Open Journal of Urology
Vol. 3 No. 2 (2013) , Article ID: 31319 , 5 pages DOI:10.4236/oju.2013.32010
Parental Consanguinity in Infertile Males*
Department of Urology, Faculty of Medicine, Erciyes University, Kayseri, Turkey
Email: #mesane@gmail.com
Copyright © 2013 Abdullah Demirtas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 5, 2013; revised April 5, 2013; accepted April 14, 2013
Keywords: azoospermia; genetics; male infertility; parental consanguinity
ABSTRACT
This study was investigated whether parental consanguinity in males has an effect on or relationship with some infertile subgroups and some semen and hormone parameters. The charts of 2651 infertile males were evaluated retrospectively for parental consanguinity ratios, sperm counts, motility parameters and hormonal values from the records of 2651 infertile males. In 1260 eligible males the first cousin parental consanguinity ratio was 22.6%. In 119 males with nonobstructive azoospermic (NOA) and 430 males with normal sperm counts, the ratios were 34.5% and 20.9%, respectively (p = 0.002). In the NOA group the parental consanguinity ratios were 27.1% (23/85) and 52.9% (18/34) in males with FSH values of >7.6 and <7.6 mIU/ml, respectively (p = 0.007). In males with normal sperm counts if the parents were first cousins, both sperm counts and motility parameters were significantly reduced when compared with the others. To our knowledge, this is the first study of consanguinity ratios among some infertile subgroups. In males with parental consanguinity lower sperm counts and motility ratios in normozoospermic males and lower FSH levels in the NOA group might show a relation with some genetically transmitted defects.
1. Introduction
Consanguineous marriages are not rare in some countries, beliefs and regions [1-3]. In our country, consanguinity among infertile couples and among their parents is not rare. Inbred marriages might increase the rate of homozygous genotype expression [4] (Baccetti B. et al., 2001). This increases the risk of recessively inherited disorders. In recent studies it has been suggested that consanguinity is highly correlated with rare genetic sperm defect syndromes [4,5]. These defects cannot be treated and may be translated to male offspring.
In most studies the evaluated parameter was whether parental consanguinity decreased the conception ratio or number of live births. It is proposed that the incidence of infertility is generally not higher in consanguineous marriages than in non-consanguineous marriages [3]. Suggested reasons are earlier marriage and so longer duration of the reproductive period.
What happens in infertile men? If consanguinity has no effect on fertility potential, no change in the ratio of parental consanguinity should exist. In the study of Inhorn et al. the opposite finding was reported. In males with severe oligospermia and nonobstructive azoospermia the ratio of parental consanguinity at first or second degrees was higher. In that study, subgroups according to sperm counts or other genetics related with infertility were not examined.
In our infertility clinic the consanguinity of parents is evaluated. Our aim was to evaluate whether some infertile subgroups are more or less effected by with first cousin parental consanguinity.
2. Materials and Methods
The data of 2651 males attending in our infertility clinic in the last 7 years were evaluated retrospectively. They all had been physically examined. All had medical and surgical records, and detailed evaluations like genetic examinations were generally performed when needed. Because of the retrospective nature of the study certain tests were absent in some subjects and sometimes there was reason for exclusion. If the parents of males were first cousins they were accepted as being consanguineous. If their parents were second or more degree cousins or were not related, they were accepted as not being consanguineous.
The male subjects were divided into groups. Group I (n: 458): pellet negative azoospermic males; group II (n: 533): males with a less than 5 million/ml sperm countincluding pellet positive males; group III (n: 363): males with a 5 to 14.9 million/ml sperm count; group IV (n: 930): males with >15 million/ml sperm count; group V (n: 119): Klinefelter’s syndrome; group VI (n: 65): males with unilateral or bilateral vasal agenesia; group VII (n: 48): congenital hypogonadotropic hypogonadism (CHH); group VIII (n: 135): unilateral or bilateral cryptorchidism.
In group I, 119 cases were objectively diagnosed as nonobstructive azoospermia with both testicular biopsy and chromosomal analysis (46 XY) and they had two testicles (subgroup I). In the other exluded 285 azoospermic cases some reasons were chemotherapy, radiotherapy, anejaculation, obstruction elsewhere in the genital tract, prior orchiectomy or atrophy related to trauma, torsion, etc. In some, biopsy or chromosomal analyses had not been performed or were absent. In groups II to IV, males were excluded from the analyses if they had varicocele, prior varicocele, hydrocele or hernia surgean, ejaculation volume of less than 1.5 ml, had one testicle or any other reasons that might be related with infertility. In the other groups no one was excluded from the analyses.
Semen analyses were performed according to 2002 WHO criteria. However, motility parameters were transformed to 2010 WHO criteria and males with sperm counts > 15 millions/ml were accepted as having normal sperm counts. Morphologic evaluations were not taken into consideration since they were not routinely performed.
Statistical analyses were performed with independent sample t and chi square tests. p values of <0.05 were considered as significant.
3. Results
In 2651 males, the parents of 627 males (23.7%) and 179 males (4.9%) were first cousins, and second or more degree relatives, respectively. Two hundred and forty five (9.2%) and 201 males (7.6%) were first cousins and second or more degree relatives with their partners, respectively.
Among 2651 males, the data of 1260 were taken into consideration for comparisons following exclusions. In 1260 males, the parents of 285 males (22.6%) and 120 males (9.5%) were first cousins with their partners.
In Table 1 the numbers and consanguinity ratios of all males are shown, and in Table 2 the numbers and consanguinity ratios in evaluable males are shown.
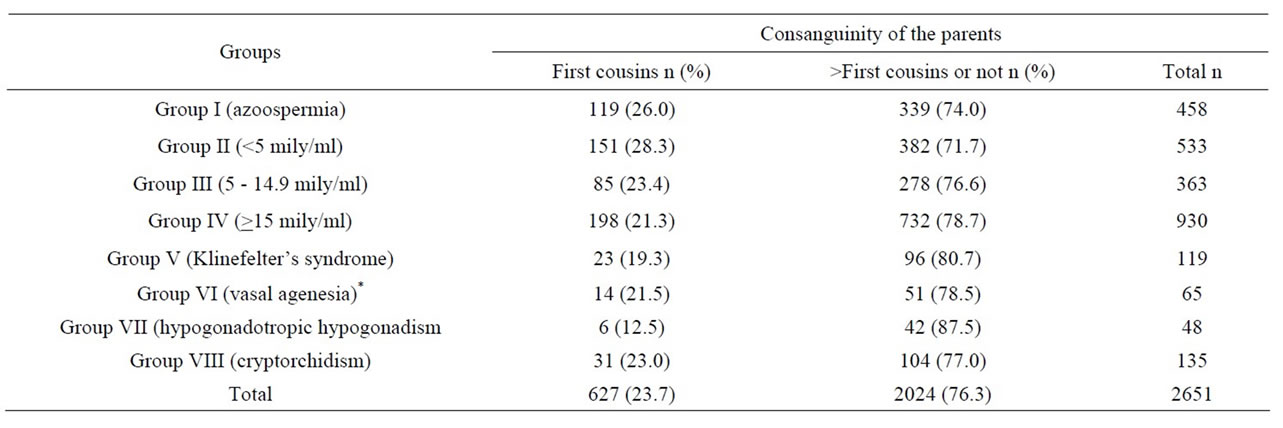
Table 1. The parental consanguinity ratios of all males according to the groups.
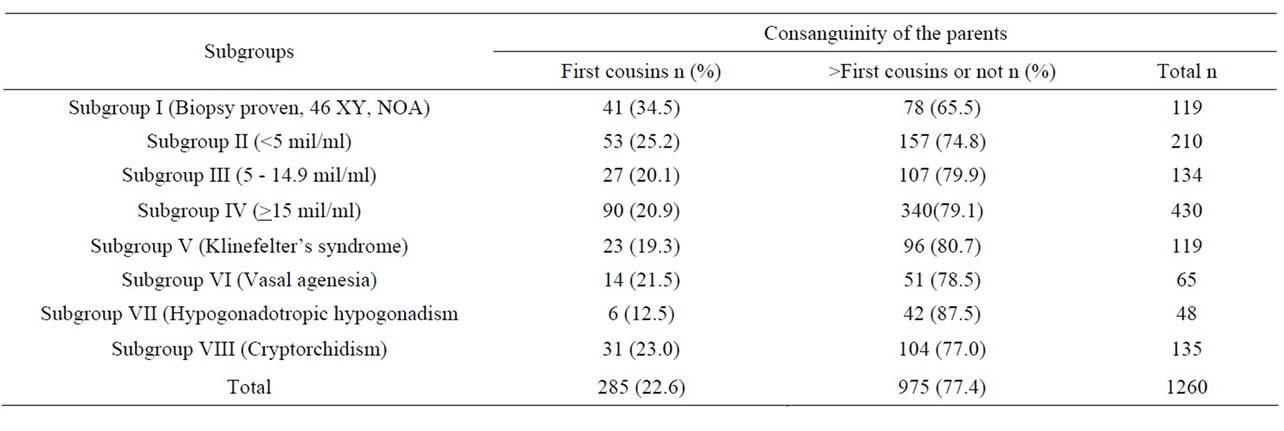
Table 2. The parental consanguinity ratios of evaluated males according to the subgroups.
In the NOA subgroup the parental consanguinity ratio was higher than in the other subgoups. This was significantly higher than in subgroups III (p = 0.01), IV (p = 0.002), V (p = 0.009), VII (p = 0.004), and VIII (p = 0.043). However, the difference was not significantly higher than subgroup VI males (p = 0.067). The parental consanguinity ratios in males with Klinefelter’s syndrome, vasal agenesia and cryptorchidism were about 20%. Parental consanguinity was the lowest with the ratio of 12.5% in the hypogonadal group. However no statistical significance could be found when compared with the other subgroups except for males in the NOA subgroup.
The subgroup of 119 NOA males were cathegorised according to the FSH values as subgroup Ia with FSH > 7.6 mIU/ml and subgroup Ib with FSH ≤ 7.6 mIU/ml (Table 3). The parental consanguinity ratio was significantly higher in males with FSH values less than 7.6 mIU/ml.
In 119 NOA males FSH, LH, TT and total testicular volumes were compared between the males with parental first cousin consanguinity and others. In 41 males with first cousin parental consanguinity and in the other 78 males without consanguinity, mean total testicular volumes were 28.9 + 10.8 and 25.2 + 9.4 ml (p = 0.06), TT values were 442.8 + 197.5 and 416.3 + 229.4 ng/dl (p = 0.27), FSH values were 13.0 + 11.8 and 18.4 + 12.2 mIU/ml (p = 0.004) and, LH values were 6.8 + 4.2 and 7.9 + 4.5 mIU/ml (p = 0.16), respectively. In subgroup II males (sperm count less than 5 million/ml) the same comparisons were made and there were no significant differences (p > 0.05 for each comparison).
In subgroup II, there were no significant differences when compared for sperm counts and motility parameters between males with and without first cousin parental consanguinity. The same was true for subgroup III males. In subgroup IV (Table 4), sperm counts, motility ratios and motile sperm counts were significantly decreased in the males with parental consanguinity. Total testicular volumes and ejaculation volumes were not different. In this subgroup when subjects were divided into 15 - 29.9, 30 - 59.9, and >60 million/ml groups empirically, the first cousin parental consanguinity ratios were 27.1% (39/144), 21.9% (40/183) and 10.7% (11/103), respectively. There were significant differences between the 15 - 29.9 million/ml group and >60 million/ml group (p = 0.002), and, the 30 - 59.9 million/ml group and >60 million/ml group (p = 0.018). In the 15 - 29.9 million/ml group when parents were first cousins sperm counts per ml were less than for the others but statistically insignificant (p = 0.058); however all the motility parameters were significantly worse (p < 0.05 for each). In the 30 - 59.9 million/ml group only progressive sperm count per ml was significantly lower in males with first cousin parental consanguinity (p = 0.012). The other motility parameters were also lower but did not reach statistical significance. In males with ≥60 million/ml sperm counts there were no significant differences in terms of motility parameters.
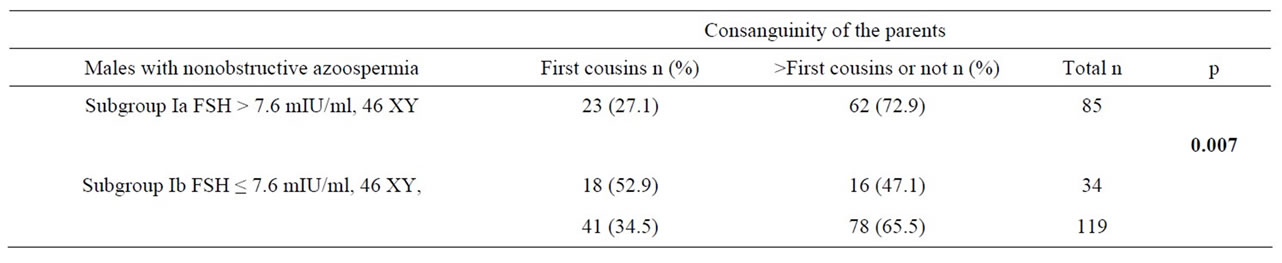
Table 3. Parental consanguinity ratios according to FSH levels of NOA males.
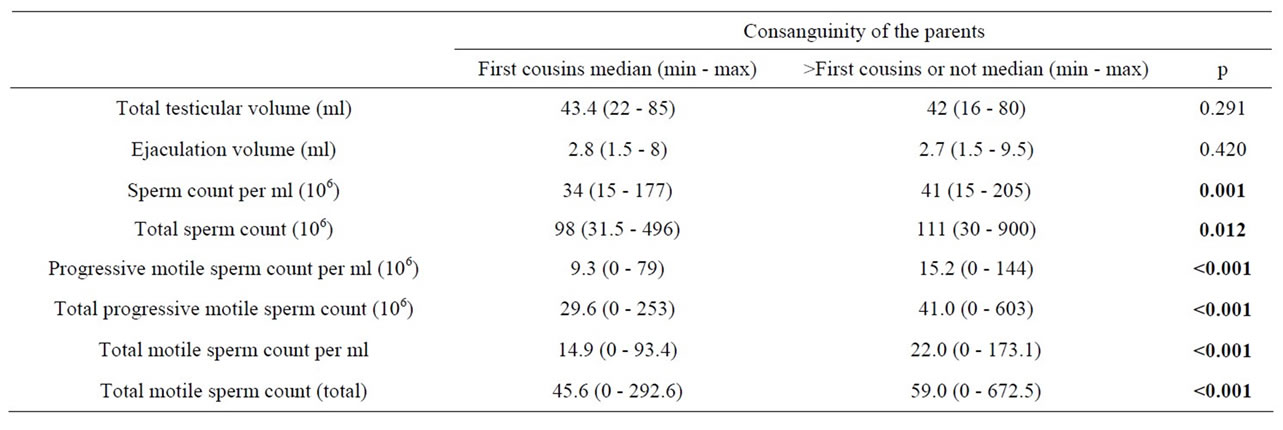
Table 4. Some comparisons in males with normal sperm counts with consanguineous and nonconsanguineous parents.
4. Discussion
Following strict exclusion criteria, the parental consanguinity of 1260 infertile males was evaluated. In the nonobstructive azoospermic (46 XY, biopsy proven) subgroup the parental consanguinity of the males was highest. In this subgroup total testicular volumes were smaller in nonconsanguineous males but did not reach statistical significance. This might have contributed to the elevated FSH and LH values but TT values were not different. Elevation of FSH generally shows significant seminiferous tubule damage. The lower FSH values in males with parental consanguinity might point to other genetically transmitted defects resulting in azoospermia.
Other interesting findings from our study was the decreased sperm counts, motility ratios and motile sperm counts in men with normal sperm counts who had parental consanguinity. This also might show a genetic background and may be a result of their ancestry being accustomed to inbred marriages in the past; however, we are unable to prove this. It is possible since older generations lived in villages or small towns and consanguineous marriages were possibly higher. In our cases, when taken together, the inbred marriage ratio was 9.5% at the first cousin level. This ratio was 22.6% in their parents.
In males with normal sperm counts, subgroup analyses showed some diverse data. In males with a sperm count of 15 - 29.9 millions/ml the parental consanguinity ratio was 27.1% and was higher than for subgoup III with sperm counts of 5 to 14.9 million/ml (20.1%) and less than subgroup II with sperm counts of less than 5 millions/ml (25.2%). Although strict exclusion criteria were applied there might be other reasons for the diminished sperm counts other than genetic reasons. Whatever the case is, it is interesting that males with sperm counts of more than 60 million per ml had the lowest ratio of parental consanguinity (10.7%). This might be stonger evidence showing the deleterious effects of consanguinous marriages on the next generations' reproduction potential.
In the group with Klinefelter syndrome, the parental consanguineous ratio was not higher than that of our infertile population in general. This syndrome has a genetic basis but the risk of translation is almost impossible through natural conception. The supernumerary X chromosome is almost equally transmitted from both the mother and the father [6,7]. However this possibly does not pose the risk of genetic translation through consanguineous partners.
In males with congenital hypogonadotropic hypogonadism the parental consanguinity ratio was the lowest (12.5%). The reason might be the low prevalence of this syndrome complex (1/4000 - 10.000 in males) [8]. There are many mutations in either sporadic or familial form [9]. In our group non of the infertile males had consanguinity with another. We did not evaluate any one or family for the mutations. In the light of new developments we should carefully evaluate and inform consanguineous couples in particular about the possibility of transmission.
How does transmission or mutation occur? Are they sporadic? Possibly this is general not the case in general. Subjects were asked as to whether cousins were uncles’ or aunts’ offspring. However the number of cases was too small for statistical analyses and power. This might be of another study. As can be seen we have a large pool from which to examine some genetically or sporadically occuring defects. This may encourage other groups to perform studies to discover for these defects, so that possible treatment options may be found.
Subjects were questioned as to whether their siblings and other cousins had infertility problems. If present, the infertile men did not generally know the reason for their infertility problems. Therefore, we could not analyze this finding. A higher number of cases are needed for this kind of evaluation.
There were lots of exluded males, because they all had one or more conditions which might be the reason for their infertility like varicocele, scrotal or inguinal surgery, prior chemo/radiotherapy, orchiectomy (except the reason of cryptorchidism) etc. Our intention was to reduce bias and to point to possible genetic reasons, if possible.
In the studies performed in Turkey more than 20 years ago, the overall parental consanguinity ratio was 21.1% (16.8 to 31.4 in different cities) [10,11]. Those men are possibly the parents of those who were included in this study. In our study the percentage of all degrees of parental consanguinity was 30.4%. It is difficult to determine if this high percentage is the result of the higher parental consanguinity ratio in infertile males being higher than in the normal population or if it is because the consanguineous marriage ratio is higher in our region because we did not have a control group from the general population. This is also a limitation of our study.
5. Conclusion
Parental consanguinity might have deleterious effects for the reproductive health of future generations. In our region, further research should be performed since there are a high numbers of cases with parental consanguinity.
REFERENCES
- M. C. Inhorn, L. Kobeissi, Z. Nassar, D. Lakkis and M. H. Fakih, “Consanguinity and Family Clustering of Male Factor Infertility in Lebanon,” Fertility and Sterility, Vol. 91, No. 4, 2009, pp. 1104-1109. doi:10.1016/j.fertnstert.2008.01.008
- J. Zlotogora, “Genetic Disorders among Palestinian Arabs: 1. Effects of consanguinity,” American Journal of Medical Genetics, Vol. 68, No. 4, 1997, pp. 472-475. doi:10.1002/(SICI)1096-8628(19970211)68:4<472::AID-AJMG20>3.0.CO;2-O
- A. H. Bittles, J. C. Grant, S. G. Sullivan and R. Hussain, “Does Inbreeding Lead to Decreased Human Fertility?” Annals of Human Biology, Vol. 29, No. 2, 2002, pp. 111- 130. doi:10.1080/03014460110075657
- B. Baccetti, S. Capitani, G. Collodel, G. Di Cairano, L. Gambera, E. Moretti and P. Piomboni, “Genetic Sperm Defects and Consanguinity,” Human Reproduction, Vol. 16, No, 7, 2001, pp. 1365-1371. doi:10.1093/humrep/16.7.1365
- D. Escalier and M. Albert, “New Fibrous Sheath Anomaly in Spermatozoa of Men with Consanguinity,” Fertility and Sterility, Vol. 86, No. 1, 2006, pp. e211-e219. doi:10.1016/j.fertnstert.2005.12.042
- F. Lanfranco, A. Kamischke, M. Zitzmann and E. Nieschlag, “Klinefelter’s Syndrome,” Lancet, Vol. 364, No. 9430, 2004, pp. 273-283. doi:10.1016/S0140-6736(04)16678-6
- J. L. Simpson, F. de la Cruz, R. S. Swerdloff, C. Samango-Sprouse, N. E. Skakkebaek, J. M. Graham, et al., “Klinefelter Syndrome: Expanding the Phenotype and Identifying New Research Directions,” Genetics in Medicine: Official Journal of the American College of Medical Genetics, Vol. 5, No. 6, 2003, pp. 460-468.
- M. Fromantin, J. Gineste, A. Didier and J. Rouvier, “Impuberism and Hypogonadism at Induction into Military service. Statistical Study,” Problemes Actuels d’Endocrinologie et de Nutrition, Vol. 3, No. 16, 1973, pp. 179- 199.
- F. Brioude, J. Bouligand, S. Trabado, B. Francou, S. Salenave, P. Kamenicky, et al., “Non-Syndromic Congenital Hypogonadotropic Hypogonadism: Clinical Presentation and Genotype-Phenotype Relationships,” European Journal of Endocrinology/European Federation of Endocrine Societies, Vol. 162, No. 5, 2010, pp. 835-851.
- N. Basaran, H. Hassa, A. Basaran, S. Artan, J. D. Stevenson and B. S. Sayli, “The Effect of Consanguinity on the Reproductive Wastage in the Turkish Population,” Clinical Genetics, Vol. 36, No. 3, 1989, pp. 168-173. doi:10.1111/j.1399-0004.1989.tb03183.x
- E. Tuncbilek and I. Koc, “Consanguineous Marriage in Turkey and Its Impact on Fertility and Mortality,” Annals of human Genetics, Vol. 58, No. 4, 1994, pp. 321-329. doi:10.1111/j.1469-1809.1994.tb00729.x
NOTES
*There is no financial disclosure of any of the authors.
#Corresponding author.

