World Journal of Condensed Matter Physics
Vol.3 No.1(2013), Article ID:28276,5 pages DOI:10.4236/wjcmp.2013.31002
Mechanical, Thermal and Chemical Durability Behaviors of CdO-Bi2O3 Boro-Phosphate Glasses Containing Fe2O3
![]()
1Department of Physics, Faculty of Science, Al-Azhar University, Women Branch, Nasr City, Cairo, Egypt; 2Department of Physics, Faculty of Science, Ain Shams University, Cairo, Egypt; 3Department of Physics, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt.
Email: *heba_saudi@yahoo.com
Received September 20th, 2012; revised October 26th, 2012; accepted November 13th, 2012
Keywords: Phosphate Glasses; Durability; Hardness; Thermal
ABSTRACT
The glass system xBi2O3-(30 – x)CdO-10B2O3-20Fe2O3-40P2O5 (0 ≤ x ≤ 30 weight %), has been prepared by the melt quenching technique. The thermal stability of the glasses, their hardness as well as their chemical durability have been investigated before and after subjecting to different gamma ray doses to insure the validity of this glass system for application to the problem of the permanent disposal for nuclear wastes. The results indicated that the glasses have well to excellent chemical durability, thermal stability and hardness with increasing Bi2O3 content on the expense of CdO, and that the effect of irradiation with gamma doses is very small.
1. Introduction
Recent interest in the vitrification of intermediate level radioactive waste (ILW) as well as high level radioactive waste (HLW) has led to the development of new waste glass compositions that have not previously been characterized [1,2]. Iron phosphate glasses have been investigated for over 10 years as a potential host matrix for the vitrification of certain radioactive wastes. Phosphate glass has poor chemical durability; however, addition of Fe2O3 improved it. Vitrification is a favored process for immobilization and destruction of dangerous wastes with high chemical durability [3]. Borosilicate glass was chosen as the first generation nuclear waste host due to the established technological base and its properties such as high chemical durability, thermal stability and the ability of this glass matrix to incorporate high-level waste components [4]. Boron was found to improve thermal stability of iron phosphate glasses [5]. Thermal stability is one of the criteria for a glass host to be considered for the vitrification of radioactive wastes, as glass host is required to resist crystallization during cooling. Especially, when melting large amounts of glass, this problem becomes more important. Boron also has a large neutron absorption cross section, so it is advantageous to have a component in glassy waste form where boron increases both the thermal and radiation stability of the waste form.
The mechanical properties of nuclear waste form glasses are also important, as they will determine the degree of cracking that may occur either on cooling or at accident handling. In a radioactive glass block that is subject to radiogenic heating, stresses are maintained due to a temperature gradient between the core and the surface. This gradient decreases with time, but persists over hundreds of years until 137Cs and 90Sr have decayed. Surface hardness measurements quantify the resistance of glass sample to both plastic and elastic deformation [6].
In the present study, we have investigated the effects of addition of bismuth on expense of CdO on the thermal, hardness as well as the chemical durability of the glass system; xBi2O3-(30 − x)CdO-10B2O3-20Fe2O3-40P2O5 (0 ≤ x ≤ 30 weight %), which was proved [7] through its attenuation to fast neutrons and gamma rays its ability to be used as a super shield, to insure its validity also for the usage as a radioactive waste disposal matrix. So it was necessary also to study the effect of γ radiation on its hardness and chemical durability.
2. Experimental
Analytically pure grade chemicals were used to prepare the following glass samples according to the formula Bi2O3-(30 – x)CdO-10B2O3-20Fe2O3-40P2O5 wt% where x = 0, 5, 10, 15, 20, 25 and 30. The batch mixtures were melted in porcelain crucibles at 1100˚C for two hours until homogeneous glasses were obtained and then annealed in a separate annealing furnace at 250˚C and then slowly cooled to the room temperature to remove any internal stresses. The glass transition temperatures (Tg) of the samples were determined by a standard Shimadzu differential scanning calorimeter (DSC). 10 mg powdered glass samples were placed in aluminum crucible and examined up to 873 K in an argon medium with flow rate of 50 ml/min.
A Vicker’s diamond indentor was used in a standard microhardness tester (Leco AMH 100, USA) for specimen indentation. A load of 500 g applied for 15 s was used to make indentations in specimens of glasses. Each sample was subjected to ten indentations at randomly selected areas; hence, errors on the measured values corresponding to the standard deviation are about 2%. The diagonal length impressions were measured and the hardness number H was calculated according to a standard formula: H = 1.854 P/d2 [8], where P is the indentation load, and d is the diagonal length impression.
The chemical durability of glasses was determined by measuring the dissolution rates from the calculated weight loss of the samples immersed in distilled water. For these measurements, the specimens have circular form with diameter of approximately 1 cm. The samples were polished using SiC papers, cleaned with acetone and distilled water, dried and weighted before suspending them in 100 ml distilled water. The beaker was placed in an oven at 90˚C. The specimens were removed, rinsed with distilled water, dried, and weighted at the end of 2, 4, 7 and 14 days. The dissolution rate (DR) was calculated from the measured weight loss (Δw) using the equation  where A is the surface area of the sample and t is the immersion time.
where A is the surface area of the sample and t is the immersion time.
Cs137 gamma cell (1500 Ci) was used as a γ-ray source with a dose rate of 1 and 10 Gy/s, at room temperature (30˚C). The investigated glasses were exposed to the same gamma dose every time.
3. Results and Discussion
3.1. Microhardness
The variation of the hardness number (H) with the Bi2O3 weight percentage is tabulated in Table 1 and Figure 1. It can be seen that the hardness increases as Bi2O3 increases and that the hardness of the glass that contains no iron and bismuth is very small compared to those containing Fe2O3 and Bi2O3. The observed increase in H is related to the increase of the rigidity of glass. These results are consistent with the density and IR results [7] which indicated that addition of Bi2O3 strengthened the bond energy between Bi-O and Fe-O. These results are also in agreement with the results obtained by Kurkjian [9].
Table 1 and Figure 1 illustrate the hardness of glass
Table 1. The hardness of glass samples after irradiation with low and high γ ray doses, and the hardness of original glass samples as a function of Bi2O3 composion in (kgf/mm²).
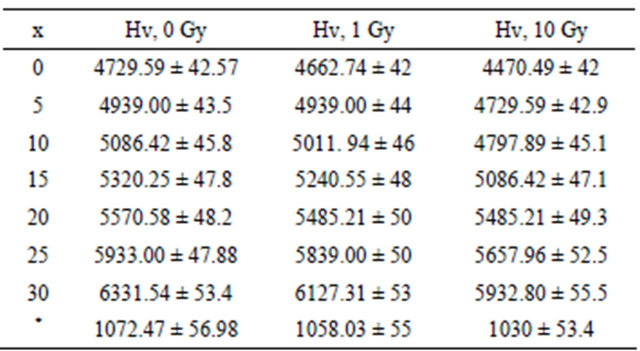
(*) No Bi2O3 or Fe2O3.
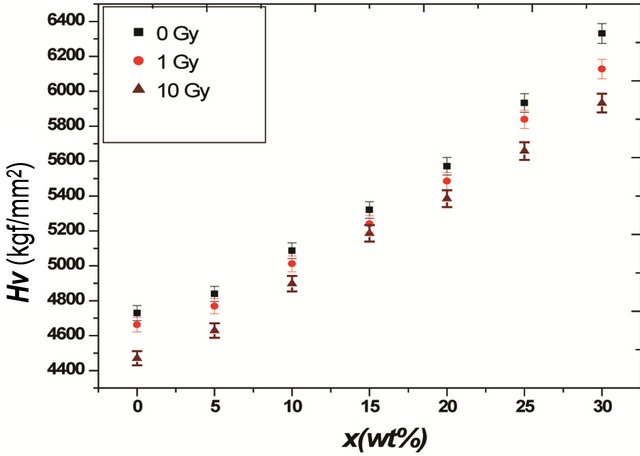
Figure 1. Variation of surface hardness as a function of Bi2O3 composition with different γ doses.
samples after irradiation by γ ray with different doses and the hardness of original samples as a function of Bi2O3 composition. It can be observed that the micro hardness is decreased slightly as compared to that of the original samples. This means that the effect of γ ray irradiation on the P-O-Bi and/or P-O-Fe bonds [7], and hence the glass hardness is small, especially at concentrations from 10 wt% to 20 wt% Bi2O3 as can be seen in Table 1 and Figure 1. The small decrease in the values of the hardness with increasing the irradiation dose to 10 Gy can be attributed to irradiation induced defects. γ irradiations can cause displacements of lattice atoms or electron defects, which involve change in the valence state of lattice or impurity atoms [10]. Thus, the glass network and the group arrangement become more asymmetrical, leading to a weakening of the network grouping vibrations, so decreasing the resistance of the glass to deformation. These irradiation-induced defects were confirmed in a previous IR studies [7] on this glass system before and after γ and neutron irradiation, which reveal the main spectral features due to phosphate network, but with some changes in the bond lengths and angels, or the breakage of some P=O or migration of some network atoms to the interstitial positions or vice versa.
3.2. Chemical Durability
As can be seen from Table 2 the dissolution rates (DR) of the glasses are found to be in the order of ~10−9 g/cm2 day, which shows that these glasses can be considered as chemical durable glasses. Low DR means that the glass has a high chemical durability [11].
It can be observed that bismuth oxide addition together with iron oxide improved the durability more than iron oxide alone. As shown in Table 2 and Figure 2 all glasses exhibit well to excellent aqueous durability except glass containing no bismuth, it has relatively higher DR, of the order of ~10−8 g/cm2 day. The glass sample containing no Bi2O3 or Fe2O3 was broken after 4 days. The often associated poorer chemical durability is explained [12,13] by smaller cross-linking density and, in case of P2O5 glass, by a localization of the П-bond on only one P-O bond in the branching point PO4 groups [14,15]. On the other hand, it can be observed also that for glasses containing Bi2O3 there is no significant change in DR with increasing the immersion periods. The higher of Bi2O3 content the higher chemical durability, i.e; Bi2O3 improves the
Table 2. The dissolution rates of glass samples as a function of Bi2O3 composition before irradiation.
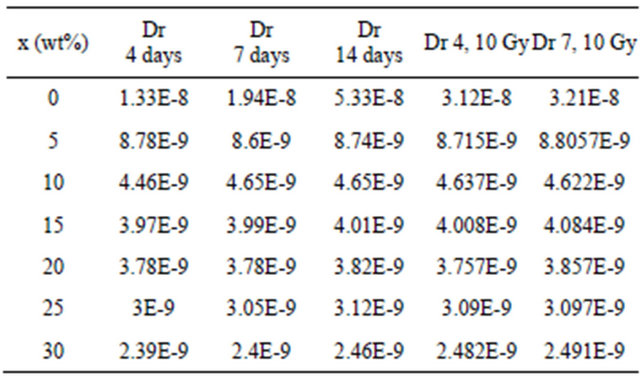
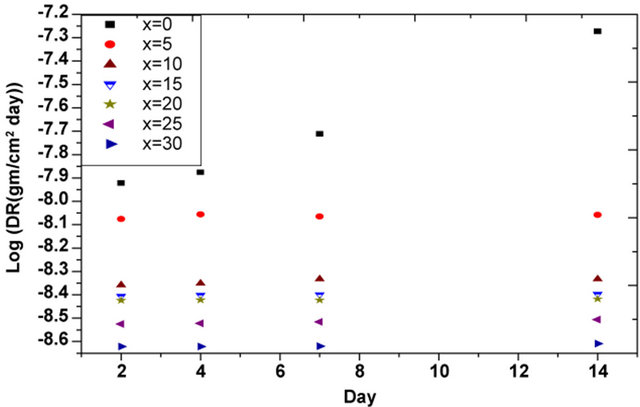
Figure 2. Dissolution rate for glasses of different Bi2O3 concentrations with different immersion periods.
water resistance ability of phosphate glass. The non linearity in the values of DR and hence in chemical durability with Bi2O3 content especially for the sample with 10 wt% Bi2O3 which showed sudden and large decrease in DR than that with 5 wt% Bi2O3 can be interpreted from the IR results [7], which proved that for the glass containing 10 wt% Bi2O3, some of Bi atoms entered the glasses as a former with an increase also in the FeO4 percentage which is proved [16] to increase in the chemical durability. This is beside the formation of P-O-Bi and/or P-O-Fe cross link with relatively high density for the present glasses with Bi2O3 of 10 wt% and 15 wt%. These cross-linking increase the water resistance ability of these glasses. So the improved durability of these glasses can be attributed to the replacement of the easily hydrated P-O-P bonds by corrosion resistant P-O-Bi and/ or P-O-Fe bonds. As the Bi2O3 content in the glass increases the number of P-O-Bi increases.
As can be seen from Table 2 and Figure 3 gamma ray irradiation affected only on the glass containing no Bi2O3 where it increases its Dr, i.e; decrease its durability. On the other hand, there is no significant change in the DR values of the glasses containing Bi2O3 after irradiation, which proves once more the role of Bi2O3 in improving the glasses properties and their resistance to each of corrosion as well as radiation effects.
3.3. Thermal Properties
DSC curves for the studied glass samples with different Bi2O3 contents have been measured. The obtained thermal properties: glass transition temperatures (Tg), onset crystallization temperatures (Tx), crystallization temperatures (Tc) as well as melting temperatures (Tm), of the glasses as obtained from the DSC results are tabulated in Table 3. It is seen that for all the glass samples studied, Tg and Tx are in the ranges of 533 - 623 K and 733 - 773 K, respectively, giving significant super cooled liquid
(SCL) regions 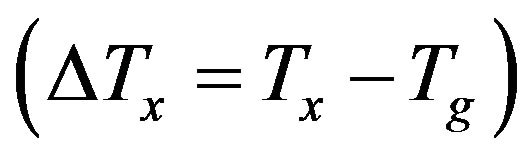 of 150 - 200 K.
of 150 - 200 K.
The value of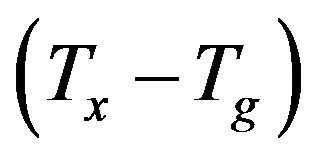 , which can be used as a measure of the thermal stability of a glass, is consistent with the glass formation boundaries within the respective systems, high thermal stability of the glassy samples indicates [17] a large supercooled liquid (SCL) region. As shown in Figure 4 sample with no iron and bismuth oxides exhibits the lower
, which can be used as a measure of the thermal stability of a glass, is consistent with the glass formation boundaries within the respective systems, high thermal stability of the glassy samples indicates [17] a large supercooled liquid (SCL) region. As shown in Figure 4 sample with no iron and bismuth oxides exhibits the lower 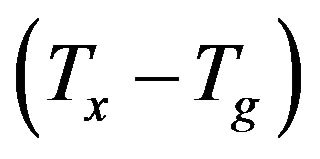 than the other glass samples since it is close to its glass formation boundary. The calculated values of ΔTx increase by increasing the Bi2O3 content with a maximum value at about 30 wt% Bi2O3, and decreased in sample with no iron and bismuth. The non-linearity in the values of ΔTx, which showed large, pronounced increase for the glasses containing 10 and 15 wt% Bi2O3, Figure 4 is consistent with the durability behavior. The sudden enhancement of chemical
than the other glass samples since it is close to its glass formation boundary. The calculated values of ΔTx increase by increasing the Bi2O3 content with a maximum value at about 30 wt% Bi2O3, and decreased in sample with no iron and bismuth. The non-linearity in the values of ΔTx, which showed large, pronounced increase for the glasses containing 10 and 15 wt% Bi2O3, Figure 4 is consistent with the durability behavior. The sudden enhancement of chemical

Figure 3. Dissolution rate for glasses of different Bi2O3 concentrations before and after irradiation with 10 Gy.
Table 3. Thermal properties of the xBi2O3-(30 – x)CdO- 10B2O3-20Fe2O-40P2O5 (0 ≤ x ≤ 30 weight %).

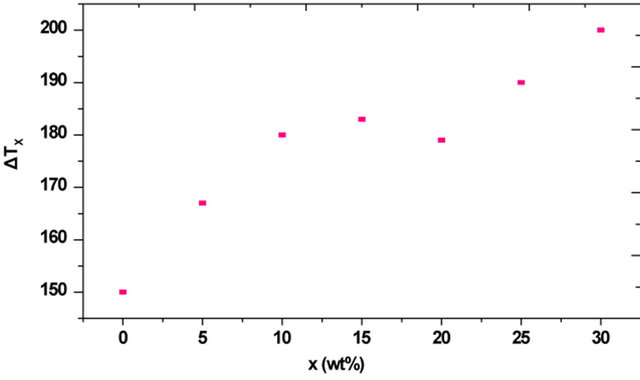
Figure 4. The effect of bismuth oxide content on the thermal stability ∆Tx.
durability with decreasing the glass transition temperature at the same time on replacing 10 wt% and 15 wt% CdO by Bi2O3 can be attributed to the structural changes that strengthen the network, as observed from IR results [7], at these Bi2O3 concentrations. As stated before [16] A. Inoue, Y. Yokoyama, Y. Shinohara and T. Masumoto, Mater. Trans. JIM 35 (1994), page 923. View record in Scopus cited by in Scopus (51) a large value of ΔTx implies that the super cooled liquid can be exist in a wide temperature range without crystallization and has a high resistance to the nucleation and growth of crystallization phases, leading to good glass forming ability.
The calculated values of the reduced glass transition temperature 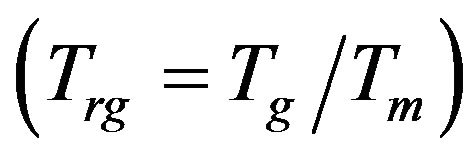 [18] for the investigated glass samples were shown in Table 3. The reduced glass transition temperature shows that,
[18] for the investigated glass samples were shown in Table 3. The reduced glass transition temperature shows that, 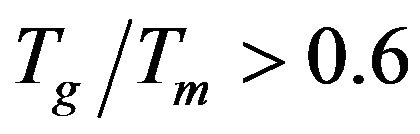 , which indicated that only surface crystallization can be observed, where crystallization is easily observed in glasses having
, which indicated that only surface crystallization can be observed, where crystallization is easily observed in glasses having 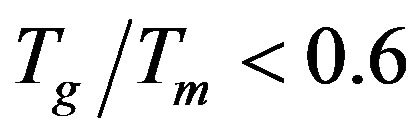 [17]. So in view of that, the calculations of the reduced glass transition temperature
[17]. So in view of that, the calculations of the reduced glass transition temperature 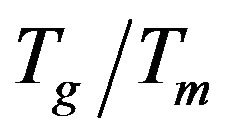 of the glass samples over all the studied range indicated that it can suffered only surface crystallization.
of the glass samples over all the studied range indicated that it can suffered only surface crystallization.
The parameter, KH [19], is often used to estimate glass stability and is given by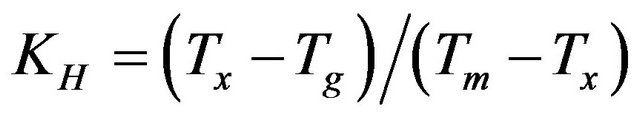 , The larger of the KH values, the greater the stability of the glass against devitrification. The aim of these calculations (KH, parameter) is, therefore, to insure the glass stability, and to investigate a possible correlation between glass stability, and the glass forming ability, for the investigated glass samples. The calculated values of KH, are given in Table 3. It is observed that the values of KH, are shifted to higher values at 15 wt% and 20 wt% Bi2O3 content. It has the same trend as the glass forming ability parameters, Trg. So a strong correlation between glass stability parameter KH, and glass forming ability is confirmed.
, The larger of the KH values, the greater the stability of the glass against devitrification. The aim of these calculations (KH, parameter) is, therefore, to insure the glass stability, and to investigate a possible correlation between glass stability, and the glass forming ability, for the investigated glass samples. The calculated values of KH, are given in Table 3. It is observed that the values of KH, are shifted to higher values at 15 wt% and 20 wt% Bi2O3 content. It has the same trend as the glass forming ability parameters, Trg. So a strong correlation between glass stability parameter KH, and glass forming ability is confirmed.
4. Conclusions
The present obtained results on the physical and chemical properties of the glass system xBi2O3-(30 – x)CdO- 10B2O3-20Fe2O3-40P2O5, with 0 ≤ x ≤ 30 prove again, in addition to the previously obtained ones on its attenuation behavior to fast neutrons and γ rays, its validity for the usage as permanent radioactive waste disposal, especially the glasses containing from 10 wt% to 20 wt% Bi2O3.
The results indicated that this glass system can be considered as chemical durable glasses; i.e it has a good water resistance ability due to the replacement of the easily hydrated P-O-P bonds by corrosion resistance P-O-Bi and/or P-O-Fe bonds. It characterized also by high thermal stability, which is one of criteria for a glass host to be considered for the vitrification of radioactive wastes. It has also good hardness and large resistance to deformation due to irradiation-induced defects. So it can resist cracking that may occur either on cooling or at accident handling.
REFERENCES
- A. J. Connelly, R. J. Hand, P. A. Bingham and N. C. Hyatt, “Mechanical Properties of Nuclear Waste Glasses,” Journal of Nuclear Materials, Vol. 408, No. 2, 2011, pp. 188-193. doi:10.1016/j.jnucmat.2010.11.034
- P. A. Bingham, N. C. Hyatt, R. J. Hand and C. R. Wilding, “Effects of Modifier Additions on the Thermal Properties, Chemical Durability, Oxidation State and Structure of Iron Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 355, No. 28-30, 2009, pp. 1526-1538. doi:10.1016/j.jnoncrysol.2009.03.008
- P. A. Bingham and R. J. Hand, “Vitrified Metal Finishing Wastes: II. Thermal and Structural Characterisation,” Journal of Hazardous Materials, Vol. 119, No. 1-3, 2005, pp. 125-133. doi:10.1016/j.jhazmat.2005.03.031
- M. Karabulut, B. Yuce, O. Bozdogan, H. Ertap and G. M. Mammadov, “Effect of Boron Addition on the Structure and Properties of Iron Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 357, No. 5, 2011, pp. 1455- 1462. doi:10.1016/j.jnoncrysol.2010.11.023
- P. A. Bingham, R. J. Hand and S. D. Forder, “Preliminary Studies of Sulphate Solubility and Redox in 60P2O5- 40Fe2O3 Glasses,” Materials Letters, Vol. 60, No. 6, 2006, pp. 844-847. doi:10.1016/j.matlet.2005.10.029
- H. E. Davies, G. E. Troxell and C. T. Wiskocil, “The Testing and Inspection of Engineering Materials,” 3rd Edition, McGraw-Hill, New York, 1994.
- H. A. Saudi, A. G. Mostafa, N. Sheta, S. U. El Kameesy and H. A. Sallam, “The Structural Properties of CdOBi2O3 Borophosphate Glass System Containing Fe2O3 and Its Role in Attenuating Neutrons and Gamma Rays,” Physica B: Condensed Matter, Vol. 406, No. 21, 2011, pp. 4001-4006. doi:10.1016/j.physb.2011.06.038
- K. K. Rao and D. B. Sirdeshmukh, “Microhardness and Interatomic Binding in Some Cubic Crystals,” Bulletin of Materials Science, Vol. 5, No. 5, 1983, pp. 449-452. doi:10.1007/BF02743923
- C. R. Kurkjian, “Mechanical Properties of Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 263-264, 2000, pp. 207-212. doi:10.1016/S0022-3093(99)00637-7
- H. A. El-Batal, F. A. Khalifa and M. A. Azooz, “Absorption and Infrared Spectra of Gamma Irradiated Ternary Silicate Glasses Containing Cobalt,” Indian Journal of Pure and Applied Physics, Vol. 42, No. 10, 2001, pp. 711-721.
- M. Karabulut, B. Yuce, O. Bozdogan, H. Ertap and G. M. Mammadov, “Effect of Boron Addition on the Structure and Properties of Iron Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 357, No. 5, 2011, pp. 1455- 1462. doi:10.1016/j.jnoncrysol.2010.11.023
- P. E. Gray and L. C. Klein, “Optical Spectra of Sodium Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 68, No. 1, 1984, pp. 75-85.
- B. C. Bunker, G. W. Arnold and J. A. Wilder, “Phosphate Glass Dissolution in Defects in Phosphate and Fluoridephosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 64, No. 3, 1984, pp. 291-316. doi:10.1016/0022-3093(84)90184-4
- J. R. Van Wazer and K. A. Holst, “Chemically Durable Nitrided Phosphate Glasses,” Journal of the American Chemical Society, Vol. 72, No. 2, 1950, pp. 639-644. doi:10.1021/ja01158a001
- U. P. Strauss, E. H. Smith and P. L. Wineman, “Polyphosphates as Polyelectrolytes. I. Light Scattering and Viscosity of Sodium Polyphosphates in Electrolyte Solutions,” Journal of the American Chemical Society, Vol. 75, No. 16, 1953, pp. 3935-3940. doi:10.1021/ja01112a017
- A. Haddi, P. Trocellier, I. Draganic, F. Farges, S. Poissonnet and P. Bonnaillie, “Ion Irradiation Behaviour and Chemical Durability of Simple Borosilicate Glasses,” Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, Vol. 266, No. 12-13, 2008, pp. 3182-3185. doi:10.1016/j.nimb.2008.03.222
- A. A. Soliman and I. Kashif, “Copper Oxide Content Dependence of Crystallization Behavior, Glass Forming Ability, Glass Stability and Fragility of Lithium Borate Glasses,” Physica B: Condensed Matter, Vol. 405, No. 1, 2010, pp. 247-253. doi:10.1016/j.physb.2009.08.154
- Y. Zhange, D. Q. Zhao, M. X. Pan and W. H. Wang, “The Structure of the Non-Crystallinematerials, Liquid and Amorphous Solids,” Journal of Non-Crystalline Solids, Vol. 315, 2003, p. 206.
- A. Hrubÿ, “Evaluation of Glass-Forming Tendency by Means of DTA,” Czechoslovak Journal of Physics B, Vol. 22, No. 11, 1972, pp. 1187-1193.
NOTES
*Corresponding author.

