Journal of Agricultural Chemistry and Environment
Vol.2 No.3(2013), Article ID:35637,8 pages DOI:10.4236/jacen.2013.23007
Chemical composition, antioxidant and antibacterial activities of essential oil from Korean Citrus unshiu peel
![]()
*Corresponding Author: sckang@daegu.ac.kr
Received 9 April 2013; revised 13 May 2013; accepted 23 May 2013
Copyright © 2013 Xiao Nan Yang, Sun Chul Kang. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
The chemical composition, antioxidant and antibacterial activities of essential oil from peel of Citrus unshiu which cultivated in South Korea were investigated. Eight compounds were identified as l-limonene (88.11%), γ-terpinene (4.66%), cyclohexane, 2,4-diisopropenyl-1-methyl-1-vinyl (1.82%), diethyl phthalate (1.02%), β-linalool (0.97%), β-myrcene (0.91%), α-farnesene (0.91%) and o-cymene (0.85%) by GC-MS. The SC50 values of this essential oil on DPPH and superoxide anion were 0.21 and 0.22% (v/v), respectively. The results of zone of inhibition, MIC, MBC and cell viability demonstrated the essential oil ofCitrus unshiu peel displayed antibacterial effect against B. cereus KCTC 14042, B. subtilis ATCC 6633 and S. aureus ATCC 6538. The release of cell material and potassium ion from the B. subtilis ATCC 6633 cells treated with essential oil was further investigated. SEM observation also revealed the damaging effect of the essential oil on the morphology of B. subtilis ATCC 6633 cells at minimum inhibitory concentration.
Keywords: Citrus unshiu; Essential Oil; GC-MS; Antioxidant; Antibacterial
1. INTRODUCTION
Essential oils (also called volatile or ethereal oils) are aromatic oily liquids obtained from plant material such as flowers, buds, seeds, leaves, twigs, barks, woods, fruits and roots. It has long been recognized that some essential oils or their components have antimicrobial [1], antiviral [2], antimycotic [3], antitoxigenic [4], antiparasitic [5], insecticidal [6] and antioxidant [7] properties. These characteristics are possibly related to the function of compounds in plants. Individual components of essential oils are also used as food flavourings, either extracted from plant materials or synthetically manufactured [8]. However, recent enhancement of interest in “green” consumerism has led to a renewal of scientific interest in these substances [9].
Orange (Citrus genus) is an ancient plant which is in the family Rutaceae. The centre of origin and diversity of this genus is believed to be Southeast Asia. Citrus are mainly cultivated in subtropical regions, and now there is also a large number of cultivation in South Korea. Citrus mainly used for dessert juice and jam production. This processing industry yields considerable amount of waste or by-product such as peels, seeds and pulps which represents 50% of the raw processed fruit [10]. Essential oils are the most important by-product usually obtained from peel of Citrus and make up the largest sector of the world production of essential oils [11]. They are widely used as flavouring agent for drinks, cakes, ice cream, airfresheners, perfumes and household products [12]. Citrus oils are not only used in food but also are generally recognized as safe [13]. Due to their great nutraceutical and economic importance, numerous investigations have been performed which aimed at identifying the chemical composition of the essential oils from peels and leaves of different Citrus species. Minh Tu, et al. [14] found that the Vietnamese pummelo (C. grandis Osbeck), orange (C. sienensis Osbeck), tangerine (C. reticulate Blanco var. tangerine) and lime (C. limonia Osbeck) peel oils consist mainly of limonene (on average 71% - 95.1%). Buettner, et al. [15] investigated the chemical composition of the peel oils from Spanishclementines (C. reticulate Blanco cv. clementine) and found that limonene and γ-terpinene were the major constituents of the peel oils, while linalool was the main one in leaf oils. In the peel oils from orange, mandarin, grapefruit, bitter orange, citrange and lemon from the same country, limonene and myrcene were identified as the major constituents [16].
Despite the substantial data on peel and leaf oils of oranges from different regions, there is a dearth of information about the chemical composition and bioactivities of Citrus which planted in Jeju, South Korea. The aim of the present investigation was to analyze the chemical composition and bioactivities of essential oil from Citrus unshiu peel which grown in South Korea.
2. MATERIALS AND METHODS
2.1. Isolation of Essential Oil
The peels of Citrus unshiu were collected from the local market in Kyoungsan City. A voucher specimen (DU-OP-001) was preserved in our laboratory for further reference. Dried peels (200 g) were subjected to hydrodistillation for 3 h using a Clevenger type apparatus. The oil was dried over anhydrous Na2SO4 and preserved in a sealed vial at 4˚C until further analysis.
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Quantitative and qualitative analysis of the essential oil was performed using a GC-MS (Model QP 2010, Shimadzu, Japan) equipped with a ZB-1 MS fused silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 µm). For GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium gas was used as a carrier gas at a constant flow rate of 1 ml/min. Injector and mass transfer line temperature were set at 220˚C and 290˚C, respectively. The oven temperature was programmed from 50˚C to 150˚C at 3˚C/min, then held isothermal for 10 min and finally raised to 250˚C at 10˚C/min. Diluted sample (1/100, v/v, in dichloromethane) of 1 µl was manually injected in the split less mode. The relative percentage of the oil constituents was expressed as percentage by peak area normalization.
Identification of components of the essential oil was based on their retention indices, relative to a homologous series of n-alkane (C8-C20) on the ZB-1 capillary column under the same operating conditions and computer matching with the NIST MS libraries.
2.3. Antioxidant Activity Assay
2.3.1. DPPH Radical Scavenging Activity
The antioxidant activity of essential oil was firstly measured on the basis of the scavenging activity to stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical with slight modification [17]. Briefly, DPPH powder was dissolved with ethanol and stored in refrigerator (4˚C) for at least 6 h to attain stable. The absorbance of DPPH solution was adjusted by ethanol to 0.7 ± 0.05 at 517 nm before using (concentration was about 0.4%). The reaction mixture contained 100 µl of sample (essential oil at 0, 0.1, 0.4, 0.8, and 1.0% in ethanol) and 900 µl DPPH solution. After shaking incubation for 30 min under dark condition at room temperature, the absorbance of each sample was recorded against blank at 517 nm. Experiments were conducted in triplicate. The scavenging percentage of DPPH radical was calculated according to the formula:
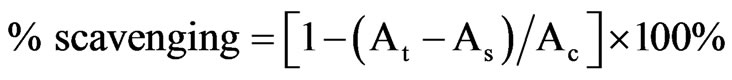
where At is the absorbance of sample treated with DPPH, As is the absorbance of sample without DPPH but equivalent volume of ethanol, Ac is the absorbance of DPPH solution without sample but equivalent volume of solvent.
2.3.2. Superoxide Anion (·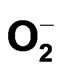 ) Radical Scavenging Activity
) Radical Scavenging Activity
The superoxide anion radical scavenging assay was carried out according to the method of Suh. et al. [18] with slight modification. The reaction mixture consisted 250 µl of 0.8 mM xanthine, 150 µl of 0.5 mM nitro-blue terazolium (NBT) and 50 µl of the sample (essential oil at various concentrations) in 0.1 mM potassium phosphate buffer (pH 7.8). After shaking incubation at 25˚C for 10 min, the reaction was started by adding 5 mU xanthine oxidase. The samples were kept at 25˚C for 20 min and then stopped by adding 500 µl of 1 N HCl. The absorbance was measured at 560 nm. The results were calculated as the percentage of scavenging according to the following formula:
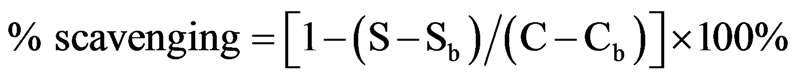
where S, Sb, C, and Cb are the absorbance of the sample treated with enzyme, the sample without enzyme, the control treated with enzyme, and the control without enzyme, respectively. Experiments were conducted in triplicate.
2.4. Antibacterial Activity
2.4.1. Microbial Strains
A panel of food-borne pathogenic bacteria including four gram-positive (Bacillus cereus KCTC 14042, Bacillus subtilis ATCC 6633, Listeria monocytogenes ATCC 10943, Staphylococcus aureus ATCC 6538) and three gram-negative (Escherichia coli ATCC 10536, Salmonella enteritidis KCTC 12243, Pseudomonas aeruginosa ATCC 15522) were used in this study. Active cultures for experimental using were prepared by transferring a loop of stock culture to Luria-Bertani (LB) broth medium and incubated at 37˚C for 24 h. Cultures of bacterial strains were maintained on LB agar medium at 4˚C.
2.4.2. Agar Disc Diffusion
Standard agar disc diffusion method was used for antibacterial assay [19]. Firstly, the active cultures were diluted with LB broth to achieve optical density of 107 CFU/ml for the test organisms at 600 nm by UV/Vis Spectrophotometer (Optizen 2120 UV). Petri plates were prepared by pouring 20 ml of LB agar medium and allowed to solidify. 0.1 ml of standardized inoculum containing 107 CFU/ml of bacterial suspension was poured on LB plate and uniformly spread, then allowed to dry for 5 min. Waterman No.1 sterile filter paper discs (6 mm diameter) were impregnated with 4 µl/disc of pure essential oil and placed on the inoculated LB agar. The plates were left at room temperature for 30 min to allow the oil diffuse into the agar, and then the plates were incubated at 37˚C for 24 h. Antibacterial activity was evaluated by measuring the diameter of the inhibition zone against the tested bacteria.
2.4.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The minimum inhibitory concentration (MIC) of essential oil was tested by the method described by Chandrasekaran, and Venkatesalu [20]. The oil was incorporated into LB broth medium tubes to obtain a final concentration from 0 to 5% (v/v). 10 µl of standardized suspension of each fresh tested organism (107 CFU/ml) was transferred to each tube. The tubes were incubated at 37˚C in a shaking incubator for 24 h. The lowest concentration of the test samples, which did not show any visual growth of tested organisms after macroscopic evaluation, was determined as MIC. Further, the concentrations showing complete inhibition of bacteria were identified and 50 µl of each culture broth was transferred on to the agar plate, incubated for specified time and temperature as mentioned above. The complete absence of growth on the agar surface in the lowest concentration of sample was defined as the minimum bactericidal concentration (MBC).
2.4.4. Effect of Essential Oil on Viable Counts of Bacteria
For viable count, each tube containing bacterial suspension (approximately 107 CFU/ml) of B. subtilis ATCC 6633, B. cereus KCTC 14042 or S. aureus ATCC 6538 in 2 ml LB broth was inoculated with the MIC level of essential oil, and then incubated at 37˚C in a shaking incubator. Sample for viable counting were taken out from each tube at 0, 30, 60, 90, 120 and 180 min time intervals. Enumeration of viable counting on LB plates was monitored as follows: 10 µl of sample was diluted into 990 µl sterile water, thereby diluted 100 times. 50 µl of dilution was spread on the surface of LB agar. The number of colony formed on LB agar was counted after incubation at 37˚C for 24 h, and recorded as A, so the CFU of original culture was calculated as A × 20 ×100. Final DATA was expressed as log CFU/ml. The controls were inoculated without essential oil for each bacterial strain with the same experimental condition as mentioned above.
2.4.5. Assay of Potassium Ions Efflux
The measurement of free potassium ions concentration in bacterial suspension was followed by the report of Paul, et al. [21]. B. subtilis ATCC 6633 was firstly incubated in LB broth for 12 h, and then the bacterial culture was exposed to essential oil (at MIC level) for 0, 30, 60 and 120 min time intervals. At each pre-established interval, the potassium efflux from cell was measured by a photometric procedure by using the Kaluim/Potassium kit (Quantofix, GmbH, Wiesbaden, Germany), and the potassium concentration of LB broth was also measured as blank. Control cells without essential oil treatment were tested using same method. Results were expressed as amount of extracellular free potassium (mM) in the media.
2.4.6. Measurement of Released Cellular Materials
The release of materials from B. subtilis ATCC 6633 cells was tested by measuring the absorbance of each supernatant at 260 nm. The bacteria was inoculated in sterile peptone water (0.1%, w/v) added of the essential oil (at MIC level). 1 ml of the cultures were taken out at 0, 30, 60, 120 min time intervals and centrifuged at 4000 rpm. The absorbance of the obtained supernatant was determined at 260 nm using an Optizen UV/Vis Spectrophotometer [22]. Control tubes without essential oil were tested at the same condition.
2.4.7. Scanning Electron Microscopy (SEM)
The method of sample preparation for scanning electron microscopy (SEM) was followed by Kockro, et al. [23] with some modifications. Bacterial cells of B. subtilis ATCC 6633 which were treated with and without essential oil at MIC level for 60 min were washed three times using 50 mM phosphate buffer solution (PBS, pH 7.3), and then centrifuged at 4000 rpm. After removing supernatant, the centrifuged cells were suspended in new PBS. A thin smear of the suspension was spread on a glass slide and fixed in 2.5% (v/v) glutaraldehyde (Electron Microscopy Science, Washington, USA) for 2 h. After fixation, the bacterial cells on each glass slide were rinsed, dehydrated using a series of graded ethanol (concentrations ranging from 50% to 100%), and dried with CO2. The dried cells were coated with gold in a sputter coater (Hitachi, Japan). Samples were observed under a Scanning electron microscope (Hitachi-S4300, Japan).
3. RESULTS AND DISCUSSION
3.1. Chemical Composition of the Essential Oil
The hydrodistillation of the Citrus unshiu yielded about 4 ml oil (2%, w/v) in the present study. Upon GC-MS analysis, eight different compounds (representing 99.26% of the total oil) were detected (Table 1). The major compounds detected were l-limonene (88.11%) and γ-terpinene (4.66%). Cyclohexane, 2,4-diisopropenyl- 1-methyl-1-vinyl (1.82%), diethyl phthalate (1.02%), β-linalool (0.97%), β-myrcene (0.91%), α-farnesene (0.91%) and o-cymene (0.85%) were found to be the minor components of Citrus unshiu peel essential oil in the present study.
3.2. Antioxidant Activity
3.2.1. DPPH Scavenging Activity
The DPPH free radical scavenging activity of essential oil from Citrus unshiu peel is shown in Figure 1. The scavenging effect of oil on DPPH exhibited dose dependent manner. 73.46% and 89.67% DPPH free radical were scavenged by 0.4 and 0.8% of essential oil, respectively. The highest DPPH scavenging concentration was observed at 1.0%, that 92.16% DPPH was scavenged by the essential oil. The SC50 (50% free radical scavenging concentration) value of oil was evaluated as 0.21%.
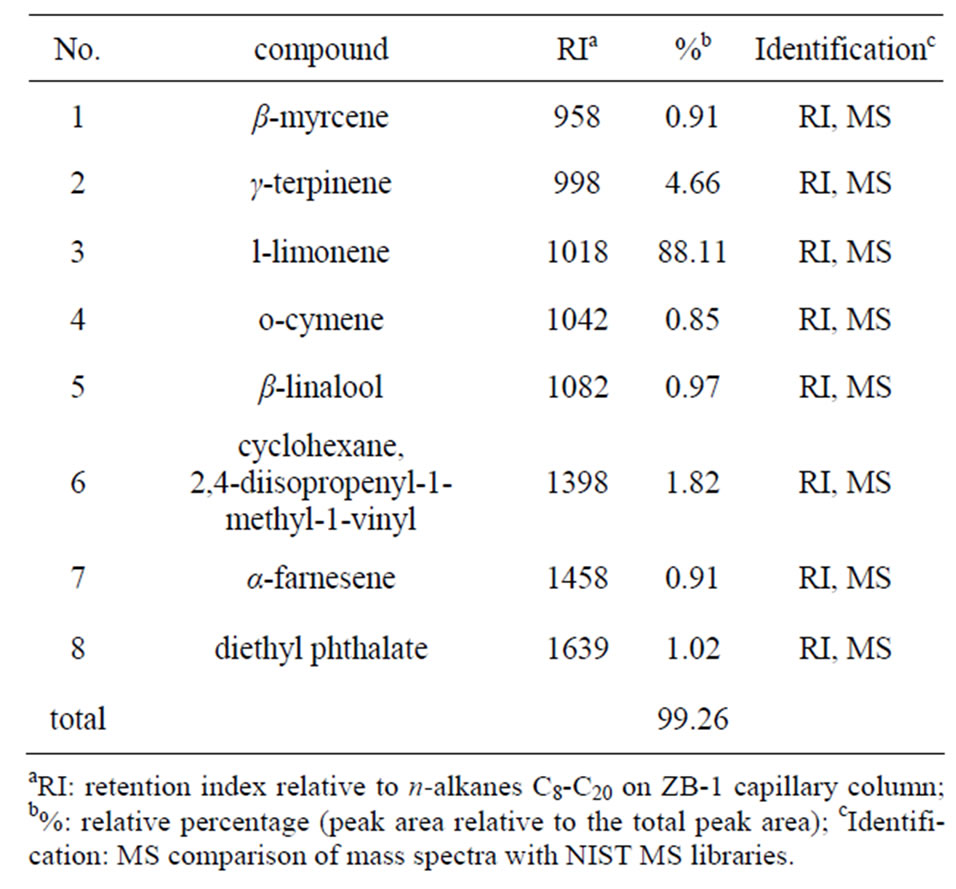
Table 1. Composition of essential oil from Citrus unshiu peel.
3.2.2. Superoxide Anion Scavenging Activity
Figure 1 also shows the effect of superoxide anion radical scavenging activity of essential oil at different concentrations. The scavenging activity on super oxide by 0.4% of oil was found as 65.36%, while the scavenging activity was 85.07% at concentration of 0.8%. The highest superoxide scavenging concentration was observed at 1.0%, that 89.86% super oxide was scavenged by the oil. The SC50 value of orange peel essential oil was found to be 0.22%. These results imply that essential oil from Citrus unshiu peel has potent antioxidant activity.
3.3. In Vitro Antibacterial Activity
3.3.1. Inhibition Zones of Essential Oil on Test Bacteria
The in vitro antibacterial activity of essential oil from Citrus unshiu peel was assessed by the presence or absence of inhibition zones (Table 2). The oil (4 µl/disc) exhibited potential antibacterial activity against B. cereus KCTC 14042, B. subtilis ATCC 6633 and S. aureus ATCC 6538 with diameter of inhibition zones were 7.9, 8.5 and 8.2 mm, respectively, while no effect was found against other bacteria.
3.3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
As shown in Table 2, the MIC value of the essential oil was found lower against gram-positive bacteria on B. subtilis ACTT 6633 (0.5%) than B. cereus KCTC 14042 and S. aureus ATCC 6538 (1.0 and 2.5%, respectively). The MBC values of the oil against the bacteria above were found to be 1.5, 2.5 and 5.0%, respectively, but not detected on other bacteria.
3.3.3. Effect of Essential Oil on Cell Viability
The reducing effects of essential oil from Citrus unshiu peel on cell viabilities of B. cereus KCTC 14042, S. aureus ATCC 6538 and B. subtilis ATCC 6633 were observed (Figure 2) during 180 min treatment. The bacteria displayed different sensitivities to the essential oil (at MIC level). More than 50% inhibition in cell viabilities were observed within 60 min of exposure time in all the three test strains. The B. subtilis ATCC 6633 exhibited higher sensetivity to the oil than the other two test strains as evidenced by the cells were completely inhibited within 90 min of exposure time, while the other two test strains were completely inhibited within 120 min.
3.3.4. Release of Potassium Ions
Figure 3 shows the result of release of potassium ions from B. subtilis ATCC 6633 treated with essentail oil at MIC level. The release of potassium ions from B. subtilis
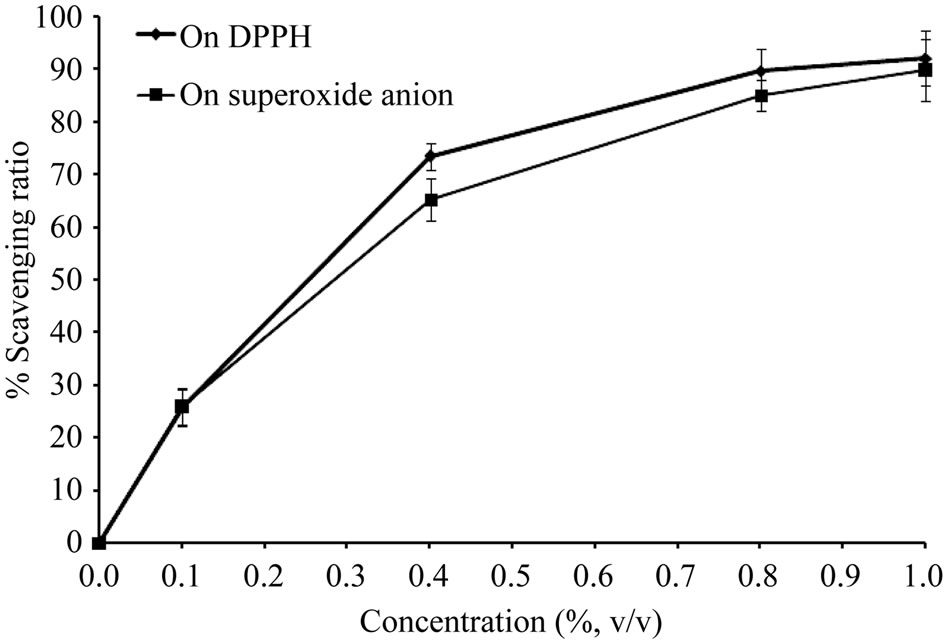
Figure 1. Free radical scavenging activity of the essential oil from Citrus unshiu peel.
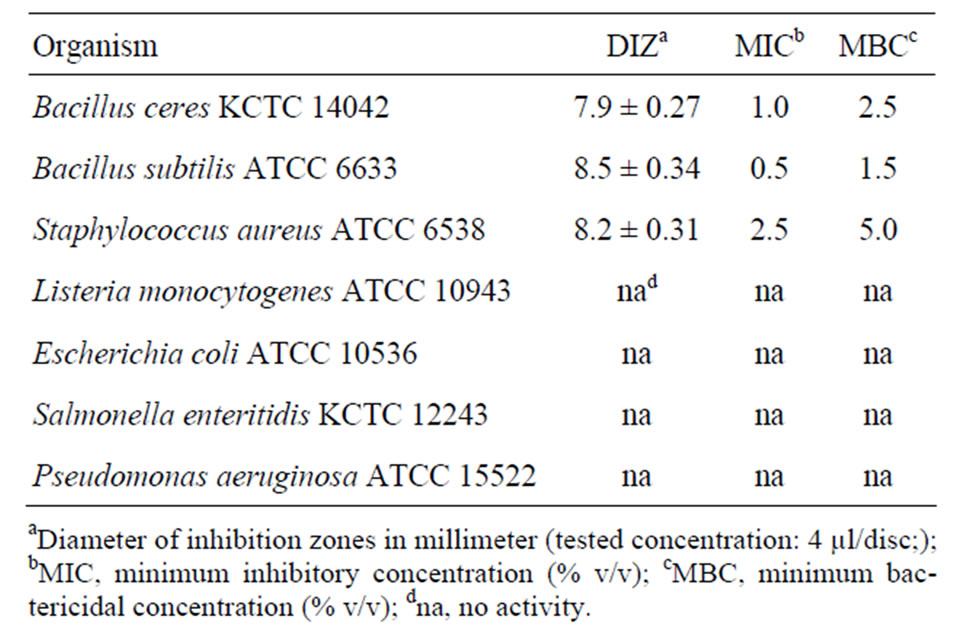
Table 2. Antibacterial activity of Citrus unshiu peel essential oil.
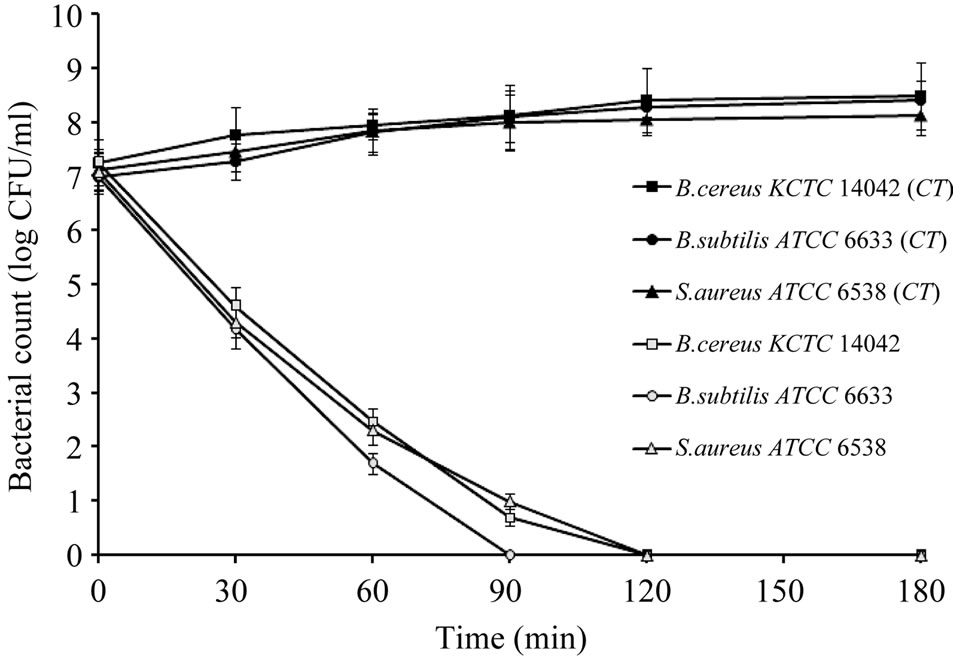
Figure 2. Effect of the essential oil from Citrus unshiu peel (MIC level) on cell viabilities of the test bacteria. CT: control without treatment.
ATCC 6633 cells immediately occurred after adding essentail oil. The concentration of extracellular potassium increased along the evaluated intervals. No potassium leakage was detected in control B. subtilis ATCC 6633 cells.
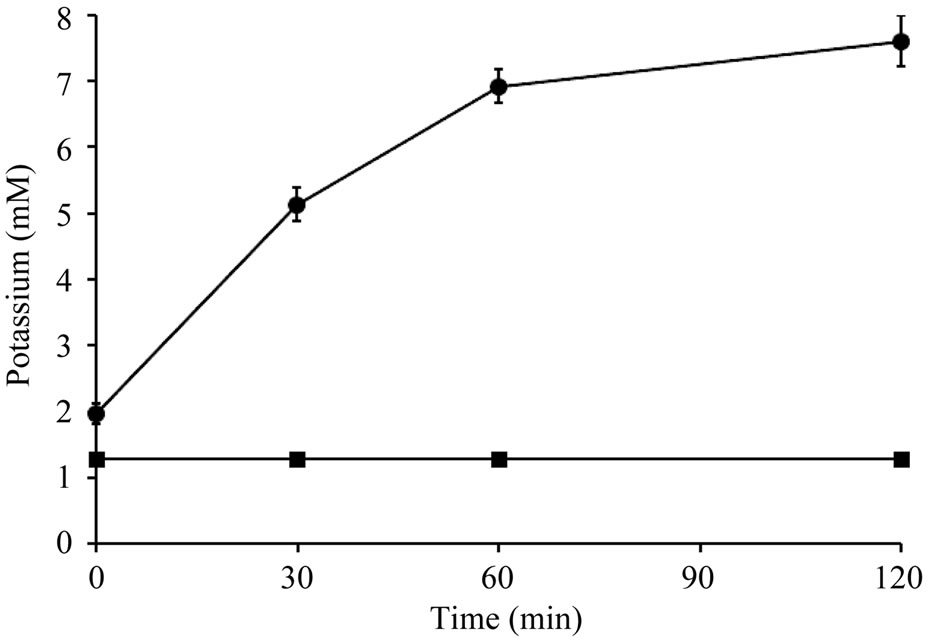
Figure 3. Potassium ions leakage from Bacillus subtilis ATCC 6633 treated with Citrus unshiu peel essential oil (● 0.5% essential oil treatment; ■: control).
3.3.5. Release of Cell Materials
As shown in Table 3, the 260 nm-absorbing materials release rate of B. subtilis ATCC 6633 cells exposed to the essential oil (at MIC level) increased according to the treated time, and 100% of material released after 120 min. In contrast, the cell materials in the control supernantent kept stable during observation period.
3.3.6. Scanning Electron Microscopy (SEM)
The essentail oil exhibited strong detrimental effect on the morphology of B. subtilis ATCC 6633 (Figure 4). Control cells (without essential oil treatment) showed a regular, smooth surface as shown in Figure 4(a). In contrast, the cells treated with essential oil at MIC level (0.5%, v/v) for 60 min appeared mophology changes of cell wall, showing cell wall lysis and pore formation (Figures 4(b) and 4(c)).
In the recent reports, much attention has been given to citrus components, since they present various pharmaceutical activities of anticarcinogenicity, antimutagenicity, antioxidative activity and antiaging [24-26]. The volatile constituents are mixed by monoterpene (limonene) and sesquiterpene hydrocarbons and their oxygenated derivatives including: aldehydes (citral), ketones, acids, alcohols (linalool) and esters [27]. Hosni, et al. [28] reported the composition of peel essential oils from four selected Tunisian Citrus species. The limonene was from 92.6% to 97.3%, while the γ-terpinene, β-myrcene and linalool were from 0.01% to 3.39%, 0.03% to 0.05% and 0.04% to 0.31%, respectively. The limonene (88.11%) of the essential oil from Korean Citrus unshiu peel in present study was less than the previous reported one. On the other hand, the content of γ-terpinene (4.66%), β-myrcene (0.91%) and linalool (0.97%) were higher than the previous reported one. That’s because the differences between the chemical compositions of essential oil are mainly influenced by genotypes, environmental

Table 3. 260 nm-absorbing cell material release rate from Bacillus subtilis ATCC 6633 treated with Citrus unshiu peel essential oil at MIC level.

Figure 4. Effect of the essential oil from Citrus unshiu peel on morphological change of Bacillus subtilis ATCC 6633. (a) Bacterium without essential oil treatment (control) showing smooth and regular cell surface; (b) and (c) Bacterium treated with essential oil at MIC level (0.5%, v/v) after 60 min. (b) and (c) are showing the cell wall lysis and pore formation, respectively.
factors and agronomic conditions [29].
Choi, et al. [7] examined DPPH scavenging effects of 37 Citrus essential oils. Radical scavenging activities of yuzu, limes, ichang lemon, hassaku, sudachi, yuko and kabosu oils showed high radical scavenging effects of over 50% at concentration of 0.5% (v/v).The previous reports support the present results that Citrus essential oils have antioxidant activity. They also have examined DPPH scavenging activities of 21 authentic compounds including γ-terpinene, limonene, linalool and myrcene, the DPPH scavenging activities of the above mentioned compounds were 227.9, 44.3, 50.3 and 28.1 mg of trolox equiv/ml, respectively. Moreover, Song, et al. [30] reported that limonene has anti-linoleic acid peroxide activity. In the present study, essential oil from Citrus unshiu peel exhibited antioxidant activity against DPPH and superoxide anion that could be attributed to the major compounds, l-limonene and γ-terpinene (presented 88.11% and 4.66%, respectively) and the minor compounds, β-linalool and β-myrcene (presented 0.97% and 0.91%, respectively), especially the high amount of γ-terpinene (4.66%).
Historically, many plant oils have been used as topical antiseptics. The use of essential oils may improve food safety and overall microbial quality. Citrus oils have been found to be inhibitory against a range of bacteria both in direct oil and vapour forms. Deans and Ritchie [31] showed Citrus oils have antimicrobial properties not only against yeast, moulds and spore forming bacteria but also food-poisoning bacteria. In 2010, Lin, et al. [32]
reported the essential oil of orange peel has significantly effects on cell population reductions of Vibrio parahaemolyticus, Salmonella typhimurium, Staphylococcus aureus and Escherichia coli at 5% in buffers. In the present study, the results of the MIC showed that the essential oil from Citrus unshiu peel exhibited potent activity against some food spoilage and food-borne pathogenic bacteria such as B. cereus KCTC 14042, B. subtilis ATCC 6633 and S. aureus ATCC 6538 at relatively low concentrations (MIC values were 1.0%, 0.5% and 2.5%, respectively). This activity could be attributed to the components such as γ-terpinene (4.66%), β-linalool (0.97%) and diethyl phthalate (1.02%). These compounds have also been found to constitute the major compositions of several essential oils, which were found to possess substantial antibacterial activities against a panel of food spoilage and food-borne pathogenic bacteria [33,34]. It shows the evidence of antibacterial activities of these components mentioned above. Also, other components are critical to the activity and may have a synergistic effect or potentiating influence.
260 nm-absorbing material and release of free potassium ions in B. subtilis ATCC 6633 were caused by exposing the cell to essential oil from Citrus unshiu peel. The results indicated that the essential oil acted to the cytoplasm membrane and caused their integrity loss and increased permeability. The membrane of cytoplasm provides a permeability barrier to the passage of ions such as H+, K+, Na+ and Ca2+ [22]. The increase in the leakage of potassium ions will indicate that the membrane structural damaged by the essential oil. Further, the SEM analysis of B. subtilis ATCC 6633 cells demonstrated the destructive effect of the essential oil on cell membrane as compared to control group.
That the gram-negative bacteria were more resistant to the action of essential oil from Citrus unshiu peel was found in this study. This is probably due to that they possess an outer membrane surrounding the cell wall, which restricts permeation of hydrophobic compounds through its lipopolysaccharide covering [35]. The hydrophobicity characteristics of essential oil and its components enable them to partition in the lipids of the bacterial cell membrane and mitochondria, disturbing the membrane structures and leading to them more permeable [36], so the essential oil showed higher inhibit activity against some gram-positive bacteria.
4. CONCLUSION
The results of the present study indicate that essential oil from Korean Citrus unshiu peel has significant antioxidant activity, and has antibacterial activities against some gram-positive bacteria. The product of Citrus unshiu peel in South Korea is abundant, therefore the essential oil of Citurs unshiu might be used as antioxidant and/or anticorrosive additive to improve the food quality and control the growth of food spoilage microbial pathogens. Also, the antioxidant compounds of the essential oil from Citrus unshiu peel might be isolated and used as antioxidant in foods.
REFERENCES
- Boyle, W. (1955) Spices and essential oils as presservatives. The American Perfumer and Essential Oil Review, 66, 25-28.
- Bishop, C.D. (1995) Antiviral activity of the essential oil of Melaleuca alternifolia (Maiden and Betche) Cheel (tea tree) against tobacco mosaic virus. Journal of Essential Oil Research, 7, 641-644. doi:10.1080/10412905.1995.9700519
- Azzouz, M.A. and Bullerman, L.B. (1982) Comparative antimycotic effects of selected herbs, spices, plant components and commercial antifungal agents. Journal of Food Protection, 45, 1298-1301.
- Ultee, A. and Smid, E.J. (2001) Influence of carvacrol on growth and toxin production by Bacillus cereus. International Journal of Food Microbiology, 64, 373-378. doi:10.1016/S0168-1605(00)00480-3
- Pandey, R., Kalra, A., Tandon, S., Mehrotra, N., Singh, H.N. and Kumar, S. (2000) Essential oil compounds as potent source of nematicidal compounds. Journal of Phytopathology, 148, 501-502. doi:10.1046/j.1439-0434.2000.00493.x
- Konstantopoulou, I., Vassilopoulou, L., Mavragani-Tsipidou, P. and Scouras, Z.G. (1992) Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Cellular and Molecular Life Sciences, 48, 616- 619. doi:10.1007/BF01920251
- Choi, H.S., Song, H.S. and Sawamura, M. (2000) Radical-scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhy-drazyl. Journal of Agricultural and Food Chemistry, 48, 4156- 4164. doi:10.1021/jf000227d
- Oosterhaven, K., Poolman, B. and Smid, E.J. (1995) Scarvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Industrial Crops and Products, 4, 23-31. doi:10.1016/0926-6690(95)00007-Y
- Nychas, G.J.E. (1995) Natural antimicrobials from plants. In: Gould, G.W., Ed., New Methods of Food Preservation, Blackie Academic and Professional, London, 58-89. doi:10.1007/978-1-4615-2105-1_4
- Anwar, F., Naseer, R., Bhanger, M.I., Ashraf, S., Talpr, F.N. and Aladedunye, F.A. (2008) Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. Journal of the American Oil Chemical Society, 85, 321- 330. doi:10.1007/s11746-008-1204-3
- Tirado, C.B., Stashenko, E.E., Combariza, M.Y. and Martinez, J.R. (1995) Comparative study of Colombian citrus oils by high-resolution gas chromatography and gas chromatography-mass spectrometry. Journal of Chromatography A, 697, 501-513. doi:10.1016/0021-9673(94)00955-9
- Ferhat, M.A., Meklati, B.Y., Smadja, J. and Chemat, F. (2006) An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. Journal of Chromatography A, 1112, 121-126. doi:10.1016/j.chroma.2005.12.030
- Food and Drug Administration (2005) GRAS notifications. www.fda.gov
- Minh Tu, N. T., Thanh, L. X., Une, A., Ukeda, H. and Sawamura, M. (2002) Volatile constituents of Vietnamese pummelo, orange, tangerine and lime peel oils. Flavour and Fragrance Journal, 17, 169-174. doi:10.1002/ffj.1076
- Buettner, A., Mestres, M., Fischer, A., Guasch, J. and Schieberle, P. (2003) Evaluation of the most odour-active compounds in the peel oil of clementines (Citrus reticulata Blanco cv clementine). European Food Research and Technology, 216, 11-14.
- Caccioni, D. R. L., Guizzardi, M., Biondi, D. M., Renda, A. and Ruberto, G. (1998) Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. International Journal of Food Microbiololgy, 43, 73-79. doi:10.1016/S0168-1605(98)00099-3
- Tepe, B., Daferera, D., Tepe, A.S., Polissiou, P. and Sokmen, A. (2007) Antioxidant activity of the essential oil and various extracts of Nepeta flavida Hub.-Mor. from Turkey. Food Chemistry, 103, 1358-1364. doi:10.1016/j.foodchem.2006.10.049
- Suh, H.J., Kim, S.R., Lee, K.S., Park, S. and Kang, S.C. (2010) Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. Journal of Photochemistry and Photobiology B: Biology, 99, 67-73. doi:10.1016/j.jphotobiol.2010.02.005
- Murray, P.P., Baron, E.J., Pfaller, M.A., Tenove, F.C. and Yolke, R.H. (1995) Manual of clinical microbiology, ASM, Washington DC.
- Chandrasekaran, M. and Venkatesalu, V. (2004) Antibacterial and antifungal activity of Syzygium jambolanum seeds. Journal of Ethnopharmacology, 91, 105-108. doi:10.1016/j.jep.2003.12.012
- Paul, S., Dubey, R.C., Maheswari, D.K. and Kang, S.C., (2011) Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control, 22, 725-731.
- Cox, S.D., Mann, C.M., Markham, J.L., Gustafson, J.E., Warmington, J.R. and Wyllie, S.G. (2001) Determining the antimicrobial actions of tea tree oil. Molecules, 6, 87- 91. doi:10.3390/60100087
- Kockro, R.A., Hampl, J.A., Jansen, B., Peters, G., Scheihing, M. and Giacomelli, R. (2000) Use of scanning electron microscopy to investigate the prophylactic efficacy of rifampin-impregnated CSF shunt catheters. Journal of Medical Microbiology, 49, 441-450.
- Nogata, Y., Yoza, K., Kusumoto, K., Kohyama, N., Sekiya, K. and Ohta, H. (1996) Screening for inhibitory activity of citrus fruit extracts against platelet cyclooxigenase and lipoxigenase. Journal of Agricultural and Food Chemistry, 44, 725-729. doi:10.1021/jf9505077
- Miyake, Y., Yamamoto, K. and Osawa, T. (1997) Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon BURM. f.) and its antioxidative activity. Food Science and Technology International, 3, 84-89.
- Sawamura, M., Song, H.S., Ozaki, K., Ishikawa, J. and Ukeda, H. (1999) Inhibitory effects of citrus essential oils and their components on the formation of N-nitrosodimethylamine. Journal of Agricultural and Food Chemistry, 47, 4868-4872. doi:10.1021/jf9903206
- Smith, D.C., Forland, S., Bachanos, E., Matejka, M. and Barrett, V. (2001) Qualitative analysis of citrus fruits extracts by GC/MS: An undergraduate experiment. The Chemical Educator, 6, 28-31. doi:10.1007/s00897000450a
- Hosni, K., Zahed, N., Chrif, R., Abid, I., Medfei, W., Kallel, M., Brahim, N.B. and Sebei, H. (2010) Composition of peel essential oils from four selected Tunisian citrus species: Evidence for the genotypic influence. Food Chemistry, 123, 1098-1104. doi:10.1016/j.foodchem.2010.05.068
- Marotti, M., Piccaglia, R. and Giovanelli, E. (1996) Differences in essential oil composition of Basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. Journal of Agricultural and Food Chemistry, 44, 3926-3929. doi:10.1021/jf9601067
- Song, H.S., Ukeda, H. and Sawamura, M. (2001) Antioxidative activities of citrus peel essential oils and their components against linoleic acid oxidation. Food Science and Technology Research, 7, 50-56. doi:10.3136/fstr.7.50
- Deans, S.G. and Ritchie, G. (1987) Antibacterial properties of plant essential oils. International Journal of Food Microbiology, 5, 165-180. doi:10.1016/0168-1605(87)90034-1
- Lin, C.M., Sheu, S.R., Hsu, S.C. and Tsai, Y.H. (2010) Determination of bactericidal efficacy of essential oil extracted from orange on the food contact surfaces. Food Control, 21, 1710-1715. doi:10.1016/j.foodcont.2010.06.008
- Bhimba, B.V., Meenupriya, J., Joel, E.L., Naveena, D.E., Kumar, S. and Thangaraj, M. (2010) Antibacterial activeity and characterization of secondary metabolites isolated from mangrove plant Avicennia officinalis. Asian Pacific Journal of Tropical Medicine, 3, 544-546. doi:10.1016/S1995-7645(10)60131-9
- Chiang, H.M., Chiu, H.H., Lai, Y.M., Chen, C.Y. and Chiang, H.L. (2010) Carbonyl species characteristics during the evaporation of essential oils. Atmospheric Environment, 44, 2240-2247. doi:10.1016/j.atmosenv.2010.02.017
- Vaara, M. (1992) Agents that increase the permeability of the outer membrane. Microbiology and Molecular Biology Reviews, 56, 395-441.
- Sikkema, J., De Bont, J.A.M. and Poolman, B. (1994) Interactions of cyclic hydrocarbons with biological membranes. Journal of Biological Chemistry, 269, 8022-8028.

