Food and Nutrition Sciences
Vol. 3 No. 6 (2012) , Article ID: 19907 , 8 pages DOI:10.4236/fns.2012.36097
Complexation of Eugenol (EG), as Main Component of Clove Oil and as Pure Compound, with β- and HP-β-CDs
![]()
Departament of Food Technology and Nutrition, Catholic University of San Antonio, Murcia, Spain.
Email: *enunez@ucam.edu
Received April 11th, 2011; revised July 17th, 2011; accepted August 6th, 2011
Keywords: Eugenol; Essential Clove Oil; β-CDs; HP-β-CDs
ABSTRACT
Eugenol, both in its pure form (EG) and included in essential clove oil (CO) was successfully solubilized in aqueous solution by forming inclusion complexes with β-cyclodextrins (β-CDs) and its modified hydroxypropyl-β-CDs (HP-β-CDs). To investigate the molecular association between β-CDs/HP-β-CDs with pure EG and essential CO, phase solubility studies were undertaken. Essential CO formed insoluble complexes with β-CDs, but not with HP-β-CDs. The work clearly demonstrates complexes formation follow an order higher than 1:1 when high essential CO and β-CDs concentrations were used, however it was 1:1 in the case of essential CO-HP-β-CDs complexes. When pure EG was studied the results indicated that EG could form 1:1 inclusion complexes with β-CDs and HP-β-CDs. Based on the studies, the Kc values for pure EG were 4555 ± 225 M−1 and 10,633 ± 614 M−1 for β-CDs and HP-β-CDs, respectively, and 2005 ± 199 M−1 for essential CO-HP-β-CDs. These finding indicate that CDs are suitable for encapsulating EG.
1. Introduction
Essential oils and extracts of various species of edible and medicinal plants, herbs, and spices constitute very potent biologically active agents. They have a complex composition, containing from a few to several hundred constituents, mainly hydrocarbons and oxygenated compounds. Both, hydrocarbons and oxygenated compounds are responsible for the characteristic odors and flavors, which are formed by aromatic plants as secondary metabolites [1].
Clove oil (CO) is an essential oil from the dried flower buds, leaves and stem of the tree Syzygium aromaticum (Eastern Hemisphere) or Eugenia caryophyllata and Eugenia aromaticum (Western hemisphere) [2]. It has been used for centuries as anesthetic for toothaches, headaches and joint pain [3,4]. Clove has received attention as an ideal fish anaesthesic [5-8] and it has been used as a fragrant and flavoring agent in a variety of food and cosmetic products [9-11]. However, irritation towards the mucosa and skin, pungent taste, volatility, light sensitivity and poor water solubility make it unsuitable to use as such.
Eugenol (EG) (4-allyl-2-methoxyphenol) is the principal constituent of the essential CO, being 90% - 95% of the total oil amount [12,13]. It has a strong phenolic smell and sharp acrid taste [14,15] and its chemical structure is represented in Figure 1.
This phenolic compound has shown several biological activities such as anti-inflammatory activity, by inhibiting the enzyme ciclooxygenase II [16]; analgesic activity, due to selective binding at capsaicin receptor [17]; antioxidative activity and anti-bacterial activity, against both gram positive and gram negative microorganisms [18- 20].
It is well established that cyclodextrins (CDs) form complexes with several molecules eliminating their unwanted effects. CDs are a group of naturally occurring cyclic oligosaccharides derived from starch with six, seven and eight glucose of residues linked by α (1 - 4) glycosidic bonds in a cylinder-shaped structure, and denominated α, β and γ-cyclodextrins, respectively. The central cavity of these molecules is hydrophobic, while the rims of the surrounding walls are hydrophilic. This hydrophobic cavity forms inclusion complexes with a wide range of organic and inorganic guest molecules [21], altering their physicochemical behavior and reducing their undesirable effects.
The inclusion of eugenol in CDs has been widely
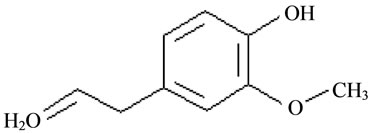
Figure 1. Chemical structure of eugenol (EG).
studied [22-25]. These inclusion complexes have been mainly characterized by using fluorescence and infrared spectroscopy (IR) [23,24], UV-visible spectroscopy (UV), powder X-Ray diffraction (XRD), thermogravimetric analysis (TA), differencial scanning calorimetry (DSC) and nuclear magnetic resonance spectroscopy (NMR) [22-24]. However, the majority of these studies are focused in the complexation of pure EG, but not in the complexation of this as main component of essential CO.
In the food industry, clove is often used in the form of ground, extracted essential oil or oleoresin, but always in a small amount because of its intense flavour. However, the use of CO could be a problem in industrial food applications due to its high volatility and low stability.
In order to optimize and design controllable and advanced essential CO-CDs carriers for food, cosmetic and other industrial applications it is necessary to study the complexes formation process between CDs and essential CO. The aim of the present work was to characterize the complexation process between pure EG and this when it is included in the essential CO with native β-CDs or its modified Hydroxypropyl-β-CDs (HP-β-CDs), by GC-MS analyses and spectrophotometric studies.
2. Materials and Methods
2.1. Materials
β-CDs were purchased from Wacker (Germany) and HP-β-CDs were supplied from TCI (Europe). Essential CO was kindly supplied by Lidervet, SA (Spain). EG was obtained from Sigma-Aldrich (Spain). All other chemicals used were of analytical grade.
2.2. Methods
2.2.1. Solubility Studies
Phase solubility studies were carried out according to the method described by Higuchi and Connors (1965). Excess amount of essential CO or pure EG were added to aqueous solutions of increasing concentrations of β-CDs, up to 13 mM, or HP-β-CDs up to 75 mM, in a final volume of 5 mL of water, at 25˚C. The samples were maintained in an ultrasonic bath at 25˚C to reach equilibrium. Later on, the tubes were shaken in a vortex. 500 mM of NaCl were added to each sample to accelerate the precipitation. Samples were centrifuged at 14,800 g at 25˚C for 30 min in a centrifuge model Biofuge Stratos. The supernatants were filtered through 0.2 µm nylon membrane filter and diluted in 80% ethanol prior to the quantification of the encapsulated pure EG or essential CO in solution by UV-spectrophotometry or GC-MS analysis. When a precipitated was observed, it was redissolved by adding 2 mL of ethanol 100%. The samples were then centrifuged at 14,800 g at 25˚C for 30 min. These supernatants were used for the quantification of the essential CO by GC-MS or UV-spectrophotometry analysis. The results obtained for supernatants or precipitates were plotted vs CDs concentration and the apparent stability constants, Kc, for essential CO or pure EG and β-CDs or Hp-β-CDs inclusion complexes were calculated from the slopes and intercept of the linear portion of the phase solubility diagrams, according to the following equation [26]:
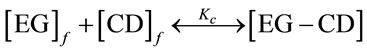 (1)
(1)
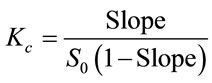 (2)
(2)
where S0 is the aqueous solubility of essential CO or pure EG, in the absence of CDs and the slope means the corresponding slope of the phase solubility diagrams, i.e. the slope of EG as main component of essential CO or pure EG vs β-CDs or HP-β-CDs concentration graph. The dissolved essential CO or pure EG in each sample, were obtained by using calibration curves prepared in the same experimental conditions by GC-MS or UV-spectrophotometry analysis.
2.2.2. Capillary GC-MS Analysis
The GC used was a Shimadzu GC-QP 2010 (Kyoto, Japan) coupled with a mass spectrometer. Helium was used as the carrier gas at an average flow rate of 0.5 mL/min. The capillary column used was a ω-WAX 250 fused silica supelco (30 m × 0.25 mm × 0.25 µm of thickness). For individual analite identification and quantification, the temperature was as follows: 3 min at 40˚C, raised up to 47˚C at 2˚C/min, held at 47˚C for 2 min, raised up to 52˚C at 2˚C/min, from 52˚C to 110˚C at 5˚C/min, ramped at 25˚C/min up to 200˚C and keep finally at 200˚C for 5 min. Peak areas of each compound were used for quantification of EG.
In the first set of experiments and in order to obtain the signal for the analite in the mass spectrometer, a control sample of essential CO was spiked. As it is known the main compound of essential CO was eugenol [12], which was used to prepare a calibration curve (Figure 2). Three replications were made for each measurement and standard error was not higher than 5%.
2.2.3. UV-Spectrophotometric Analyses
Formation of the inclusion complexes was verified spectrophotometrically in a Shimadzu model UV-1603 spectrophotometer at the absorption maximum of the essential CO, which was coincident with that for EG at 282 nm (Figure 3).
For this purpose calibration curves were prepared by using increasing concentrations of essential CO and pure EG ethanolic solution (from 0.005 to 0.05 mg/mL).
3. Results and Discussion
Prior to the aqueous solubility studies of essential CO in the presence of CDs, a GC-MS characterization of essential CO was carried out. The major peak obtained was
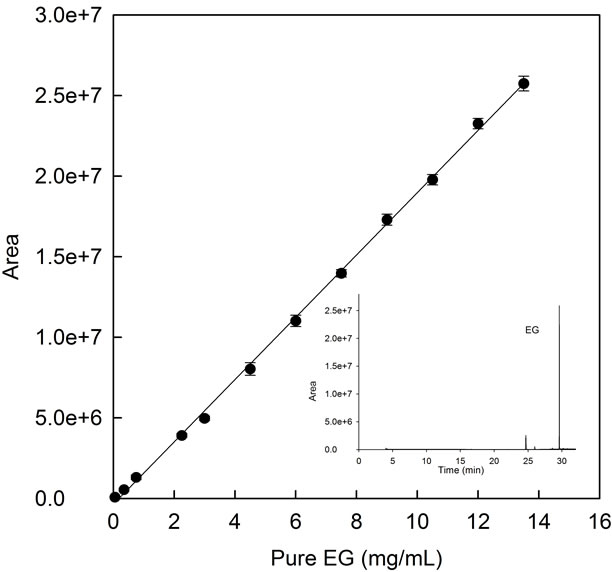
Figure 2. Essential CO (●) calibration curve by GC-MS. Inset: Total ion chromatogram of essential CO representing the main compound, eugenol (EG), of essential CO.

Figure 3. UV-Vis absortion spectra of increasing concentrations of essential CO (0.04, 0.03 mg/mL) (a, b, solid line) and pure EG (0.04 - 0.05 mg/mL) (c, d) discontinue line.
EG (Figure 2, inset), as could be demonstrated by the mass spectrum (data not shown). The pure compound (EG) was spiked and a calibration curve was prepared (Figure 2).
Once the main compound of essential CO was identified, its UV-spectrum was study. Pure EG absorption spectra were the same that absorption spectra of essential CO, showing a maximum at 282 nm (Figure 3). The slope of both, essential CO and pure EG, calibration curves were very similar (Essential CO: y = 0.014 + 13.8x; EG: y = 0.021 + 15.91x) (Figure 4).
Therefore, either the calibration curve of pure EG obtained by GC-MS (Figure 2) or by spectrophometry (Figure 4, open squares) were used to know the concentration of encapsulated essential CO in CDs solution and to compare the efficiency of both methods in EG quantification, due to the fact that the main compound of essential CO is EG.
3.1. Methods Validation
First of all, the validation of EG quantification methods used in this study (GC-MS and UV-spectrophotometry) was carried out.
3.1.1. Linearity
Figures 2 and 4 (open squares), show the linear relationship between absorbance at 282 nm or the peak area for UV-spectophotometry or GC-MS analysis of pure EG, respectively. The regression analysis in Figure 4 (open squares) and Figure 2 points to the linear response between the EG concentration and the peak area or absorbance at
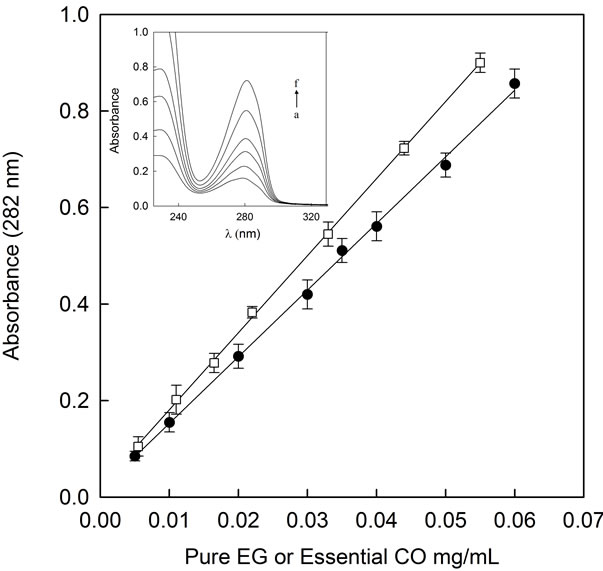
Figure 4. Essential CO (●) and pure EG (□) calibration curve at 282 nm Inset: Absortion spectra of pure EG from 2.2 × 10−3 to 0.05 mg/mL. a to f represent the increasing pure EG concentrations.
282 nm, yielding the following equations:
y = 242022 + 1516664x (GC-MS) (1)
y = 0.021 + 15.91x (UV-Spectrophotometry) (2)
with a correlation coefficient r2 of 0.999 for both, GCMS and UV-spectrophotometric analysis (Table 1).
3.1.2. Limit of Quantification (LOQ) and Limit of Detection (LOD)
The limit of detection or least detectable dose (LOD) is the smallest concentration of the analyte that produces a signal significantly different from zero with a stated degree of confidence. There is a general consensus in favor of selecting the analyte dose providing 3 times the standar deviation (SD) from the mean measurement of the blank dose signal. The LOD for GC-MS or spectrophotometric method was stimed by analysis of five sets of 12 replicates of the zero standars. The mean peak area or absorbance at 282 nm values plus 3-fold SD correspond to an estimated limit of detection of 3.2 × 10−4 and 2.2 × 10−3 mg/mL of EG for GC-MS and spectrophotometric analysis, respectively (Table 1).
The limit of quantification (LOQ) is the smallest concentration of analyte that can be measured in samples so as to yield a predicted concentration with a stated relative precision and accuracy. Commonly, the selected LOQ is defined by the mean peak area or absorbance at 282 nm value plus 10-fold SD. The LOQ of both methods were calculated on the basis of the analysis of 20 samples with 1.1 × 10−3 mg/mL for GC-MS analyses and 7.2 × 10−3 mg/mL for spectrophotometric analyses (Table 1).
3.1.3. Precision
Assay precision was tested by repeatability (intraday variation) and reproductibility (interday variation) studies. Six samples were fortified at 5 mg/mL for GC-MS and 25 µg/mL for spectrophotometric analyses, and EG concentration was measured 6 times (by triplicate), on the same day (repeatability) and of five different days (reproducibility). The SD and intra and interday coefficient variation (% CV) of the peak area for GC-MS and absorbance at 282 nm for spectophotometric values were calculated giving values of 1.5% and 4.6% for GC-MS analyses and 1.1% and 4.1% for spectrophotometric analyses (Table 1).
3.1.4. Ruggedness
To determine the ruggedness of both GC-MS and spectrophotometric methods, 5 mg/mL of EG for GC-MS analyses and 25 µg/mL of EG for spectrophotometric analyses, were analyzed for 30 days, during which peak area and absorbance at 282 nm presented a CV of 6.3% for GC-MS and 6.6% for spectrophotometry, confirming the reliability of both assays (Table 1).
3.2. Complexation Study
When the aqueous solubility of essential CO was studied in the presence of increasing CDs concentrations, different patrons of behavior were observed in the presence of the native β-CDs vs the modified HP-β-CDs.
In the case of β-CDs (Figure 5, filled circles), the total essential CO (refers always to its main compound EG concentration) dissolved increased with β-CDs concentrations up to 2 mM. At this point, the maximum level of essential CO in solution was reached (12 mM). Above β-CDs 2 mM, the essential CO concentration decreased until reach a constant level of 6 mM, creating a B-type phase-solubility profile. These results indicated the formation of CO-β-CDs complexes with limited aqueous solubility (typical for native β-CDs).
Specifically, phase solubility diagram obtained was a Bs-type. As shown in Figure 5, β-CDs concentration above 2 mM did not improve the essential CO solubility. The solubility limit of essential CO-β-CDs complexes were reached at β-CDs 2 mM and 12 mM of essential CO and further addition of CDs resulted in the formation of less soluble inclusion complexes as has been previously described by Grant and Higuchi (1990) [27]. At this point (12 mM of essential CO) no more essential CO-β- CDs soluble inclusion complexes were formed, and further addition of β-CDs led to the precipitation of less soluble complexes. Over 2 mM β-CDs concentration, a gradually increase in essential CO-β-CDs insoluble complexes were produced until reach a maximum essential CO level of 550 mg/g precipitate (Figure 5, open circles). Waleczek, et al. in 2002 [28] reported a phase solubility study of camomile essential oil with β-CDs and its major compound β-bisabolol, obtaining also B-type profiles.
To further investigate, the complexation of pure EG, the major compound of essential CO, with β-CDs was carried out. Surprisingly, the profile of pure EG using

Table 1. Values of more representative validation parameters.
β-CDs (Figure 6, open squares) was not exactly the same as that obtained for essential CO (Figure 6, filled circles) in the solubility studies.
In the case of pure EG the saturation level of dissolved compound was 12 mM and it was reached with 2 mM β-CDs concentration. It is important to note that at this point (β-CDs 2 mM) the complexation of essential CO also reached its maximum solubility level (12 mM).
Despite of the fact of reaching a constant level of pure EG-β-CDs soluble complexes, no precipitate was observed when β-CDs concentration increased above 2 mM (Figure 6, open squares), comparing with essential CO (Figure 6, filled circles). Looking at the phase solubility
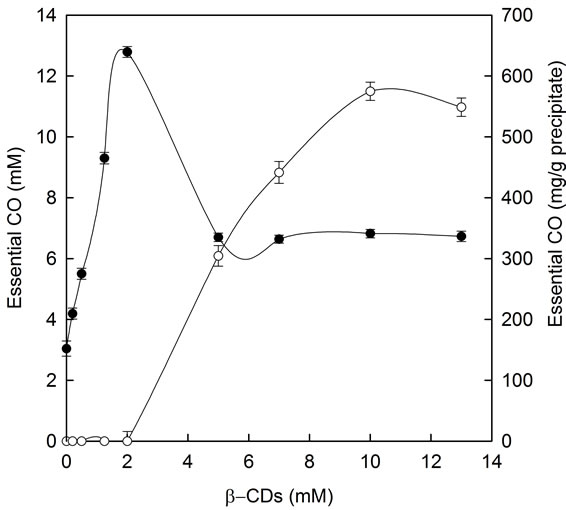
Figure 5. Phase solubility diagram for essential CO in presence of increasing β-CDs concentrations: soluble (●) and insoluble (○) essential CO-β-CDs complexes.
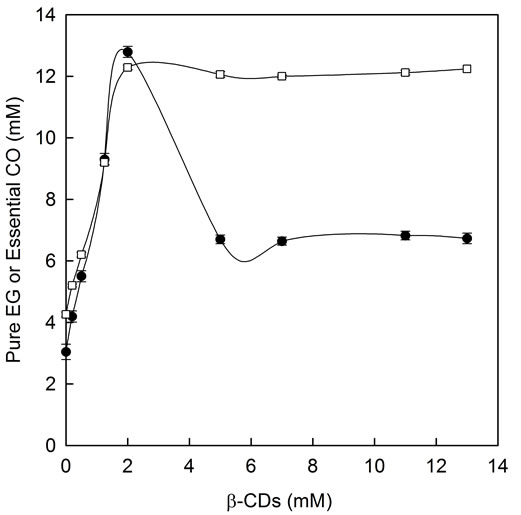
Figure 6. Phase solubility diagram for pure EG and essential CO in the presence of increasing β-CDs concentrations: pure EG-β-CDs complexes (□) and soluble essential CO-β- CDs complexes (●).
profile of pure EG showed in Figure 6 (open squares), the results clearly demonstrate that the complexation limit of pure EG with β-CDs was reached, however, the use of β-CDs concentration below its aqueous solubility limit (13 mM) did not permite us to see the formation of pure EG-β-CDs insoluble complexes.
These results could be explained by the presence of other essential CO minority compounds which although prevent the complexation process between EG and β- CDs they dismishes the EG-β-CDs complexes solubility. Similar effect was previously described by Waleczek et al. (2002) [28] for camomile essential oil.
When the slope value of the linear portion of phase solubility diagram of pure EG was calculated (0 - 2 mM β-CDs) (Figure 6, open squares), it was approximately 5, indicating the formation of complexes with stoichiometry higher than 1:1 with respect to EG.
To further investigate the relation between binding of pure EG and β-CDs, the complexation behavior of soluble pure EG at low β-CDs was examinated by spectrophotometric analysis. In this experiment the concentration of pure EG was kept constant at 0.07 mM and the CDs concentration ranged from 0 to 3 mM. When increasing concentrations of β-CDs were added to the reaction medium an exponential decay was obtained in the range of 0 - 1 mM of β-CDs concentration, when plotting the absorbance at 282 nm vs β-CDs concentration (Figure 7).
The absorbance at any wavelength (A) can be related to the CDs concentration by the equation [29]:
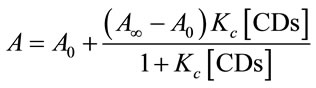 (3)
(3)
where A∞ is the absorbance when total EG has been complexed in CDs and A0 is the absorbance of pure EG in the absence of CDs.
Figure 7 shows that absorbance at 282 nm decreased at the beginning of the curve and then a slowly flattened out at β-CDs concentration values higher than 0.5 mM. This behavior suggested that the majority of EG had been included inside of the CDs cavity forming inclusion complexes. The association constant between EG-β-CDs was calculated asuming a 1:1 stoichiometry, by applying the Benesi-Hildebrand treatment to the absortion curve [30]:
 (4)
(4)
The experimental data from Figure 7 were replotted as a function of 1/[CDs] vs 1/A − A0 in the new Figure 7, inset. Fitting the data of Figure 7 inset, to Equation (4), a Kc value of 10,633 ± 614 M−1 was obtained. Zhan et al. (2008) [24] reported similar experiments with pure EG
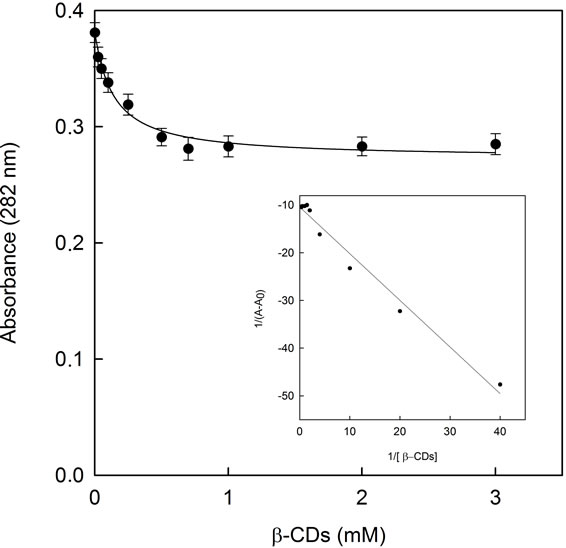
Figure 7. Absorbance of pure EG at 282 nm in the presence of increasing vs β-CDs concentrations (0 to 3 mM). The line show the best fit to Equation (3). Inset: Benesi-Hildebrat plot.
by using fluorescence spectroscopy, giving a smaller Kc value (357.46 M−1) for β-CDs. Waleczek et al. (2002) [29] investigated molecular association of β-CDs with a pure component as β-bisabolol or β-bisabolol as the major component of camomile essential oil undertaking phase solubility studies with β-CDs. In contrast with our results, they found very similar profiles for both, giving a Kc values of 273 and 304 M−1, and no insoluble complexes were formed.
It is important to note that the study of pure EG complexation with β-CDs carried out with pure EG concentrations below its aqueous solubility limit (S0), clearly reflects the 1:1 stoichiometry complexes formation, as has been previously described by Yang and Lee, (2005) [23]. However, when the complexation studies between EG (pure or as essential CO) and β-CDs were carried out at EG concentrations above S0 and high β-CDs concentrations (until 13 mM), higher order stoichiometry inclusion complexes were formed (reflected by a slope > 1 in the phase solubility diagrams). Moreover, complexes with low aqueous solubility were formed even leading to the precipitation of them.
In the essential CO solubility study in the presence of HP-β-CDs a linear response was obtained as CDs concentration increased (Figure 8, filled circles) showing an AL-type profile, previously described by Higuchi and Connors (1965) [26]. The Kc value for the complexes formation between essential CO and HP-β-CDs was calculated by using the Equation (2) obtaining a value of 2005 ± 199 M−1. This linear relationship with a slope value lower than 1, suggests a 1:1 stoichiometry for essential CO-HP-β-CDs complexes. This linear profile is
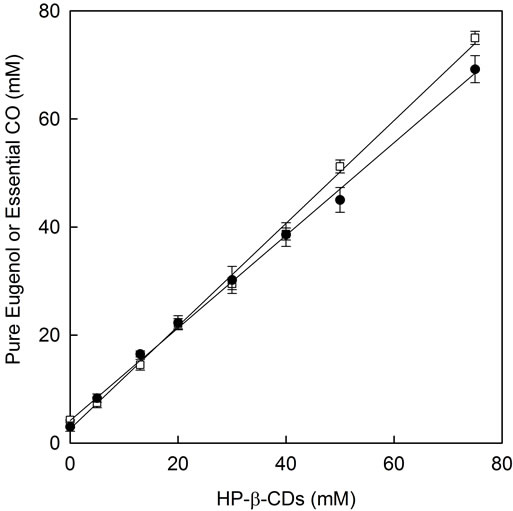
Figure 8. Phase solubility diagram for essential CO and pure EG with HP-β-CDs: essential CO-HP-β-CDs (●) and pure EG-HP-β-CDs (□).
typical for modified β-CDs and it indicates a high aqueous solubility limit of the formed complexes. These results differ from those described previously for β-CDs, in which case insoluble essential CO-β-CDs complexes were formed.
Moreover it is important to note that due to the high solubility of HP-β-CDs and the formed complexes it is possible to solubilize a high quantity of essential CO (i.e. 70 mM using 75 mM of HP-β-CDs), whereas in the case of β-CDs the highest level of soluble essential CO is 12.7 mM using 13 mM of β-CDs (its aqueous solubility limit).
When the complexation of pure EG (the major compound of essential CO) with HP-β-CDs was studied, a linear response was also obtained (Figure 8, open squares).
The apparent stability constant, Kc, for pure EG and HP-β-CDs inclusion complexes were calculated and a value of 4555 ± 225 M−1 was obtained. Garg, Gupta, Prakash and Singh (2010) [31] reported results for pure EG-HP-β-CDs giving a stability constant four times smaller (1206.4 M−1) than us.
The Kc values for pure EG and with β-CDs was higher than this obtained for its modified HP-β-CDs, (10,633 ± 614 M−1 and 4555 ± 225 M−1, respectively). Whereas the stability constants were in the same order in all cases, β-CDs showed the high stability constant. Choi et al. (2009) [25] studied molecular inclusion with β-CDs and they found that β-CDs was more effective than its modified HP-β-CDs for pure EG encapsulation. It is supposed that the side chain of HP-β-CDs might interrupt EG inclusion within the cavity of HP-β-CDs molecule.
In conclusion, this work demonstrated that EG as essential CO or as pure compound could be successfully encapsulated with β-CDs and HP-β-CDs, but there were differences in the encapsulation characteristics. Essential CO with β-CDs allows the formation of insoluble complexes, whereas no insoluble complexes were obtained in the case of HP-β-CDs. The work further make clear the complexes formation with an order higher than 1:1 with respect to EG as essential CO for β-CDs, when saturating essential CO and high β-CDs concentrations were used. The same experiment with HP-β-CDs permit a 1:1 complexes formation. In global terms and despite of the fact that all the Kc values are in the same order of magnitude, pure EG showed higher values than EG encapsulated as essential CO. These results suggest that both β-CDs and its modified HP-β-CDs could be an alternative to solve out the practical application of the essential CO and at the same time facilitate the controlled release of its constituents in food and medical industries.
4. Acknowledgements
We gratefully acknowledge Lidervet for material support. P.H.S. is a holder of a research grant from the Programa Nacional de Formación de Personal Investigador (FPI) of Fundación Séneca.
REFERENCES
- S. N. Ebrahimi, J. Hadian, M. H. Mirjalili, A. Sonboli and M. Yousefzadi, “Essential Oil Composition and Antibacterial Activity of Thymes caramanicus at Different Phonological Stages,” Food Chemistry, Vol. 110, 2008, pp. 927-931. doi:10.1016/j.foodchem.2008.02.083
- R. Schmid, “A Resolution of the Eugenia-Syzygium Controversy (Myrtaceae),” American Journal of Botany, Vol. 59, No. 4, 1972, pp. 423-436. doi:10.2307/2441553
- L. A. Shelef, “Antimicrobial Effects of Spices,” Journal Food Safety, Vol. 6, 1983, pp. 29-44. doi:10.1111/j.1745-4565.1984.tb00477.x
- C. G. Soto and Burhanuddin, “Clove Oil as Fish Anaesthesic for Measuring Length and Weight of Rabbit Fish (Siganus lineatus),” Aquaculture, Vol. 136, No. 1-2, 1995, pp. 149-152. doi:10.1016/0044-8486(95)01051-3
- J. L. Ackerman and D. R. Bellwood, “Comparative Efficiency of Clove Oil vs Rotenone for Sampling Tropical Reef Fish Assemblages,” Journal Fish Biology, Vol. 60, 2002, pp. 893-901. doi:10.1111/j.1095-8649.2002.tb02416.x
- G. N. Wagner, T. D. Singer and M. McKinley, “The Ability of Clove Oil and MS-222 to Minimize Handling Stress in Rainbow Trout (Oncohynchus Mykiss Walbaum),” Aquaculture Research, Vol. 34, No. 13, 2003, pp. 1139-1146. doi:10.1046/j.1365-2109.2003.00916.x
- P. Hoskonen and J. Pirhonen, “The Effect of Clove Oil Sedation on Oxygen Consumption of Six TemperateZone Fish Species,” Aquaculture Research, Vol. 35, No. 10, 2004, pp. 1002-1005. doi:10.1111/j.1365-2109.2004.01084.x
- R. Roubach, L. C. Gómez, F. A. Fonesca and A. L. Val, “Eugenol as an Efficacious Anaesthesic for Tambaqui, Colosoma macropomum (Cuvier),” Aquaculture Research, Vol. 36, 2005, pp. 1056-1061. doi:10.1111/j.1365-2109.2005.01319.x
- T. Atsumi, I. Iwaka, S. Fujisawa and T. Ueha, “Reactive Oxygen Species Generation and Photo-Cytotoxicity of Eugenol in Solutions of Various pH,” Biomaterials, Vol. 22, No. 12, 2001, pp. 1459-1466. doi:10.1016/S0142-9612(00)00267-2
- S. Fujisawa, T. Atsumi, Y. Kadoma and H. Sakagami, “Antioxidant and Prooxidant Action of Eugenol Related Compounds and Their Toxicity,” Toxicology, Vol. 177, No. 1, 2002, pp. 39-54. doi:10.1016/S0300-483X(02)00194-4
- M. Ito, K. Murakami and M. Yoshino, “Antioxidant Action of Eugenol Compound: Role of Mental Ion in the Inhibition of Lipid Peroxidation,” Food Chemistry Toxicology, Vol. 43, 2005, pp. 461-466. doi:10.1016/j.fct.2004.11.019
- J. L. Briozzo, J. Chirife, L. Herzage and M. Dáquino, “Antimicrobial Activity of Clove Oil Dispersed in a Concentrated Sugar Solution,” Journal of Applied Bacteriology, Vol. 66, 1989, pp. 69-75. doi:10.1111/j.1365-2672.1989.tb02456.x
- I. Gülçin, M. Elmastas and H. Y. Aboul-Enein, “Antioxidant Activity of Clove Oil: A Powerful Antioxidant Source,” Arabian Journal Chemistry, in Press.
- V. Mouchrek, “Analytical Studies and Chemical Modifications by Methylation and Acetylation of Eugenol of Essential Oil Extracted from the Leaves of Pimenta Dioica Lindl,” PhD Thesis, Universidade São Paulo, São Paulo, 2000.
- A. Ozturz and H. Ozbek, “The Anti-Inflamatory Activity of Eugenia Caryophyllata Essential Oils: An Animal Model of Anti-Inflammatory Activity,” European Journal of General Medicine, Vol. 2, 2005, pp. 159-163.
- K. H. Son, S. Y. Kwon, H. P. Kim, H. W. Chang and S. S. Kang, “Constituents of Syzigium Aromaticum Merr et Perry,” Journal of natural Products, Vol. 4, 1998, pp. 263-267.
- T. Ohakubo and M. Shibata, “The Selective Capsaicin Receptor Antagonist Capsazepine Abolishes the Anticonceptive Actions of Eugenol and Guaiacol,” Journal of Dental Research, Vol. 76, 1997, pp. 848-851. doi:10.1177/00220345970760040501
- H. C. Ou, F. P. Chou, T. M. Lin, C. H. Yang and W. H. Sheu, “Protective Effects of Eugenol Against Oxidized LDL-Induced Cytotoxicity and Adhesion Molecule Expression in Endothelial Cells,” Food Chemistry Toxicology, Vol. 44, 2006, pp. 1485-1495. doi:10.1016/j.fct.2006.04.011
- G. M. Laeckeman, L. V. Hoof, A. Haemers, D. A. Vandem and A. G. Herman, “Eugenol: A Valuable Compound for in Vitro Experimental Research and Worthwhile for Further in Vivo Investigation,” Phytoterapy Research, Vol. 4, 2006, pp. 90-96. doi:10.1002/ptr.2650040304
- D. Kalemba and A. Kunicka, “Antibacterial and Antifungical Properties of Essentials Oils,” Current Medicinal Chemistry, Vol. 10, No. 10, 2003, pp. 813-829. doi:10.2174/0929867033457719
- Y. Cai, S. H Gaffney, T. H. Lilley, D. Magnolato, R. Martin, C. M. Spencer and E. Haslam, “Polyphenol Interactions, Part 4: Model Studies with Caffeine and Cyclodextrins,” Journal of the American Chemical Society, Vol. 2, 1990, pp. 2197-2209.
- S. Divakar and M. M. Maheswaran, “Structural Studies on Inclusion Compounds of β-Cyclodextrins with Some Substitued Phenols,” Journal of Inclusion Phenomena and Macrocyclic Chemistry, Vol. 27, No. 2, 1997, pp. 113-126. doi:10.1023/A:1007949215051
- Y. Yang and L. X. Song, “Study on the Inclusion Compounds of Eugenol with α-, β-, γ and Heptakis (2,6- di-O-methyl) β-Cyclodextrins,” Journal Inclusion Phenomenom Macrocyclic Chemistry, Vol. 53, 2005, pp. 27- 33. doi:10.1007/s10847-005-0247-4
- H. Zhan, Z. Jiang, Y. R. Wang and T. Dong, “Molecular Microcapsules and Inclusion Interactions of Eugenol with β-Cyclodextrins and Its Derivatives,” European Food Research Technology, Vol. 227, 2008, pp. 1507-1513. doi:10.1007/s00217-008-0873-3
- M. J. Choi, A. Soottitantawat, O. Nuchuchua, S. G. Min and U. Ruktanonchai, “Physical and Light Oxidative Properties of Eugenol Encapsulated by Molecular Inclusion and Emulsion-Diffusion Method,” Food Research International, Vol. 42, No. 1, 2009, pp. 149-156. doi:10.1016/j.foodres.2008.09.011
- T. Higuchi and K. A. Connors, “Phase Solubility Techniques,” Advances in Analitical Chemistry Instrument, Vol. 4, 1965, pp. 56-63.
- D. J. Grant and T. Higuchi, “Solubility Behaviour of Organics Compounds. Techniques of Chemistry,” Wiley Interscience Publication 21, New York, 1990.
- K. J. Waleczek, H. M. Cabral-Marques, B. Hempel and P. C. Schmidt, “Phase Solubility Studies of Pure α-Bisabolol and Camomile Essential Oil with β-Cyclodextrins,” European Journal Pharmacia Biopharmacia, Vol. 55, 2002, pp. 247-251. doi:10.1016/S0939-6411(02)00166-2
- K. A. Connors, “Binding Constants,” Wiley, New York, 1987, pp. 103-108.
- J. H. Benesi-Hildebrant, “A Spectrophotometric Investigation on the Interaction on the Interaction of Iodine with Aromatic Hydrocarbons,” Journal of the American Chemical Society, Vol. 71, No. 8, 1949, pp. 2703-2707. doi:10.1021/ja01176a030
- A. Garg, B. Gupta, R. Prekash and S. Singh, “Preparation and Characterization of Hydroxypropyl-b-Cyclodextrins (HP-β-CD) Inclusion Complex of Eugenol: Differential Pulse Voltammetry and HNMR,” Chemical Pharmacollogy bulletin, Vol. 58, 2010, pp. 1313-1319.
NOTES
*Corresponding author.

