Food and Nutrition Sciences
Vol. 4 No. 10 (2013) , Article ID: 36805 , 5 pages DOI:10.4236/fns.2013.410130
NMR Spectral Analysis and Hydrolysis Studies of Rebaudioside N, a Minor Steviol Glycoside of Stevia rebaudiana Bertoni
![]()
Natural Ingredient Development, Blue California, Rancho Santa Margarita, USA.
Email: *saipc@bluecal-ingredients.com
Copyright © 2013 Venkata Sai Prakash Chaturvedula et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 1st, 2013; revised September 1st, 2013; accepted September 8th, 2013
Keywords: Stevia rebaudiana; Diterpene Glycoside; Isolation; Structure Elucidation; Spectral Data; Hydrolysis Studies
ABSTRACT
The complete proton and carbon NMR spectral assignments of a diterpene glycoside isolated from the commercial extract of the leaves of Stevia rebaudiana Bertoni, 13-[(2-O-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy] ent-kaur-16-en-19-oic acid-[(2-O-α-L-rhamnopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl) ester] (1); also known as rebaudioside N, was achieved by the extensive 1D and 2D NMR (1H and 13C, COSY, HMQC, HMBC) as well as mass spectral data. Further, hydrolysis studies were performed on rebaudioside N using acid and enzymatic studies to identify aglycone and sugar residues in its structure.
1. Introduction
Stevia rebaudiana (Bertoni) is a perennial shrub belonging to the family of Asteraceae (Compositae) native to Brazil and Paraguay, but now grown commercially in a number of areas, particularly in Japan, Taiwan, Korea, Mainland China, Thailand and Indonesia [1,2]. Extracts of the leaves of S. rebaudiana have been used for decades to sweeten food and beverages in Japan, South America and China. The major constituents in the leaves of S. rebaudiana are the potently sweet glycosides namely steviolbioside, stevioside, rebaudiosides A and E, dulcoside A and rubusoside; which are glycosides of the diterpene steviol, ent-13-hydroxykaur-16-en-19-oic acid [3,4]. These compounds are also known as Stevia sweeteners.
Recently Ohta et al. have reported several minor steviol glycosides from S. rebaudiana Morita including rebaudioside N [5], however, they have not reported isolation or complete spectral assignment of pure rebaudioside N. As a part of our research related to the discovery of natural sweeteners and sweetener enhancers, we are herewith describing the isolation, characterization and complete 1H and 13C NMR spectral assignments for the diterpene glycoside 13-[(2-O-β-D-glucopyranosyl-3- O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-ent-kau r-16-en-19-oic-acid-[(2-O-α-L-rhamnopyranosyl-3-O-βD-glucopyranosyl-β-D-glucopyranosyl) ester] (1) which is also known as rebaudioside N (Figure 1) isolated from the commercial extract of Stevia rebaudiana Bertoni. The complete NMR assignments were achieved on the basis of 1D (1H and 13C) and 2D (COSY, HMQC and HMBC) NMR as well as high resolution mass spectroscopic data. Acid and enzymatic hydrolysis studies on compound 1 were carried out to identify aglycone and sugar residues.
2. Experimental
2.1. General Instrumentation Procedures
HPLC analysis was performed using a Dionex UPLC ultimate 3000 system (Sunnyvale, CA), including a quaternary pump, a temperature controlled column compartment, an auto sampler and a UV absorbance detector. Phenomenex Luna NH2 with guard column, 150 × 3.0 mm, 3 µm (100A) were used for the characterization of rebaudioside N (1). NMR spectra were acquired on a Varian INOVA 600 MHz instrument with

Figure 1. Structure of rebaudioside N (1) and other compounds.
a 5 mm HCN probe using standard pulse sequences. The NMR spectra were performed in C5D5N; chemical shifts are given in d (ppm), and coupling constants are reported in Hz. The spectral data was referenced to the residual solvent signal (dH 7.19, and dC 123.5 for pyridine-d5). IR spectral data was acquired using a Perkin Elmer 400 Fourier Transform Infrared (FT-IR) Spectrometer with Universal attenuated total reflectance (UATR) polarization accessory. MS and MS/MS data were generated with a Thermo LTQ-FTMS mass spectrometer (100,000 resolutions) equipped with a Nano spray ionization source. Samples were diluted with methanol and introduced via infusion using the onboard syringe pump.
2.1.1. Isolation of Compound 1
Compound 1 was purified by repeated isocratic elution (72% acetonitrile in water) of the commercial extract of Stevia rebaudiana Bertoni using Dionex UPLC ultimate 3000 system with Phenomenex Luna NH2 guard column. Collected the peak eluting at tR 7.03 min; and dried the corresponding solution under nitrogen yielded 1.
2.1.2. Characterization of 13-[(2-O-β-DGlucopyranosyl-3-O-β-D-Glucopyranosyl- β-D-Glucopyranosyl) oxy] Ent-Kaur-16-en-19 -Oic Acid-[(2-O-α-L-Rhamnopyranosyl-3-O-β- D-Glucopyranosyl-β-D-Glucopyranosyl) Ester] (Rebaudioside N, 1)
White powder; IR νmax: 3317, 2945, 1725, 1063, 914 cm−1; 1H-NMR (600 MHz, C5D5N, δ ppm) and 13C-NMR (150 MHz, C5D5N, δ ppm) spectroscopic data see Table 1; HRMS (M + H)+ m/z 1275.5493 (calcd. for C56H91O32: 1275.5478).
2.1.3. Acid Hydrolysis of Compound 1
To a solution of compound 1 (5 mg) in MeOH (10 ml) was added 3 ml of 5% H2SO4 and the mixture was refluxed for 24 hours. The reaction mixture was then neutralized with saturated sodium carbonate and extracted with ethyl acetate (EtOAc) (2 × 25 ml) to give an aqueous fraction containing sugars and an EtOAc fraction containing the aglycone part. The aqueous phase was concentrated and compared with standard sugars using the TLC systems EtOAc/n-butanol/water (2:7:1) and CH2Cl2/MeOH/water (10:6:1) [6-8]; the sugars were identified as D-glucose and L-rhamnose.
2.1.4. Enzymatic Hydrolysis of Compound 1
Compound 1 (1 mg) was dissolved in 10 ml of 0.1 M sodium acetate buffer, pH 4.5 and crude pectinase from Aspergillus niger (50 uL, Sigma-Aldrich, P2736) was added. The mixture was stirred at 50˚C for 96 hr. The product precipitated out during the reaction and was filtered and then crystallized. The resulting product obtained from the hydrolysis of 1 was identified as steviol (4) by comparison of its co-TLC with standard compound and 1H NMR spectral data [9].
3. Results and Discussion
Compound 1 was isolated as an crystalline material and its molecular formula has been deduced as C56H90O32 on the basis of its positive ESI TOF mass spectrum which showed [M + H]+ ion at m/z 1275.5493, and this composition was supported by 13C NMR spectral data. The 1H NMR spectrum of 1 showed the presence of two methyl singlets at δ 1.17 and 1.51, two olefinic protons as singlets at δ 5.05 and 5.69 of an exocyclic double bond, nine methylene and two methine protons between δ 0.72 - 2.60, characteristic for the ent-kaurane diterpenoids isolated earlier from the genus Stevia [10-12]. The basic skeleton of ent-kaurane diterpenoids was supported by COSY (H-1/H-2; H-2/H-3; H-5/H-6; H-6/H-7; H-9/H-11; H-11/H-12) and HMBC (H-1/C-2, C-10; H-3/C-1, C-2, C-4, C-5, C-18, C-19; H-5/C-4, C-6, C-7, C-9, C-10, C-18, C-19, C-20; H-9/C-8, C-10, C-11, C-12, C-14, C-15; H-14/C-8, C-9, C-13, C-15, C-16 and H-17/C-13, C-15, C-16) correlations. The 1H NMR spectrum of 1 showed the presence of six sugar units in its structure by the presence of the anomeric protons resonating at δ 5.03, 5.06, 5.37, 5.58, 6.22, and 6.32; which was further supported by the MS/MS spectrum of 1 in the positive ESI mode showed the fragment ions at m/z 1113, 951, 805, 643, 481 and 319. Acid hydrolysis of


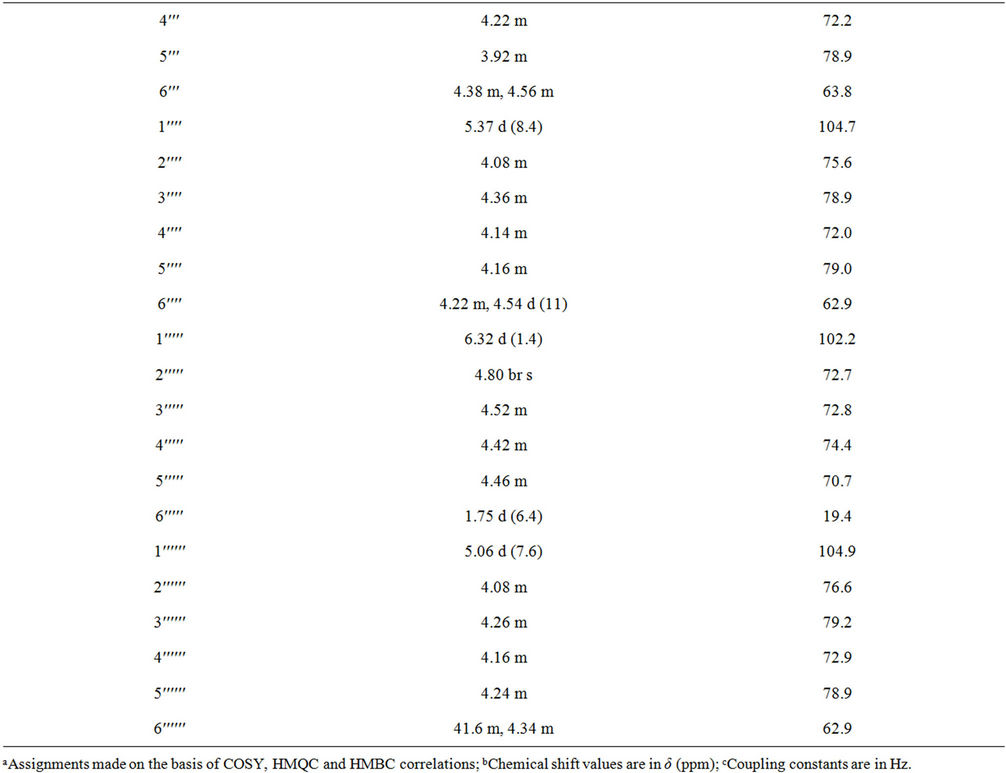
Table 1. 1H and 13C NMR spectral data (chemical shifts and coupling constants) for rebaudioside N (1) in d5-pyridine (C5D5N)a-c.
1 with 5% H2SO4 afforded the sugars D-glucose and L-rhamnose, which were identified by direct comparison with authentic samples by TLC [6-8]. Enzymatic hydrolysis of 1 furnished an aglycone which was identified as steviol (4) by comparison of 1H NMR and co-TLC with standard compound [9]. The large coupling constants observed for the five anomeric protons of the glucose moieties at δ 5.03 (d, J = 7.8 Hz), 5.06 (d, J = 7.6 Hz), 5.37 (d, J = 8.4 Hz), 5.58 (d, J = 7.8 Hz), and 6.22 (d, J = 8.4 Hz), suggested their β-orientation as reported for steviol glycosides [9-12]. The sixth anomeric sugar corresponding to that of L-rhamnosyl unit was identified as a doublet at δ 6.32 (J = 1.4 Hz) suggesting its α-orientation [5]. The 1H and 13C NMR values for all the carbons in 1 were assigned on the basis of COSY, HSQC and HMBC correlations (Table 1).
Based on the results from NMR spectral data and hydrolysis experiments of 1, it was concluded that there are five β-D-glucosyl units and an α-L-rhamnosyl unit in its structure connected to the aglycone steviol. A close comparison of the 1H and 13C NMR values of 1 with rebaudioside M (2) and rebaudioside A (3) [5] suggested the presence of a 2,3-disubstituted β-D-glucosyl unit at C-13 in the form of ether linkage and another 3-substituted β-D-glucosyl unit at C-19 position in the form of an ester linkage, leaving the assignment of the additional α-L-rhamnosyl unit. The downfield shift for both the 1H and 13C chemical shifts at C-2′ of sugar I suggested that the additional α-L-rhamnosyl moiety has been attached at this position. This was confirmed by the key HMBC correlations: H-2′/C-1′, C-3′, C-1′′′′′ and H-1′′′′′/C-2′, C-2′′′′′, C-3′′′′′. Based on the results from chemical and spectral studies, 1 was assigned as 13-[(2-O-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl) oxy] ent-kaur-16-en-19-oic acid-[(2-O-α-L-rhamnopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl) ester]. The structure was further supported by the key COSY and HMBC correlations as shown in Figure 2.
To the best of our knowledge, this is the first report of the isolation of rebaudioside N (1) from S. rebaudiana Bertoni. Though partial NMR spectral data has been reported earlier for rebaudioside N (1) by Ohta et al. [5], this

Figure 2. Key COSY and HMBC correlations of 1.
is the first report of complete 1H and 13C NMR spectral assignments based on 1D (1H and 13C) and 2D (COSY, HMQC and HMBC) NMR as well as high resolution mass spectroscopic data which was supported by enzymatic and acid hydrolysis studies.
4. Conclusion
We are herewith reporting the isolation, complete 1H and 13C NMR spectral assignments for 13-[(2-O-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-ent-kaur-16-en-19-oic-acid-[(2-O-α-L-rhamnopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)
ester], also known as rebaudioside N (1) on the basis of extensive 1D and 2D NMR as well as high resolution mass spectral data. Further, acid hydrolysis furnished D-glucose suggesting the presence of two sugar units that were identified as β-D-glucose and α-L-rhamnose; and enzymatic hydrolysis furnished steviol.
5. Acknowledgements
We wish to thank Dr. Shaoxiong Wu, and Dr. Bing Wang of Emory University, Atlanta, USA for obtaining some selected spectral data and other chemistry related help.
REFERENCES
- S. E. Mosettig and W. R. Nes, “Stevioside. II. The Structure of the Aglucon,” Journal of Organic Chemistry, Vol. 20, No. 7, 1955, pp. 884-899. http://dx.doi.org/10.1021/jo01125a013
- S. E. Mosettig, U. Beglinger, F. Dolder, H. Lichiti, P. Quitt and J. A. Waters, “The Absolute Configuration of Steviol and Isosteviol,” Journal of American Chemical Society, Vol. 85, No. 11, 1963, p. 2305. http://dx.doi.org/10.1021/ja00898a025
- J. E. Brandle, A. N. Starrratt and M. Gijen, “Stevia rebaudiana: Its Agricultural, Biological and Chemical Properties,” Canadian Journal of Plant Sciences, Vol. 78, No. 4, 1998, pp. 527-536. http://dx.doi.org/10.4141/P97-114
- E. S. Wayne and L. Lin, “NMR Studies of the Conformation of the Natural Sweetener Rebaudioside A,” Carbohydrate Research, Vol. 344, No. 18, 2009, pp. 2533- 2538. http://dx.doi.org/10.1016/j.carres.2009.10.005
- M. Ohta, S. Sasa, A. Inoue, T. Tamai, I. Fujita, K. Morita and F. Matsuura, “Characterization of Novel Steviol Glycosides from Leaves of Stevia rebaudiana Morita,” Journal of Applied Glycoscience, Vol. 57, No. 3, 2010, pp. 199-209. http://dx.doi.org/10.5458/jag.57.199
- E. Bedir, N. J. Toyang, I. A. Khan, L. A. Walker and A. M. Clark, “A New Dammarane Type Triterpene Glycoside from Polyscias fulva,” Journal of Natural Products, Vol. 64, No. 1, 2001, pp. 95-97. http://dx.doi.org/10.1021/np0003589
- V. S. P. Chaturvedula, J. K. Schilling, J. S. Miller, R. Andriantsiferana, V. E. Rasamison and V. D. G. I. Kingston, “New Cytotoxic Oleanane Saponins from the Infructescences of Polyscias amplifolia from the Madagascar Rainforest,” Planta Medica, Vol. 69, 2003, pp. 440- 444. http://dx.doi.org/10.1055/s-2003-39711
- V. D. Huan, S. Yamamura, K. Ohtani, R. Kasai, K. Yamasaki and N. T. Nham, “Oleanane saponins from Polyscias fructicosa,” Phytochemistry, Vol. 47, No. 3, 1998, pp. 451-457. http://dx.doi.org/10.1016/S0031-9422(97)00618-3
- K. Ohtani, Y. Aikawa, R. Kasai, W. Chou, K. Yamasaki and O. Tanaka, “Minor Diterpene Glycosides from Sweet Leaves of Rubus suavissimus,” Phytochemistry, Vol. 31, No. 5, 1992, pp. 1553-1559. http://dx.doi.org/10.1016/0031-9422(92)83105-8
- V. S. P. Chaturvedula and I. Prakash, “A New Diterpenoid Glycoside from Stevia rebaudiana,” Molecules, Vol. 16, No. 4, 2011, pp. 2937-2943. http://dx.doi.org/10.3390/molecules16042937
- V. S. P. Chaturvedula and I. Prakash, “Structures of the Novel Diterpene Glycosides from Stevia rebaudiana,” Carbohydrate Research, Vol. 346, No. 8, 2011, pp. 1057- 1060. http://dx.doi.org/10.1016/j.carres.2011.03.025
- V. S. P. Chaturvedula and I. Prakash, “Additional Minor Diterpene Glycosides from Stevia rebaudiana,” Natural Product Communications, Vol. 6, No. 8, 2011, pp. 1059- 1062.
NOTES
*Corresponding author.

