Vol. 35, No. 5, 2004, pp. 455-463. doi:10.1097/00126334-200404150-00003
- D. Scott-Algara, L. X. Truong, P. Versmisse, A. David, T. T. Luong and N. V. Nguyen, “Cutting Edge: Increased Nk Cell Activity in HIV-1-Exposed but Uninfected Vietnamese Intravascular Drug Users,” Journal of Immunology, Vol. 171, No. 11, 2003, pp. 5663-5667.
- K. R. Fowke, R. Kaul, K. L. Rosenthal, J. Oyugi, J. Kimani and W. J. Rutherford, “HIV-1-Specific Cellular Immune Responses among HIV-1-Resistant Sex Workers,” Immunology and Cell Biology, Vol. 78, No. 6, 2000, pp. 586-595.
- G. Alter and M. Altfeld, “NK Cells in HIV-1 Infection: Evidence for Their Role in the Control of HIV-1 Infection,” Journal of Internal Medicine, Vol. 265, No. 1, 2009, pp. 29-42. doi:10.1111/j.1365-2796.2008.02045.x
- G. Alter, N. Teigen, B. T. Davis, M. M. Addo, T. J. Suscovich and M. T. Waring, “Sequential Deregulation of Nk Cell Subset Distribution and Function Starting in Acute HIV-1 Infection,” Blood, Vol. 106, No. 10, 2005, pp. 3366-3369. doi:10.1182/blood-2005-03-1100
- S. D. Douglas, S. J. Durako, N. B. Tustin, J. Houser, L. Muenz and S. E. Starr, “Natural Killer Cell Enumeration and Function in HIV-Infected and High-Risk Uninfected Adolescents,” AIDS Research and Human Retroviruses, Vol. 17, No. 6, 2001, pp. 543-552. doi:10.1089/08892220151126643
- R. Ahmad, S. T. Sindhu, P. Tran, E. Toma, R. Morisset and J. Menezes, “Modulation of Expression of the Mhc Class I-Binding Natural Killer Cell Receptors, and Nk Activity in Relation to Viral Load in HIV-Infected/AIDS Patients,” Journal of Medical Virology, Vol. 65, No. 3, 2001, pp. 431-440. doi:10.1002/jmv.2053
- Y. Koyanagi, W. A. O’Brien, J. Q. Zhao, D. W. Golde, J. C. Gasson and I. S. Chen, “Cytokines Alter Production of HIV-1 from Primary Mononuclear Phagocytes,” Science, Vol. 241, No. 4873, 1988, pp. 1673-1675. doi:10.1126/science.3047875
- D. Emilie, M. C. Maillot, J. F. Nicolas, R. Fior and P. Galanaud, “Antagonistic Effect of Interferon-Gamma on Tat-Induced Transactivation of HIV Long Terminal Repeat,” The Journal of Biological Chemistry, Vol. 267, No. 29, 1992, pp. 20565-20570.
- M. Clerici, A. Clivio and G. M. Shearer, “Resistance to HIV Infection: The Genes Are Only Part of the Solution,” Trends in Microbiology, Vol. 5, No. 1, 1997, pp. 2-4. doi:10.1016/S0966-842X(97)81762-3
- S. Kottilil, T. W. Chun, S. Moir, S. Liu, M. McLaughlin and C. W. Hallahan, “Innate Immunity in Human Immunodeficiency Virus Infection: Effect of Viremia on Natural Killer Cell Function,” Journal of Infectious Diseases, Vol. 187, No. 7, 2003, pp. 1038-1045. doi:10.1086/368222
- M. R. Goodier, N. Imami, G. Moyle, B. Gazzard and F. Gotch, “Loss of the CD56hiCD16‑ NK Cell Subset and Nk Cell Interferon-Gamma Production During Antiretroviral Therapy for HIV-1: Partial Recovery by Human Growth Hormone,” Clinical and Experimental Immunology, Vol. 134, No. 3, 2003, pp. 470-476. doi:10.1111/j.1365-2249.2003.02329.x
- C. Tomescu, J. Chehimi, V. C. Maino and L. J. Moontaner, “Retention of Viability, Cytotoxicity, and Response to IL-2, IL-15, or IFN-α by Human NK Cells after CD107a Degranulation,” Journal of Leukocyte Biology, Vol. 85, No. 5, 2009, pp. 871-876. doi:10.1189/jlb.1008635
- E. Martinelli, C. Cicala, R. D. Van, D. J. Goode, K. Macleod and J. Arthos, “HIV-1 gp120 Inhibits TLR9-Mediated activation and IFN-α Secretion in Plasmacytoid Dendritic Cells,” Proceedings of National Academy of Sciences USA, Vol. 104, No, 9, 2007, pp. 3396-3401. doi:10.1073/pnas.0611353104
- Y. J. Liu, “IPC: Professional Type 1 Interferon-Producing Cells and Plasmacytoid Dendritic Cell Precursors,” Annual Review of Immunology, Vol. 23, 2005, pp. 275-306. doi:10.1146/annurev.immunol.23.021704.115633
- A. Meier, J. J Chang, E. S. Chan, R. B. Pollard, H. K. Sidhu S. Kulkarni, et al., “Sex Differences in the Toll-Like Receptor-Mediated Response of Plasmacytoid Dendritic Cells to HIV-1,” Nature Medicine, Vol. 15, No. 8, 2009, pp. 955-959. doi:10.1038/nm.2004
- J. S. Finke, M. Shodell, K. Shah, F. P. Siegal and R. M. Steinma, “Dendritic Cell Numbers in the Blood of HIV-1 Infected Patients before and after Changes in Antiretroviral Therapy,” Journal of Clinical Immunology, Vol. 24, No. 6, 2004, pp. 647-52. doi:10.1007/s10875-004-6250-5
- J. C. Tilton, M. M. Manion, M. R. Luskin, A. J. Johnson, A. A. Patamawenu and C. W. Hallahan, “Human Immunodeficiency Virus Viremia Induces Plasmacytoid Dendritic Cell Activation in Vivo and Diminished Alpha Interferon Production in Vitro,” The Journal of Virology, Vol. 82, No. 8, 2008, pp, 3997-4006.
NOTES
*Competing Interests: The authors declare no Financial/Non-Financial conflict of interest.
#Corresponding author.

