Agricultural Sciences
Vol.5 No.8(2014), Article
ID:47642,8
pages
DOI:10.4236/as.2014.58069
Bioavailability of Chlorpyrifos in Wheat Plants (Triticum aestivun)
Sylvia V. Copaja*, Rosa Vergara, Héctor R. Bravo
Departamento de Química, Facultad de Ciencias, Universidad de Chile, Santiago, Chile
Email: *scopaja@uchile.cl
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 29 April 2014; revised 3 June 2014; accepted 28 June 2014
ABSTRACT
Adsorption processes of chlorpyrifos in two Chilean agro soils (Calera: C; San Esteban: SE) in relation with the bioaccumulation in wheat plants (Triticum aestivun) were studied. RP-HPLC method was developed to determine the chlorpyrifos content in soils, roots and seedling tissues. The two soils showed high adsorption capacity (C = 78%, SE = 92%). The values are not in relation with the contents of organic matter (C = 3.9% and SE = 2.0%) and clay (C = 12.7% and SE = 10.1%) determined in the soils. Persistence and mobility of chlorpyrifos in the soils were estimated from halflife values (Csoil = 23 d, SEsoil = 14 d) and the Guss index (Csoil = 0.89, SEsoil = 0.25) respectively. These values are in the range of non-leaching compounds, and suggest that there should be no pollutant in the ground water. Wheat plants grown in both soils incubated with chlorpyrifos bioaccumulate residues in roots and seedling tissues. Root tissues showed the greatest contents (C = 22.3 mg/k. f. wt; SE = 51.8 mg/kg. f. wt.). Germination and growth of wheat young plants were not inhibited for the contents in the tissues. A relation between the levels of chlorpyrifos residues in the soils and the bioaccumulation in wheat tissues was estimated from the BAI parameter. Values showed that the bioaccumulation is dependent on the residues contents in soils. These results suggest that bioavailability of chlorpyrifos in wheat plants may be a harmful pollutant for mammals if it remains stable at all growth stage of the plant. Further research should be considered to see if bioavailability in forage and/or grain can occur.
Keywords:Soil, Chlorpyrifos, Wheat, Persistence, Bioavailability

1. Introduction
Pesticide application is almost essential in modern agriculture to maintain high productivity. Increased awareness of the environmental damage caused by continued use of conventional synthetic pesticides has raised great interest in their behavior in the ecosystem and their effects on the biota. It has been estimated that less than 0.1% of the pesticide applied to crops actually reaches the target pest; the rest enters the environment, gratuitously contaminating soil, water and air, where it can poison or otherwise adversely affect non-target organisms [1] -[3] . Greater amounts of the pesticides applied in agriculture are included in the soil.
In general, pesticides in the environment are governed by the transformation process, which can include pesticide molecule breakdown by chemical, photochemical or biological degradation or by transfer processes such as adsorption/desorption, runoff, volatilization and leaching. Among transfer processes, adsorption is a key process that largely controls the behavior of pesticides in soil, determining their distribution in the soil and the aquatic systems [4] -[8] . The dynamics of pesticides in the soil is therefore considered to be highly complex.
Persistence is a parameter that encompasses all the processes involved in the dynamics of pesticides in the soil. Persistence may determine that non-target organism accumulates the residues of pesticides or their degradation products. Particularly important can be accumulation in plant tissues, which can be harmful for mammals when they are used as foodstuffs. Organophosphorous (OP) insecticides are widely used in the control of insect pests. OPs are not very persistent in soil and they are known to be considerably toxic in that they inhibit a number of hydrolytic enzymes and have neurotoxic action in insects and mammals [8] [9] .
Dynamics of OPs in soils, particularly the chlorpyrifos insecticide has been studied [10] -[14] . However, data on the interactions with plants are relatively limited [15] -[17] .
Chlorpyrifos (Figure 1(a)) is the OP insecticide most used in Chile. It is used in the control of insect pest in fruit and cereal cultivars. To gain a deeper understanding of the availability and bioaccumulation in plants of this chemical, we study the persistence in two Chilean agro soils and the bioavailability in wheat plants (Triticum aestivum) under controlled conditions.
An RP-HPLC method was developed to determine the chlorpyrifos content in soils and plants. The physicochemical characteristics of the two soils were determined.
2. Materials and Methods
2.1. Chemicals
Chlorpyrifos was purchased as a 99% pure commercial product (Sigma Chemical Company). A commercial formulation was obtained from ANASAC (48%). The purity of the chemicals was confirmed by HPLC. Solvents used in the study were HPLC grade (Merck) and all inorganic reagents were laboratory grade.
2.2. Soil Samples
Two Chilean agricultural soils, Calera (C) and San Esteban (SE) (VI region of Chile) (0 - 20 cm), were selected. The soils were dried at room temperature and sieved (˂2 mm). Particle size distribution (texture) was determined by the pipette method [18] ; pH, electrical conductivity (EC) and organic carbon were determined by described methods [19] . All measurements were performed in duplicate. Geographical location of both soils are: Soil Calera, 32˚52' latitude S and 70˚30' longitude and San Esteban soil 32˚45' latitude S and 70˚38' longitude.
2.3. Chemical Analysis
The residues of chlorpyrifos in the soils and plants were determined using a liquid chromatograph (Waters 1525) high resolution (HPLC) method under the following conditions: Columns Waters Symmetry C18; mobile phase:
 (a)
(a) (b)
(b)
Figure 1. Structure of chlorpyrifos (0,0-diethyl-0-(3,5,6-trichloro-2-pyridil)-phosphothioate) (C9H11Cl3NO3PS) and (b) 3,5,6 trichloro-2-pyridinol (C5H2Cl3N).
acetonitrile/water 70:30; flow rate 1.0 mL/min and injection volume 20 µL. Detection was performed using a diode array detector (PDA, Waters 2996) at 220 nm. Retention time (tR) of the chlorpyrifos standard was 9.42 ± 0.20 min. For both solutions, standard pure and commercial formulation, tR and UV spectra were compared. The linear range, detection limit and quantification limit were determined in the pure standard and commercial formulation. In Figure 2 are show the chromatogram of standard solution (pure compounds) and commercial formulation solution of chlorpyrifos.
2.4. Bioaccumulation Assay
50 wheat seeds were planted in plastic pots containing 250 g of soils (C and SE) and watered with 50 mL of a 1000 mg∙L−1 solution of chlorpyrifos. Controls were watered with MiliQ grade water (Simplicity, Millipore). The temperature in the growth room was 25˚C ± 3˚C; the relative humidity range was 55% - 65% and continuous cold white fluorescent light with an intensity of 200 ft-c. was used. Each assay was performed with two replicates.
Forty-one d after sowing, between 150 - 250 mg of wheat seedling or root tissue were macerated 3 times in succession with 5 mL of acetonitrile using a pestle and mortar. Extracts were centrifuged at 4000 rpm for 15 min; supernatants were filtered (0.45 µm) and were evaporated under vacuum and the residues were dissolved in 2 mL of acetonitrile and analyzed by the HPLC method.
2.5. Persistence Assay
20 plastic pots with 10 g of soils (C and SE) were treated with 5 mL of solution 1000 mg∙L−1 solution of chlorpyrifos. Incubated soils were irrigated with water Milli-Q grade (Simplicity, Millipore) periodically to maintain the field capacity humidity. Incubation was conducted for a period of 60 d in the same conditions of temperature
 (a)
(a) (b)
(b)
Figure 2. Chromatogram of Chlorpyrifos: (a) pure compounds and (b) commercial product.
and humidity as the bioaccumulation assays. Each 7 d, two pots of C soil, SE soil and control soil were treated three times with 5 mL of acetonitrile and stirred for 30 min. Samples were centrifuged at 4000 rpm for 15 min. supernatants were filtered (0.45 µm) and evaporated under vacuum and the residues were dissolved in 2 mL of acetonitrile and analyzed by HPLC. The amount of chlorpyrifos in the soil was obtained by linear regression from a calibration curve.
2.6. Adsorption Studies
1 g of soil samples, were treated in 250 mL plastic bottles with 10 mL solution of chlorpyrifos in a concentration range from 0 to 1000 mg∙L−1. Stock solutions pesticide were prepared in acetonitrile (HPLC grade) and a 0.01 M solution of CaCl2 (Fluka p.a.) was used to complete the total volume (10 mL). The suspension was agitated at 23˚C ± 2˚C in a batch system for 24 h, long enough to reach equilibrium. Subsequently, samples were centrifuged for 15 minutes at 4000 rpm, and supernatants were stored in cold for further analysis. Each adsorption experiment was performed in duplicate. Losses during the process were monitored by including two blank controls in each test: one plastic bottle that only had a chemical solution without any adsorbent and another with only the adsorbent and CaCl2 solution without chemical solution. The amount of each compound adsorbed in the soil phase, Cs (mg∙kg−1) was obtained by subtracting from the amount of compound in the initial solution the amount measured the aqueous phase. Thus it was assumed that processes such as degradation, volatilization or photolysis were not significant during the 24 h period.
3. Results and Discussion
3.1. Physical and Chemical Properties of Soils
Table 1 shows the textural characteristics, pH, electrical conductivity (EC) and organic carbon (OC) for the two soils studied. Clay and organic matter (OM; OC × 1.724) content are two principal parameters involved in the dynamics of pesticides in the soil [20] -[25] . Our soils showed relatively similar percentages of clay, and the C soil had more organic matter content (3.9%), The pH were slightly alkaline and the EC values corresponded to the range of non-saline soils.
3.2. Persistence of Chlorpyrifos in Soils
The transfer processes of chlorpyrifos in the C and SE soils were evaluated over a period of 63 d. Both soils showed high capacity to absorb chlorpyrifos. In the first measurement (to, after approximately 1 h) the adsorption in C and SE soils were 78% and 92%, respectively. Adsorption of neutral compounds in soils has been extensively investigated, and appears to depend on the organic (OM) and inorganic (clay matter) components of the soils [26] -[28] . The mobility of pesticides in soils with low organic matter content (˂5%) appears to be largely governed by inorganic fractions (type and percentage of clay).
The soils studied did not have significant differences in clay content (Table 1), thus the different amounts adsorbed cannot be related to this parameter. On the other hand, the C soil with greater organic matter content showed lower adsorption. Figure 3 show the adsorption isotherms of chlorpyrifos in both soils.
The behavior was similar and L-type curve according the classification of Giles et al. (1974), were observed [29] . These results suggest that part of the organic matter may be inaccessible to the pesticide if it’s is associated in a solid state with the humic particles or clay aggregate, reducing the effective level of active organic carbon in the soil. Therefore, other properties such as the molecular nature of the organic matter or the type of clay matter may influence the adsorption process of chlorpyrifos.
The dynamics of dispersion for the residues, 22% and 8% in C and SE soil respectively, are shown in Figure 4. D50 values, a measure of the time when the content of compound decreases by 50% with respect to the starting
Table 1 . Physic-chemical characterization of Calera (C) and San Esteban (SE) soils (SD of two replicates).
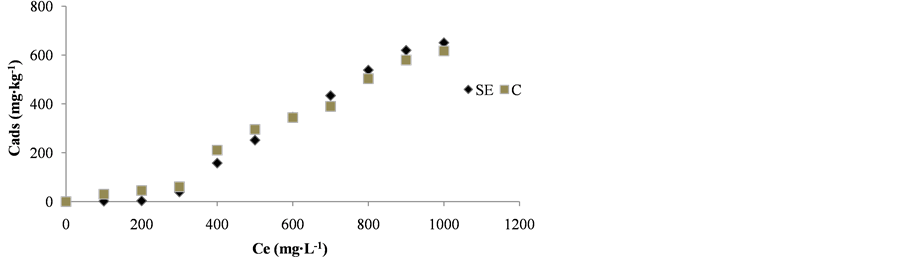
Figure 3. Adsorption curves of chlorpyrifos in San Esteban (SE) and Calera (C) soils.
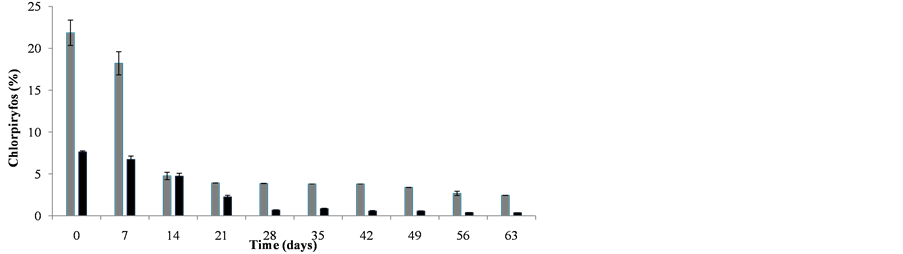
Figure 4. Persistence of chlorpyrifos in La Calera (C) and San Esteban (SE) soils.
point, were derived from these data. D50 values for C and SE soils were 7 d and 14 d, respectively. This result shows a greater affinity of chlorpyrifos for the SE soil.
Degradation is fundamental for attenuating pesticide residue levels in soil [4] [7] [30] . It is governed by biotic and abiotic factors and the adsorption-desorption process. Organophosphorous (OP) insecticides may hydrolyze under alkaline, neutral and acidic conditions. For most OPs acidic hydrolysis is fairly slow and is thus negligible under environmental reaction conditions [10] , whereas neutral and alkaline hydrolysis are of concern, the latter usually being the most important hydrolytic degradation pathway [11] [12] . Chlorpyrifos is hydrolyzed to its primary metabolite, 3,5,6 trichloro 2-pyridinol (Figure 1(b)) in the soil [16] [31] [32] . Our soils are slightly alkaline, thus part of the dispersion of chlorpyrifos may depend on hydrolytic degradation, although degradation by microorganisms could be more important [14] [33] . From our experimental conditions the metabolite B was not detected.
Persistence of chlorpyrifos in the soils was estimated from half-life (t1/2) values. The t1/2 values for C and SE soils were 23 d and 14 d respectively. The mobility of chlorpyrifos in the soils was also estimated from the Gus index [34] (Gustafson, 1989), a measure of the leaching or diffusion the compound toward groundwater. The values for C and SE soils were 0.89 and 0.25 respectively. These values are in the range of non-leaching compounds, thus the contents of chlorpyrifos in both soils should not be polluting the groundwater. All these results suggest that chlorpyrifos is available in both soils.
3.3. Bioaccumulation in Plant Tissues
The amount of chlorpyrifos absorbed by wheat plants after 41 d in C and SE soils incubated with chlorpyrifos is shown in Table2
The content of chlorpyrifos in plants from SE soil was higher than the content in plants from C soil. Root tissues accumulated more compound than seedling tissues. Persistence data suggest that the available chlorpyrifos content is low in the both soils, but this result shows that they are sufficient to be absorbed by wheat plants. The SE soil displayed lower bioavailable chlorpyrifos content, but the tissues showed greater accumulation.
Table 2. Chlorpyrifos content in roots and seedling tissues of wheat cultivated in C and SE soils and the bioaccumulation index (BAI).
This behavior can be related the greater affinity of chlorpyrifos for SE soil. Clarity about the relation between the content of chlorpyrifos in the tissues plant and the content in the soil was obtained from the bioaccumulation index (BAI) [35] (Kabara-Pendias and Pendias, 2000). The values obtained are given in Table2 How is observed SE soil displayed the larger BAI values particularly with the root tissues.
Therefore, the BAI index should be an appropriate parameter to estimate the levels of pollution of chlorpyrifos in soils with different psychochemical characteristics and their relation to its bioaccumulation in wheat cultivars.
In order for a pesticide to injure a plant a certain threshold concentration of the chemical at the site of action must be reached and maintained for a certain period of time. The length of the period required is affected by condition of the plant and the environmental conditions under which the plant is growing. In our experimental conditions we observed that the germination and growth of young wheat plants was not affected by the chlorpyrifos content in tissues.
Further research should be considered to see if chlorpyrifos remain stable at all growth stage of this cultivar.
Although the concentrations in this study are probably great than in field, the accumulation and persistence in cultivars of great agricultural importance should be occurring. Consequently the accumulation in wheat straw used how forages and the grain can be a harmful pollutant for mammals.
4. Conclusions
The adsorption of chlorpyrifos by both soils was dominated by interaction with the solid state of the soils.
Adsorption capacity did not depend upon the organic matter content in the soil.
The Gus index suggests that chlorpyrifos would not be a pollutant in the groundwater.
The BAI index value should be an appropriate parameter to evaluate the levels of pollution of chlorpyrifos residues in soils in relation to the bioaccumulation in wheat cultivars.
Germination and growth of wheat plants were not affected by the chlorpyrifos accumulation in the tissues.
Acknowledgments
The authors thank the Chemistry Department, Sciences Faculty. Chile University
References
- Pimentel, D. and Levitan, L. (1996) Pesticides Amounts Applied and Amounts Reaching Pests. Bioscience, 36, 86-91.http://dx.doi.org/10.2307/1310108
- Rao, P.S.C, Mansell, A.S., Baldwin, L.B. and Laurent, M.F. (1983) Pesticides and their Behavior in Soil and Water. Soil Science. Fact Sheer Florida Cooperative Extension Service, Institute of Food and Agricultural Science, University of Florida.
- Aktar, M.I., Sengupta, D. and Chowdury, A. (2009) Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdisciplinary Toxicology, 2, 1-12. http://dx.doi.org/10.2478/v10102-009-0001-7
- Arias-Esteves, M., Lopez-Periago, E., Martinez-Caraballe, E., Simal-Gandra, I., Mejuto, J.C. and García-Ries, L. (2008) The Mobility and Degradation of Pesticides in Soils and Pollution of Ground Water Resources. Agriculture, Ecosystems & Environment, 123, 247-260. http://dx.doi.org/10.1016/j.agee.2007.07.011
- Linn, D.M., Carski, I.H., Brusseau, M.L. and Chiang, F.H. (1993) Sorption and Degradation of Pesticides and Organic Chemical in Soil. Soil Science Society of America, Madison, 260.
- Guo, L., Jury, W.A., Wagenet, R.J. and Flury, M. (2000). Dependence of Pesticide Degradation on Sorption Nonequilibrium Model and Application to Soil Reactors. Journal of Contaminant Hydrology, 43, 45-62.http://dx.doi.org/10.1016/S0169-7722(99)00097-2
- Chapman, R.A. and Cole, C.M. (1982) Observation on the Influence of Water and Soil pH on the Persistence of Insecticides. Journal of Environmental Sciences and Health, 17, 457-504.
- Milbrath, D.S, Eton, M. and Casida, J.E. (1978) Distribution and Fate in Mammals of the Potent Convulsing and GABA Antagonist t-Butyl-Bicyclophosphste and Its Methyl Analog. Toxicology and Applied Pharmacology, 46, 411-415. http://dx.doi.org/10.1016/0041-008X(78)90086-8
- Darlington, W.A., Partes, R.D. and Ratts, K.W. (1971) Correlation of Cholinesterase Inhibition and Toxicity in Insect and Mammals I Ethylphosphonates. Toxicology and Applied Pharmacology, 18, 542-547.http://dx.doi.org/10.1016/S0041-008X(71)80007-8
- Awasthi, M.D. and Prakash, N.B. (1997) Persistence of Chlorpyrifos in Soils under Different Moisture Régimes. Pesticide Science, 50, 1-4. http://dx.doi.org/10.1002/(SICI)1096-9063(199705)50:1<1::AID-PS549>3.0.CO;2-X
- Geetzin, L.W.E. (1981) Degradation of Chlorpyrifos in Soil Influence of Autoclaving Soil Moisture and Temperature. Journal of Economic Entomology, 74, 158-162.
- Chu, X., Fang, H., Pan, X., Wang, X., Shau, M., Feng, B. and Yu, Y. (2008) Degradation of Chlorpyrifos Alone and in Combination with Chlorothalonil and Their Effects on Soil Microbial Populations. Journal of Environmental Sciences, 20, 464-469. http://dx.doi.org/10.1016/S1001-0742(08)62080-X
- Van Emmerik, T., Angove, M., Johnson, B. and Walls, J. (2007) Sorption of Chlorpyrifos to Selected Minerals and Their Effect of Humic Acid. Journal of Agricultural and Food Chemistry, 55, 7527-7533.http://dx.doi.org/10.1021/jf071084z
- Racke, K.D., Laskowski, D.A. and Schultz, M.R. (1990) Persistance of Chlorpyrifos to Enhanced Biodegradation in Soil. Journal of Agricultural and Food Chemistry, 38, 1430-1436. http://dx.doi.org/10.1021/jf00096a029
- Zhang, X., Shen, Y., Yu, X. and Liu, X. (2012) Dissipation of Chlorpyrifos and Residue Analysis in Rice, Soil and Water under Paddy Field Conditions. Ecotoxicology and Environmental Safety, 78, 276-280.http://dx.doi.org/10.1016/j.ecoenv.2011.11.036
- Sardar, D. and Cole, R.K. (2005) Metabolim of Chlorpyrifos in Relation to Its Effect on the Availability of Some Plant Nutrients in Soil. Chemosphere, 61, 1273-1280. http://dx.doi.org/10.1016/j.chemosphere.2005.03.078
- Roger, M.R. and String, W.T. (2009) Partitioning of Chlorpyrifos to Soil and Plants in Vegetated Agricultural Drainage Ditches. Chemosphere, 75, 109-114. http://dx.doi.org/10.1016/j.chemosphere.2008.11.036
- Day, P. (1965) Particle Fractionation and Particle-Size Analysis. In: Black, C.A., Ed., Methods of Soil Analysis, American Society of Agronomy, Madison, 545-566.
- Sadzawka, M.A., Carrasco, M.A., Grez, R., Mora, M.L., Flores, H. and Neaman, A. (2006) Métodos de Análisis recomendados para los Suelos de Chile. Instituto de Investigaciones Agropecuarias (INIA), Serie Actas INIA, No. 34, 145.
- Pignatello, J.J. (1998) Soil Organic Matter as a Nonoporous Sorbent of Organic Pollulants. Advances in Colloid and Interface Science, 76-77, 445-467. http://dx.doi.org/10.1016/S0001-8686(98)00055-4
- Gao, J.P., Maguhn, J., Epitzaner, P. and Kottrup, A. (1998) Sortion of Pesticide in the Sediment of the Tenfolsweiher Pond (Southern Germnay) I. Equilibrium Assessments Effects of Organic Carbon Content and pH. Water Research, 32, 1662-1672. http://dx.doi.org/10.1016/S0043-1354(97)00377-1
- Spark, K.M. and Swift, R.S. (2002) Effect of Soil Composition and Dissolved Organic Matter on Pesticide Sorption. Science of the Total Environment, 298, 147-161. http://dx.doi.org/10.1016/S0048-9697(02)00213-9
- Abmad, R., Nelson, P.N. and Koodana, S. (2006) The Molecular Composition of Soil Organic Matter as Determined by 13C-NMR and Elemental Analysis and Correlation with Pesticide Sorption. European Journal of Soil Science, 57, 883-893. http://dx.doi.org/10.1111/j.1365-2389.2005.00784.x
- Copaja, S.V., Bravo, H.R. and Muñoz, P. (2012) Adsorption of the Fungicides in Chilean Soils Incubated with Biosolids. Journal of the Chilean Chemical Society, 57, 1091-1094. http://dx.doi.org/10.4067/S0717-97072012000200006
- MacNamara, G.M. and Toth, S.J. (1970) Adsorption of Linuron and Malathion by Soil and Clay Minerals. Soil Science, 104, 234-240. http://dx.doi.org/10.1097/00010694-197004000-00006
- Coquet, Y. (2003) Sorption of Pesticide Atrazine, Isoproturon and Metamitron in Vadose Zone. Vadose Zone Journal, 2, 40-51. http://dx.doi.org/10.2136/vzj2003.0040
- Dabus, I.G., Barriuso, E. and Calvet, R. (2001) Sorption of Weak Organic Acids in Soils: Clorfencet, 2,4-D and Salicylic Acids. Chemosphere, 45, 767-774. http://dx.doi.org/10.1016/S0045-6535(01)00108-4
- Clausen, L. and Fabricius, I. (2002) Atrazine, Isoproturon, Mecogerop, 2,4-D, and Bentazone Adsortion onto Iron Oxides. Journal of Environmental Quality, 30, 858-869. http://dx.doi.org/10.2134/jeq2001.303858x
- Giles, C., Smith, D. and Huitson, A. (1974) A General Treatment and Classification of the Solute Adsorption Isotherm I. Theoretical. Journal of Colloid and Interface Science, 47, 755-765. http://dx.doi.org/10.1016/0021-9797(74)90252-5
- Boivin, A., Cherrier, R., Perrin-Ganier, C. and Schiavon, M. (2004) Time Effect on Bentazone Sorption and Degradation in Soil. Pest Management Science, 60, 809-814. http://dx.doi.org/10.1002/ps.889
- Macalady, D.L. and Wolfe, N.L. (1985) Effects of Sediment Sorption on Abiotic Hydrolysis. 1. Organophosphorothioate Esters. Journal of Agricultural and Food Chemistry, 33, 167-173. http://dx.doi.org/10.1021/jf00062a003
- Liang, B., Yang, C.L., Gong, M.B., Zhang, J., Zhu, C.X., Jiang, J.D. and Li, S.P. (2011) Adsorption and Degradation of Triazophos, Chlorpyrifos and Their Main Hydrolyte Metabolites in Paddy Soil from Choahin Lake, China. Journal of Environmental Management, 92, 2229-2234. http://dx.doi.org/10.1016/j.jenvman.2011.04.009
- Singh, B.K., Walker, A., Morgan, J.A. and Wright, D.J. (2003) Effects of Soil pH on the Biodegradation of Chlorpyrifos and Isolation of a Chlorpyrifos-Depredating Bacterium. Applied and Environmental Microbiology, 69, 5198-5206. http://dx.doi.org/10.1128/AEM.69.9.5198-5206.2003
- Gustafson, D.I. (1989) Groundwater Ubiquity Score: A Simple Method for Assessing Pesticide Leachability. Enviromental Toxicology and Chemistry, 8, 339-357. http://dx.doi.org/10.1002/etc.5620080411
- Kabara-Pendias, A. and Pendias, H. (2000) Trace Elements in Soil and Plants. 3rd Edition, CRC Press, Boca Raton, 27.
NOTES

*Corresponding author.


