Journal of Agricultural Chemistry and Environment
Vol.1 No.1(2012), Article ID:24890,5 pages DOI:10.4236/jacen.2012.11002
Residue patterns of buprofezin and teflubenzuron in treated peaches@NolistTemp#
Email: kslee@cnu.ac.kr
![]() 1Department of Bio-Environmental Chemistry, Chungnam National University, Daejeon, South Korea; *Corresponding Author:2Syngenta Korea Inc., Seoul, South Korea
1Department of Bio-Environmental Chemistry, Chungnam National University, Daejeon, South Korea; *Corresponding Author:2Syngenta Korea Inc., Seoul, South Korea
Received 3 September 2012; revised 13 October 2012; accepted 24 October 2012
ABSTRACT
The biological half-life and final residue levels of buprofezin and teflubenzuron were examined in peaches over a 14-day cultivation period. The residue levels of buprofezin and teflubenzuron were analyzed by chromatographic method with recovery ranging from 84.0% to 96.6%. The biological half-lives of buprofezin and teflubenzuron were 4.88 and 11.49 days at the standard dose, and 4.40 and 10.86 days at a triple dose, respectively. The initial concentration of buprofezin exceeded the maximum residue limit (MRL) set in Korea, but the concentration decreased to below the MRL within 6 days after application. The initial and persisting concentrations of teflubenzuron were all below the prescribed MRL. The final residue levels of buprofezin and teflubenzuron were 0.17 and 0.10 mg∙kg−1 following a standard single dose, and 0.20 and 0.23 mg∙kg−1 following a triple dose, respectively. The final residue levels of buprofezin and teflubenzuron were also compared with the good agricultural practices standards of the United States and Italy.
Keywords: Biological half-life; Buprofezin; Peach; Residue; Teflubenzuron
1. INTRODUCTION
Pesticides play a beneficial role in agriculture, combating a variety of pests that destroy crops. However, residues of pesticides can still be detected in the food supply under certain circumstances, even when the pesticides are applied in accordance with good agricultural practices (GAP). These residues constitute a potential risk to human health due to their sub-acute and chronic toxicity. To prevent such a risk and to ensure the quality of crops, the evaluation of pesticide residues based on crop monitoring to verify compliance with statutory maximum residue limits (MRLs) [1], is of considerable importance to certify the safety of harvests.
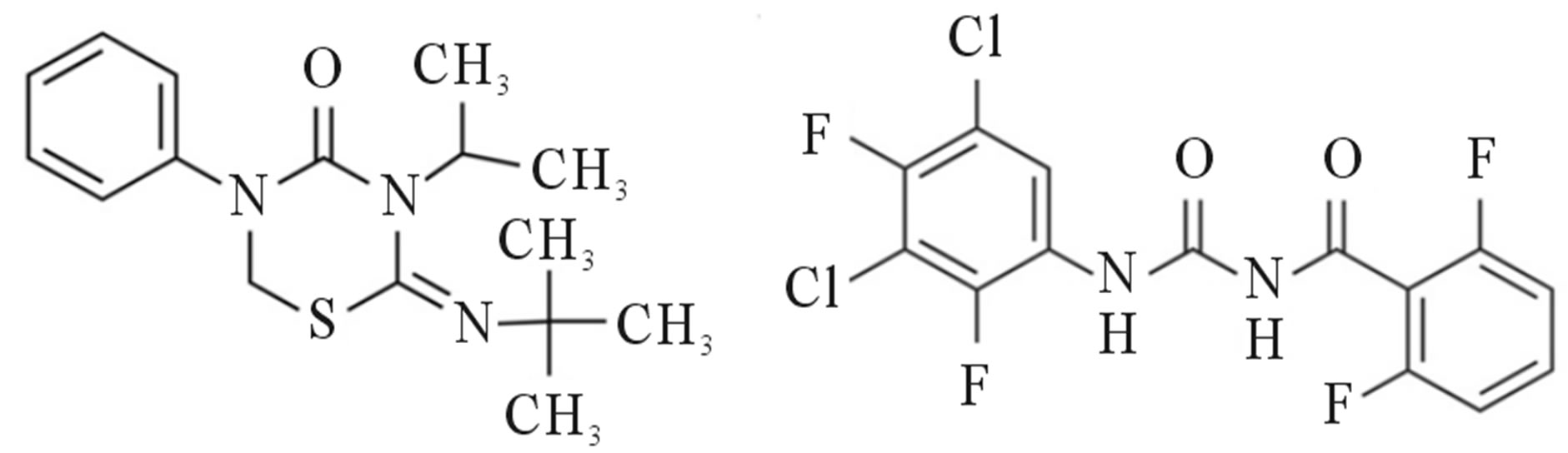
Buprofezin [(Z)-2-tert-butylimino-3-isopropyl-5-phenyl- 1,3,5-thiadiazinan-4-one; Figure 1(a)] is a persistent insecticide with larvicidal action against Acarina, and some Coleoptera and Hemiptera, through the inhibition of chitin biosynthesis and effects on the hormone levels of nymphs. Buprofezin is known to be a contact and stomach poison, but this toxicity is not translocated to the plant. It inhibits the molting of nymphs and larvae, leading to death, and also suppresses oviposition by adults such that exposed insects lay sterile eggs [2].
Teflubenzuron [1-(3,5-dichloro-2,4-difluorophenyl)-3- (2,6-difluorobenzoyl)urea; Figure 1(b)] is a nonsystemic insect growth regulator with stomach action belonging the benzoylurea family of insecticides [3]. It disrupts chitin synthesis in shellfish and kills lice by preventing molting. It is effective on larval-stage molting and preadult lice, but it has no effect on adult lice [4]. Teflubenzuron is used to control the oriental fruit moth (Grapholita molesta), an insect native to China that has spread throughout the world and is considered the most important insect pest of peaches and nectarines [5]. Teflubenzuron is also recommended for the control of Lepidoptera, Coleoptera, Diptera, Aleyrodidae, Phylliidae, and Hemiptera larvae on other crops, such as pome, stone, and citrus fruits, and vegetables [3].
This study aimed to examine the biological half-life and residue patterns of two pesticides, buprofezin and teflubenzuron, in peaches during cultivation. The final
 (a) (b)
(a) (b)
Figure 1. Chemical structures of (a) buprofezin and (b) teflubenzuron.
residue levels in peaches remaining at harvest time was also predicted according to the GAP standards of the United States and Italy.
2. MATERIALS AND METHOD
2.1. Chemicals
Analytical standards of buprofezin (99.0% pure) and teflubenzuron (99.5% pure) were purchased from Chem Service, Inc. (West Chester, PA, USA) and Dr. Ehrenstorfer GmbH (Augsburg, Germany), respectively. Stock (1000 mg∙L−1) and working standard solutions were prepared in acetonitrile and used as external standards throughout the study. A florisil solid phase extraction (SPE) cartridge (1000 mg/6mL) was purchased from Phenomenex (Torrance, CA, USA). Florisil (0.150 - 0.250 mm) and silica gel (0.063 - 0.200 mm) were purchased from Merck (Darmstadt, Germany) and activated at 130˚C for more than 5 hours prior to use. Acetonitrile and water were of high performance liquid chromatography (HPLC) grade, and the other solvents were of pesticide residue grade. All solvents were obtained from J.T. Baker (Philipsburg, NJ, USA).
2.2. Field Treatment and Sampling
The trial was conducted at a peach orchard located in Jochiwon-eup, Yeongi-gun, Chungcheongnam-do, Korea. Forty five peach trees (five plots of nine trees) were used for three replicates of experiments with a randomized complete block design. Treatments were carried out with the following commercial products: Baekseung SC (20% buprofezin) at the standard single dose of 400 g a.i. ha−1 and a triple dose of 1200 g a.i. ha−1, and Nomolt SC (5% teflubenzuron) at a standard single dose of 100 g a.i. ha−1 and a triple dose of 300 g a.i. ha−1. Pesticides were applied at 7-day intervals using a rechargeable battery sprayer (KS-PK 2000; Kwang Sung, Daejeon, Korea) with a spray volume of 0.4 L∙m−2. During the experimental period, total rainfall was 139 mm in July and 140.5 mm in August. The average relative humidity ranged from 64.3% to 95.8%, with maximum and minimum average daily temperatures of 28.0˚C and 23.2˚C, respectively. The control peach samples were collected from a control plot where no buprofezin or teflubenzuron had been applied. After spraying, representative peach samples were collected at 0 (2 hours), 2, 4, 6, 8, 10, 12, and 14 days. Edible parts of the peach were blended and stored in a freezer at –20˚C until analysis.
2.3. Extraction and Partitioning
A 30 g sample of peach was put into an Erlenmeyer flask with 100 mL acetone and shaken for 30 min with a mechanical shaker (Vision Scientific Co., Ltd., Daejeon, Korea). The sample was then filtered under a vacuum through a Buchner funnel. The container and filter cakes were washed with 30 mL acetone and the rinsate was combined with the previous filtrate. The filtrate was transferred to a 1 L separatory funnel, and 300 mL distilled water and 15 g sodium chloride were added. Partitioning was carried out twice with 70 mL portions of nhexane. After vigorous shaking, the organic layer was allowed to separate clearly, then dried over anhydrous sodium sulfate and combined. The solvent was completely evaporated with a vacuum rotary evaporator and dissolved in 1 mL n-hexane for buprofezin clean up, and 3 mL n-hexane for teflubenzuron clean up.
2.4. Clean Up
2.4.1. Buprofezin
The florisil SPE cartridge was preconditioned with 5 mL n-hexane and all of the solution from the partitioning step was added to the cartridge. To remove impurities, the SPE cartridge was washed with 5 mL n-hexane and eluted with 15 mL acetone/n-hexane (30:70, v/v). The eluted mixture was completely evaporated to dryness in a vacuum rotary evaporator at 40˚C, and the residue was redissolved in 3 mL acetone for gas chromatography with an electron capture detector (ECD).
2.4.2. Teflubenzuron
A chromatographic column (15 mm i.d. × 30 cm) was plugged with glass wool, dry packed with 2 g activated florisil and 3 g activated silica gel, and topped with a 2 cm layer of anhydrous sodium sulfate. The column was pre-washed by passing through 20 mL n-hexane. All of the hexane extract from the partitioning step was poured into the column. When the liquid drained to the sodium sulfate layer, the column was eluted with 30 mL acetone/n-hexane (10:90, v/v), and the fraction was discarded. The column was then eluted again with 30 mL acetone/n-hexane (10:90, v/v). The eluted solution was completely evaporated in a vacuum rotary evaporator at 40˚C, and the residue was redissolved in 3 mL acetonitrile for HPLC analysis.
2.5. Confirmation of the Residue
2.5.1. Gas Chromatography
An Agilent 6890 gas chromatograph (Santa Clara, CA, USA) equipped with an ECD and DB-5 column (30 m × 0.25 mm i.d., 0.25 μm film thickness) was used to determine the level of buprofezin residue. A 2 μL sample was injected in splitless mode, and the injector and detector temperatures were 260˚C and 320˚C, respectively. Oven temperature programming was initialized with a 2 min hold at 180˚C, then the temperature was increased at 10˚C∙min−1 to 300˚C, where it was finally held for 4 min, giving a total run time of 18 min. Ultrapure nitrogen was used as the carrier gas with a flow rate of 0.9 mL∙min−1, and the retention time of buprofezin was 9.88 min.
2.5.2. High Performance Liquid Chromatography
A high performance liquid chromatograph (SCL-10vp; Shimadzu, Kyoto, Japan) equipped with an ultraviolet diode array detector and a Phenomenex Luna C18 column (250 × 4.60 mm, 5 μm) maintained at 40˚C was used for teflubenzuron residue analysis. The mobile phase was acetonitrile/water, delivered at a flow rate of 1 mL∙min−1 with a gradient composition of: acetonitrile/ water (55:45, v/v, 0 - 2 min) → acetonitrile/water (65:35, 2 - 5 min) → acetonitrile/water (75:25, 5 - 10 min) → acetonitrile/water (55:45, 15 - 18 min). The injection volume was 20 μL and the detection wavelength was set at 250 nm. Under these conditions, the retention time of teflubenzuron was 16.7 min.
2.5.3. Validation of the Analytical Method
Recovery studies were performed with fortification levels of 0.05 mg∙kg−1 [10× limit of quantification (LOQ)] and 0.25 mg∙kg−1 (50× LOQ), obtained from control samples. Three replicates were analyzed at each fortification level. Average recoveries were 81.8% - 96.6%, whereby the coefficient of variation was within 10%, generally considered satisfactory for residue quantification. Results of the recovery studies are presented in Table 1.
2.6. Statistical Analysis
The statistical analysis of data was done by using Microsoft Excel and statistical software, SPSS version 19.
3. RESULTS AND DISDUSSION
Field trials were carried out over 14 days after spraying treatments that maintained GAP to assess the residual levels of buprofezin and teflubenzuron.
3.1. Buprofezin
Residues of the two pesticides were determined in samples by comparing the area of the buprofezin concentration peak with that of the external standard method. A
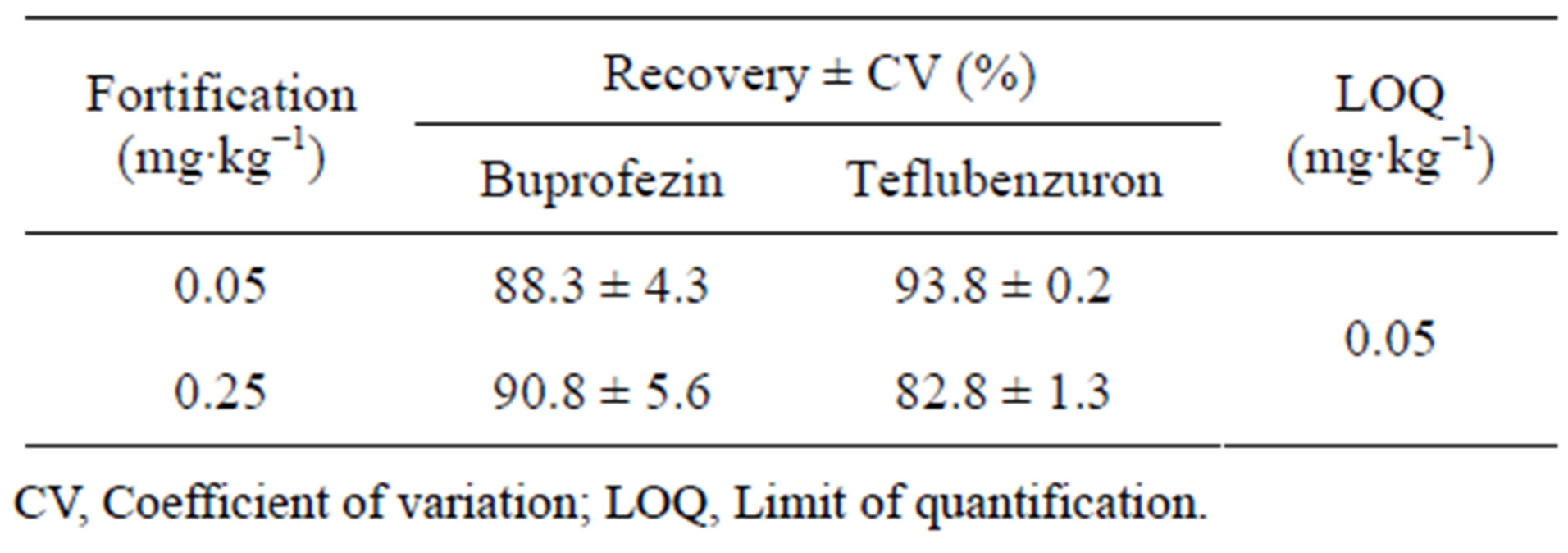
Table 1. Recovery and limit of quantification of the analytical method for two pesticides in peach.
seven point calibration curve from 0.05 to 10 mg∙kg−1 was constructed for quantitative analysis, and the detector response was linear throughout the entire range of concentrations, with a correlation coefficient (R2) of 0.999***.
Initial (2 hours) residues were 1.37 and 2.08 mg∙kg−1 at the recommended and triple doses, respectively. These residue values were higher than the MRL of 1 mg∙kg−1 used in Korea. Fourteen days after application, the remaining residues were 0.17 and 0.20 mg∙kg−1; i.e., 87.60% and 90.39% of the initial chemical had dissipated. Figure 2" target="_self"> Figure 2 shows the changes in buprofezin residue for eight sample times at 0 - 14 days after spraying. The kinetics of buprofezin can be described with the following equations: y = 1.5751e−0.1421t (R2 = 0.9597***) and y = 2.4683e−0.1574t (R2 = 0.9495***). The biological half-lives of buprofezin in peach were 4.88 and 4.40 days at the recommended and triple doses, respectively.
The final residue levels were compared when buprofezin was sprayed according to the maximum GAP values for peach in the United States [2.26 kg a.i. ha−1 × 2, and not more than 3.37 kg a.i. ha−1 per growing season, 14 day pre-harvest interval (PHI)], and predicted based on the initial amount sprayed and the coefficient of regression [6]. Buprofezin residues remaining over time, according to application amount, are given in Figure 3. After spraying with 4.52 kg∙ha−1 (2.26 kg a.i. ha−1 × 2), the final residue was predicted to be 1.45 mg kg−1 at harvest. After spraying with 3.56 kg∙ha−1 (1.78 kg ha−1 × 2), the predicted residue was 1.14 mg∙kg−1. From this prediction, the buprofezin residue in peaches was expected to be above the MRL of the United States (1 mg∙kg−1) up to 17 and 15 days after spraying with 4.52 kg a.i. ha−1 and 3.56 kg a.i. ha−1, respectively. The level of residue should not exceed 1.77 kg∙hg−1, the maximum level of application according to the GAP for the buprofezin WP formulation in the United States. Therefore, the application of other formulations should be recommended as an
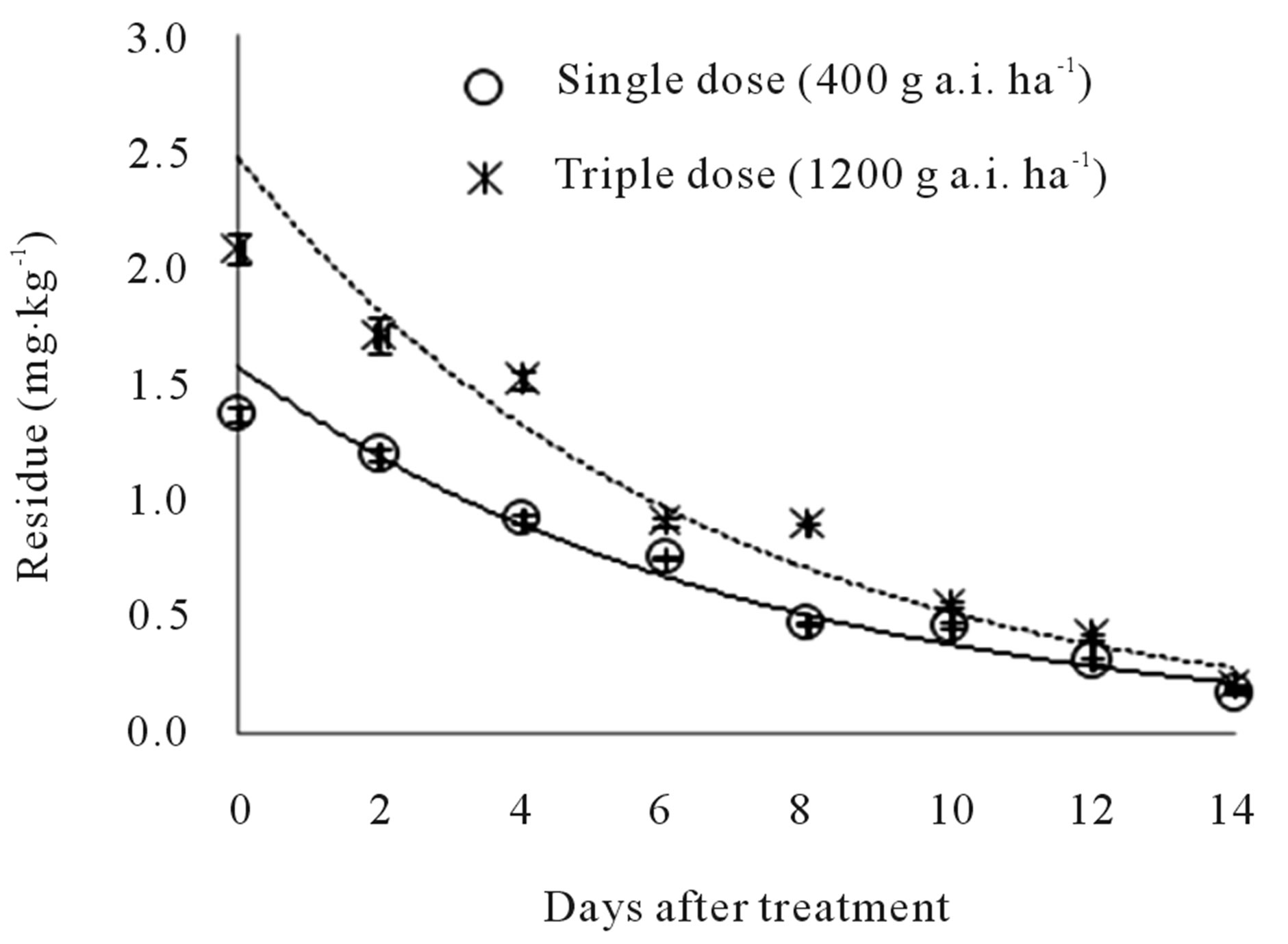
Figure 2. Dissipation of buprofezin in peach.
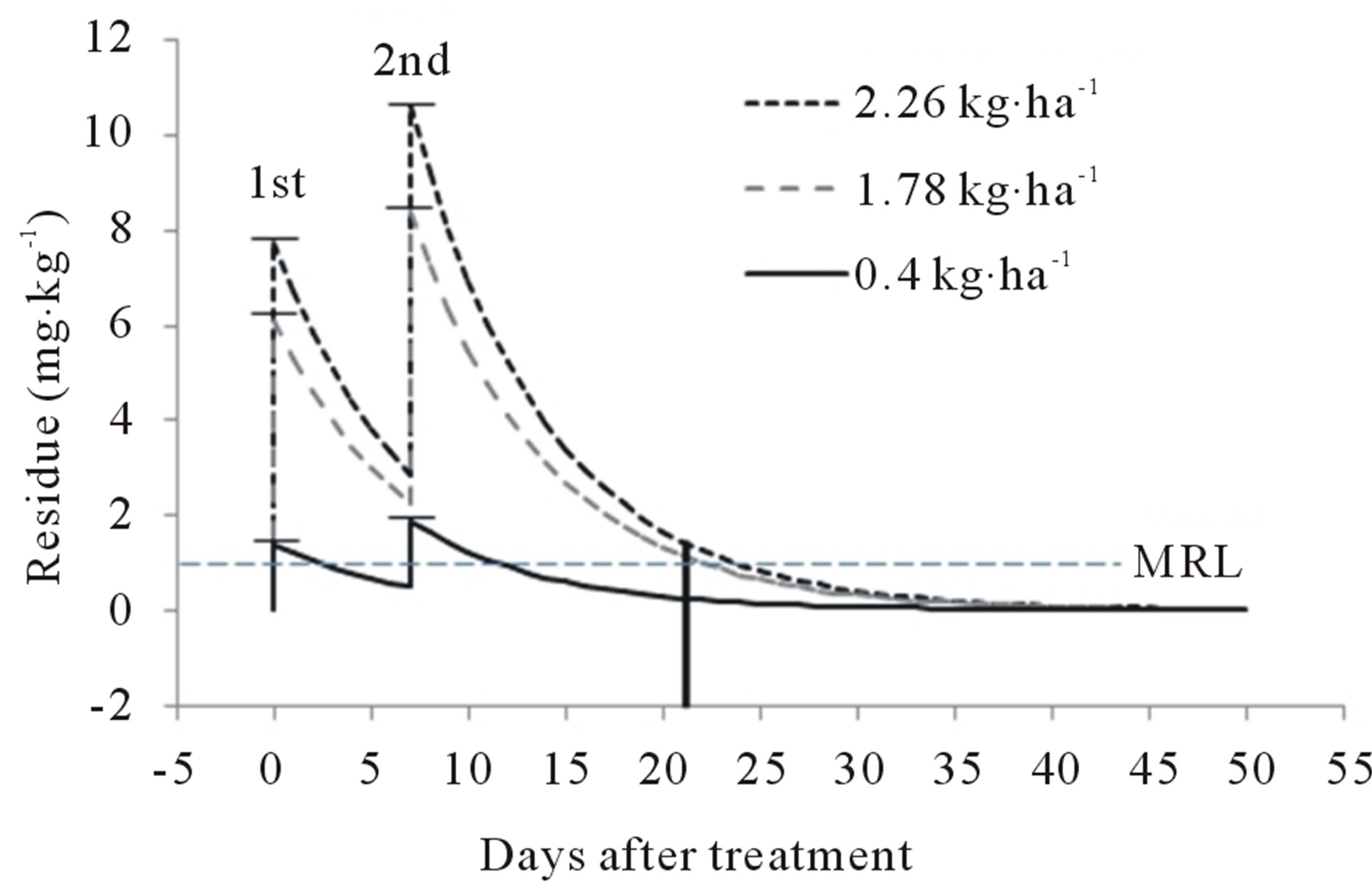
Figure 3. Predicted final residue levels of buprofezin according to application amount. MRL, maximum residue limit.
alternative to WP.
3.2. Teflubenzuron
Under the chromatographic conditions described, the calibration graphs (external standard method) showed strong linearity in the range of 0.05 - 5 mg∙kg−1 with a correlation coefficient (R2) of 0.999*** (peak height vs. concentration).
Initial (2 hours) residues were 0.26 and 0.71 mg∙kg−1 at the recommended and triple doses, respectively. Fourteen days after application, the remaining residues were 0.10 and 0.23 mg∙kg−1, respectively. In other words, 61.54% and 67.61% of the initial residue levels had dissipated. Figure 4 shows the changes in teflubenzuron residue for eight sample times at 0 - 14 days after spraying. The kinetics of teflubenzuron can be described with the following equations: y = 0.2709e−0.0603t (R2 = 0.9434***) and y = 0.6133e−0.0638t (R2 = 0.8828***). The biological half-lives of teflubenzuron in peach at the recommended and triple doses were 11.49 and 10.86 days, respectively.
The final residue levels in peach were compared when teflubenzuron was sprayed according to the GAP standard for peach in Italy, registered with 1 - 3 treatments of 0.12 kg a.i. ha−1 and a 21 day PHI [7]. Teflubenzuron residues according to application amount are given in Figure 5. After spraying with 0.36 kg ha−1 (0.12 kg a.i. ha−1 × 3), the final residue level was predicted to be 0.18 mg∙kg−1 at harvest. According to these values and Italy’s MRL (1 mg∙kg−1), residues of teflubenzuron found in peach were generally lower than the MRL value. Therefore, the use of teflubenzuron according to GAP produces harvested fruit with residue levels below the MRL.
The average weight of each representative peach after 0 - 14 days was 154.5 g (n = 48) at day 0 and increased to 273.5 g (n = 48) at day 14. Over 14 days, peach weight increased about 1.77-fold.
The pesticide dilution effect (f') was calculated from remaining pesticide and weight of peach over 10 days [8].
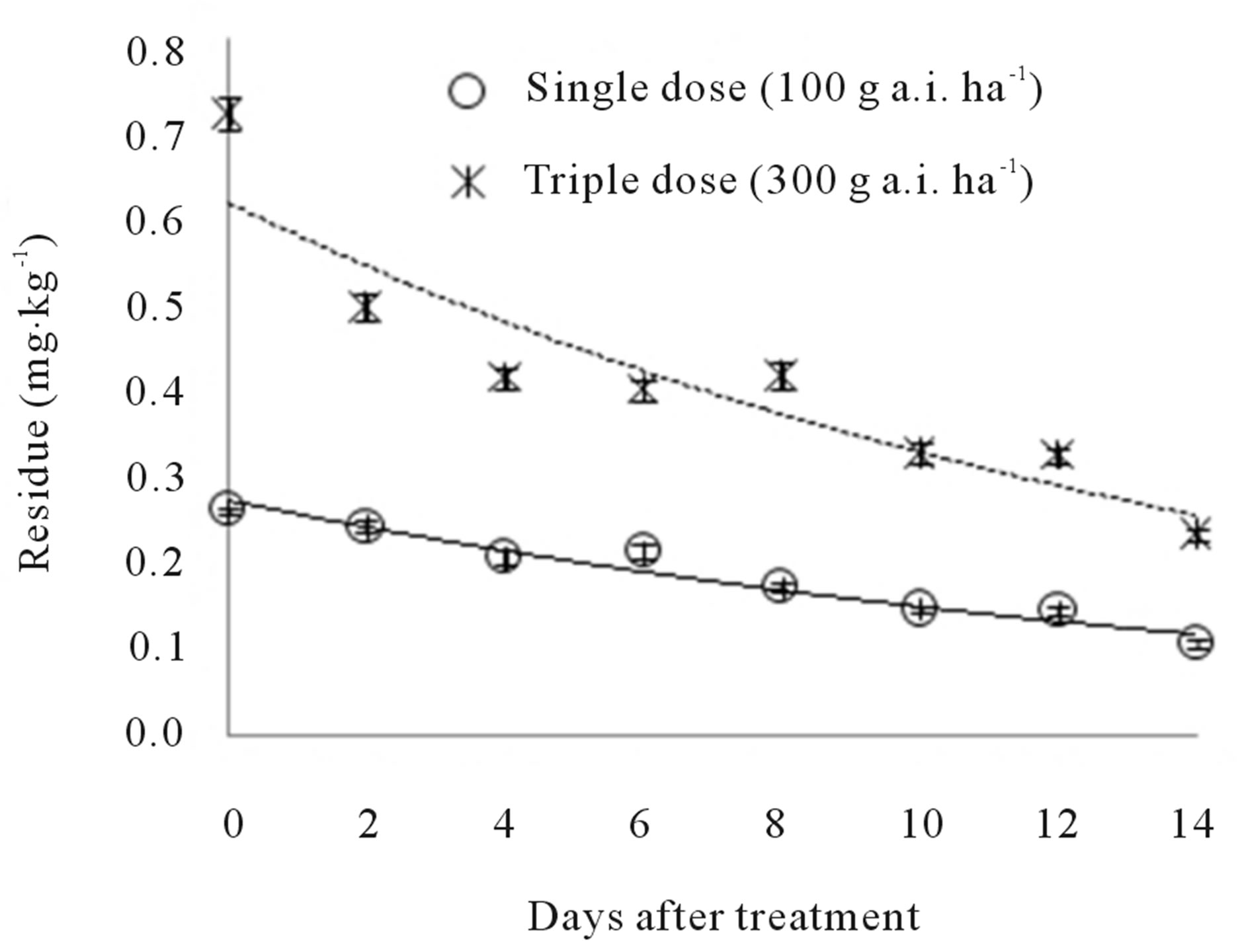
Figure 4. Dissipation of teflubenzuron in peach.
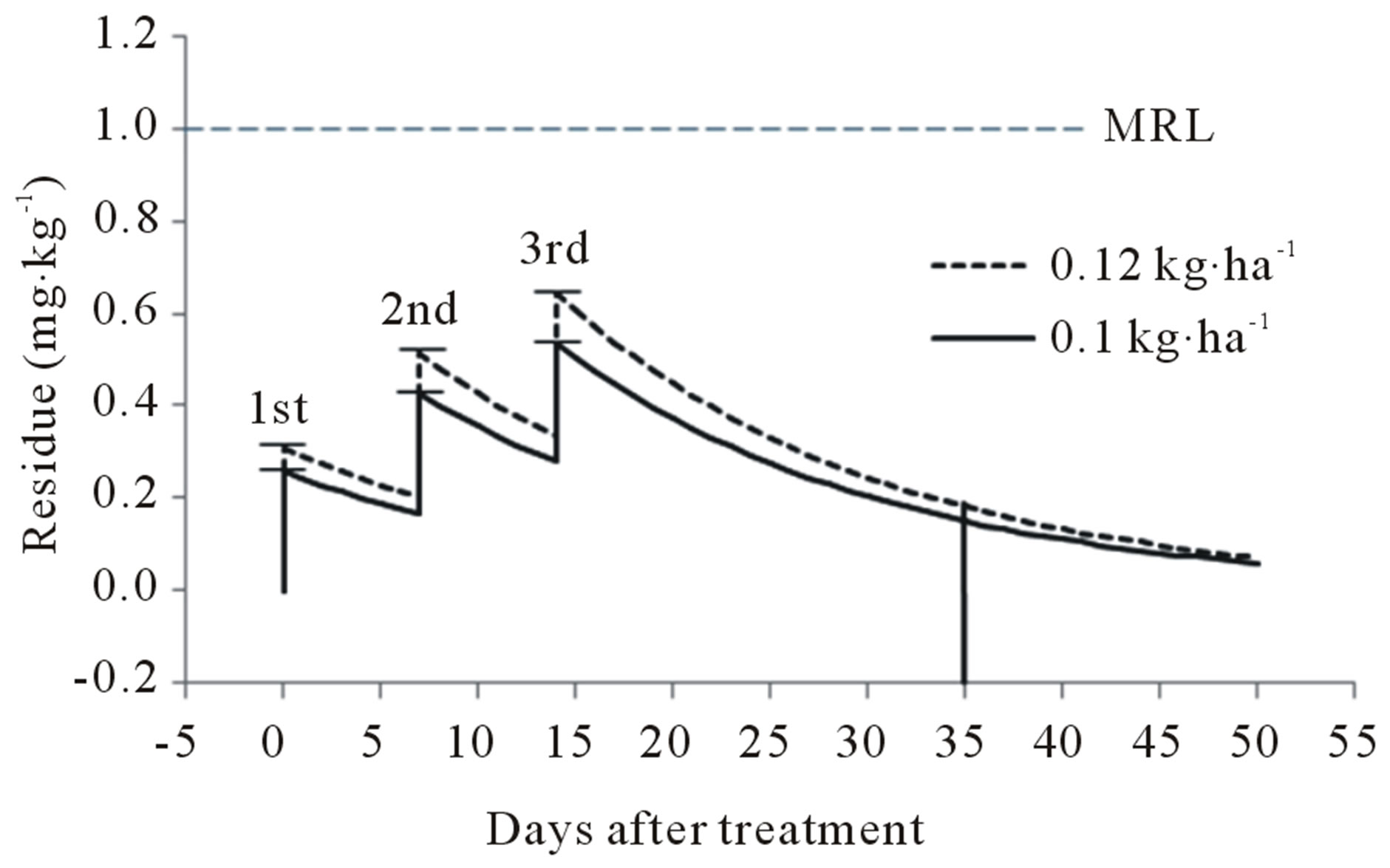
Figure 5. Predicted final residue levels of teflubenzuron according to application amount. MRL, maximum residue limit.
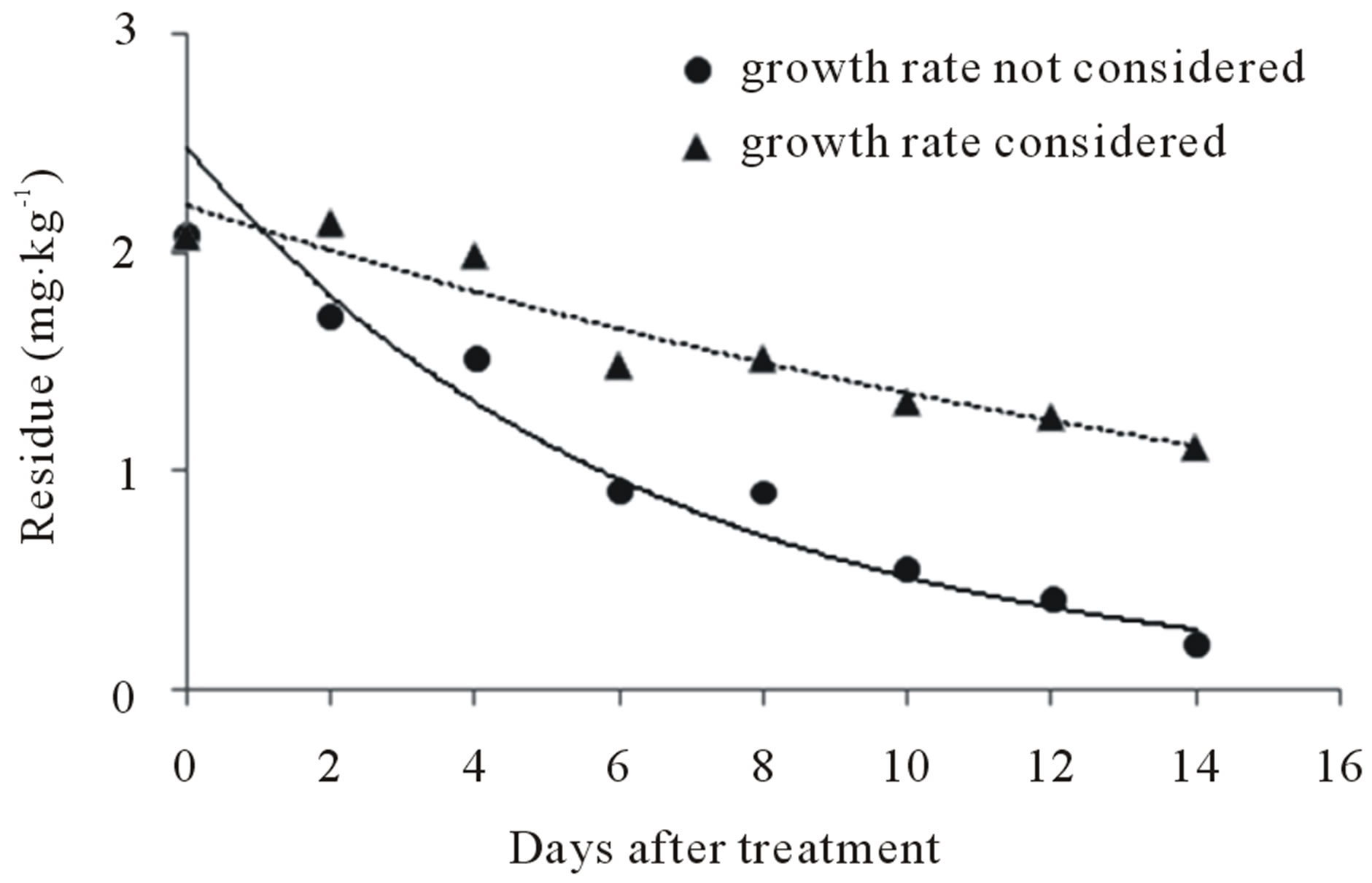
For the two cases in which growth rate was not considered and those in which the 1.77-fold growth rate was considered, the patterns of residue in relation to growth at the triple dose are given in Figure 6.
The biological half-lives of buprofezin were 13.97 and 4.40 days, respectively, when growth rate was not considered (y = 2.2159e−0.0496t) and when the 1.77-fold growth rate was considered (y = 2.4683e−0.1574t). The half-lives of teflubenzuron were 56.35 and 10.86 days, respectively when growth rate was not considered (y = 0.6622e−0.0123t) and when the 1.77-fold growth rate was considered (y = 0.6133e−0.0638t).
 (1)
(1)
Therefore, the growth rate of peaches influenced the levels of buprofezin and teflubenzuron residue. When growth rate was not considered, teflubenzuron residues remained remarkably stable for the entire cultivation period (14 days), which is attributed to the stability of the insecticide molecule against biological and environmental factors [3].
 (a)
(a)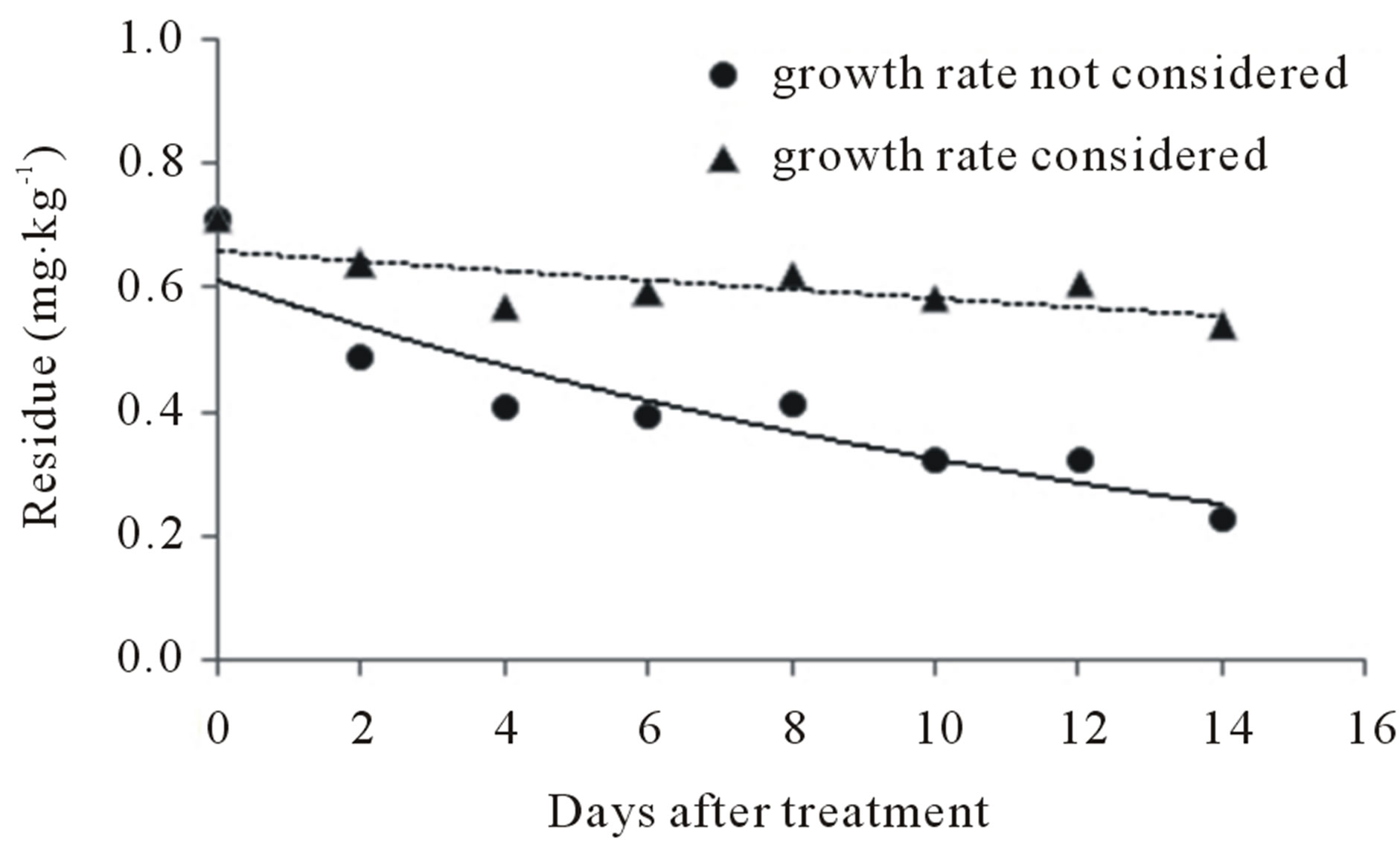 (b)
(b)
Figure 6. Dilution effects of (a) buprofezin and (b) teflubenzuron on peach in relation to fruit growth rate.
4. ACKNOWLEDGEMENTS
This study was financially supported by the National Agricultural Products Quality Management Services, Republic of Korea.
REFERENCES
- Ioannis V.Z., Dimitra A.L., Theocharis G.D., Despoina T.K. and Triantafyllos A.A. (2009) Assessment of pesticide residues in fresh peach samples produced under integrated crop management in an agricultural region of northern Greece. Food Additives & Contaminants, 26, 1256-1264. doi:10.1080/02652030903045122
- Lee, Y.D. and Jang, S.W. (2010) Determination of buprofezin residues in rice and fruits using HPLC with LC/ MS confirmation. Korean Journal of Environmental Agriculture, 29, 247-256. doi:10.5338/KJEA.2010.29.3.247
- Nicholas, G.T., Pipina, G.A. and George, E.M. (1999) Evaluation of teflubenzuron residue levels in grapes exposed to field treatments and in the must and wine produced from them. Journal of Agricultural and Food Chemistry, 47, 4583-4586. doi:10.1021/jf990010n
- Yoon, M.H., Jeong, S.A., Heo, S.J., Park, H.R. and Hur, J.H. (2011) Residual analysis and establishment of standard for safe use of teflubenzuron 5% SC in the Peach (Prunus persica L.). Journal of Agricultural, Life and Environmental Sciences, 23, 34-39.
- Rothschild, G.H.L. and Vickers R.A. (1991) Biology, ecology and control of the oriental fruit moth. In: van der Geest L.P.S and Evenhuis, H.H., Eds., Totricid Pests: Their Biology, Natural Enemies, and Control, Elsevier Science Publishers, Amsterdam, 389-412.
- Kim, S.W., Lee E.M., Lin, Y., Park, H.W., Lee, H.R., Riu, M.J., Na, Y.R., Noh, J.E., Keum, Y.S. and Kim, J.H. (2009) Establishment of pre-harvest residue limit (PHRL) of insecticide bifenthrin during cultivation of grape. Korean Journal of Pesticide Science, 13, 241-248.
- FAO (1997) Plant production and protection paper. 142. http://www.fao.org/docrep/W5897E/w5897e4n.htm#pears
- Hong, J.H., Lee, C.R., Lim, J.S. and Lee, K.S. (2011) Comparison of analytical methods and residue patterns of pymetrozine in Aster scaber. Bulletin of Environmental Contamination and Toxicology, 87, 649-652. doi:10.1007/s00128-011-0407-8

